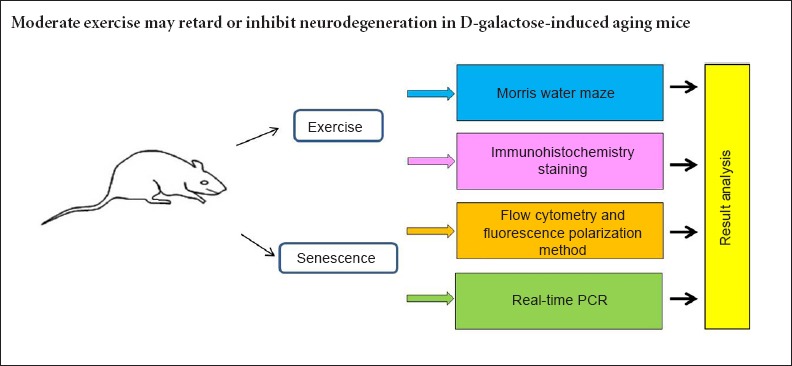
Keywords: nerve regeneration, D-galactose, brain aging, behavioral performance, brain-derived neurotrophic factor, neuronal apoptosis, glucose transporters, synaptic plasticity, neurodegeneration, neural regeneration
Abstract
D-galactose has been widely used in aging research because of its efficacy in inducing senescence and accelerating aging in animal models. The present study investigated the benefits of exercise for preventing neurodegeneration, such as synaptic plasticity, spatial learning and memory abilities, in mouse models of aging. D-galactose-induced aging mice were administered daily subcutaneous injections of D-galactose at the base of the neck for 10 consecutive weeks. Then, the mice were subjected to exercise training by running on a treadmill for 6 days a week. Shortened escape latency in a Morris water maze test indicated that exercise improved learning and memory in aging mice. The ameliorative changes were likely induced by an upregulation of Bcl-2 and brain-derived neurotrophic factor, the repression of apoptosis factors such as Fas and Bax, and an increase in the activity of glucose transporters-1 and 4. The data suggest moderate exercise may retard or inhibit neurodegeneration in D-galactose-induced aging mice.
Introduction
Aging populations are increasing worldwide, and many health problems, such as cognitive decline, cardiovascular disease, oxidative stress, cancer, stroke, and hypertension, can seriously affect elderly individuals. Lifestyle interventions, such as physical activity and exercise, environmental enrichment, and energy restriction, were demonstrated to prevent and possibly restore health problems in aged individuals (van Praag et al., 2005; Mora et al., 2007; Adams et al., 2008). Regardless of exercise intensity, physical activity and exercise might be an accessible way to protect or enhance cognitive brain functions of elderly individuals. However, the mechanism that mediates physical activity impact on the rate of cognitive decline is still under investigation. The mouse hippocampus, the memory area of the brain, is stimulated by exercise, and running increases neurogenesis in the granule cell layer of the adult hippocampus (Ferrari, 2007). In addition to increasing the proliferation of hippocampal cells, exercise also up-regulates neurotrophic factors, and most research has focused on brain-derived neurotrophic factor (BDNF) (Duman, 2005). Exercise also enhances the functional brain capabilities by acting on synapses and changing the number, structures, and functional features of synapses, termed as “synaptic plasticity”. Synaptic plasticity is essential for learning and memory, and it is believed necessary to preserve or restore brain function during aging or injury (Kim and Linden, 2007). Therefore, this study used D-galactose-induced aging mice to investigate the factors involved in mediating the positive effects of exercise on spatial learning and memory capacity.
Materials and Methods
Ethics statement
Animal studies were approved by the Care And Use of Animals for Scientific Purposes in China and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Precautions were taken to minimize suffering and the number of animals used in each experiment.
Aging model establishment and exercise treatment
A total of 40 healthy 3-month-old Institute of Cancer Research (ICR) male mice were obtained from the Experimental Animal Center of Capital Medical University in China (license No. SCXK (Jing) 2012-0006), and were housed individually in specific pathogen-free conditions with 12-hour light-dark cycles. All animals were fed standard laboratory rodent chow and tap water ad libitum, and were weighed weekly. For specific intervention, mice were randomly assigned to one of four groups (10 in each group): aging model groups with (ECA) or without (SECA) exercise, and normal control groups with (ECN) or without (SECN) exercise.
Aging was induced in mice by administering daily subcutaneous injections of D-galactose (Sigma, St. Louis, MO, USA) (100 mg/kg body weight in 0.9% saline), at the base of the neck for 10 consecutive weeks (Gao et al., 2015; Yu et al., 2015; Zhou et al., 2015). Mice were subjected to exercise training by running on a mouse treadmill (Respironics, Bend, OR, USA) for 6 days a week with 1 day of rest. Initially, they ran for 10 minutes per day at a speed of 15 m/min, with a progressive increase of time (5 minutes) and speed (5 m/min) per week until reaching a total of 20 minutes at 25 m/min per day during the 4th week. Then the mice were maintained on this exercise regimen until the end of the 10th week (Jeong et al., 2015; Shibuya et al., 2015). Normal control mice in both the sedentary and exercise training groups were administered subcutaneous injections of the vehicle only (0.9% saline) in an equivalent volume (same volume as 100 mg/kg body weight D-galactose in 0.9% saline) at the base of the neck for 10 consecutive weeks (Figure 1).
Figure 1.
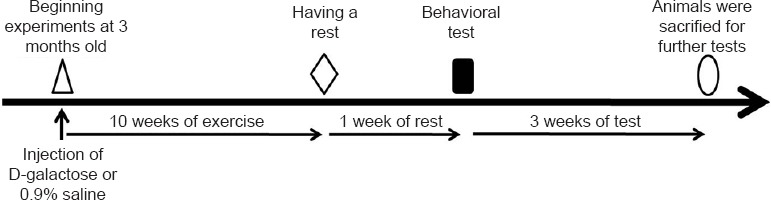
Experiment schedule of the establishment of the aging model and exercise treatment.
Morris water maze test
After 10 weeks of exercise, the mice had 1 week of rest. Then the Morris water maze task was used to evaluate the spatial memory performance of the animals (Morris, 1984; Li et al., 2015). The water maze required the mice to swim and find a submerged platform 1 cm below the surface of the water for safety. The pool had a diameter of 1.20 m and a depth of 0.5 m, and was filled with 30 cm of water at 22–24°C. For each trial, a mouse entered the pool from an ambulatory position for a maximum of 2 minutes. The mouse used spatial cues held at fixed positions in the room to find the escape platform. If the platform was not located in time, the mouse was directed to the platform and allowed to stay there for 15 seconds. Two trials were conducted per day for each animal with an interval of 2 hours between trials for 7 days. All trials were recorded by a monitor system (Nodulus, Wageningen, Netherlands). For data analysis (escape latency, distance moved), the last 5 days’ test records were used.
Apoptosis analysis by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end-labeling (TUNEL) staining
Following the water maze test, five animals from each group were sacrificed by cervical dislocation under intraperitoneal sodium pentobarbital anesthesia (40 mg/kg). The brains were rapidly removed and the hippocampi (Głombik et al., 2015) were bilaterally dissected on ice. These two regions from one hemisphere were homogenized with 3 mL of phosphate-buffered saline (PBS) (pH 7.0). After the homogenate was centrifuged, the sediments were collected and prepared for mono cell suspension (the cell mortality was less than 5%, and the cell counts were 1 × 106/mL). TUNEL staining was performed using the TUNEL apoptosis detection kit (Roche, Mannheim, Germany) according to the manufacturer's instructions. Briefly, the number of apoptotic cells was determined by nuclear DNA fragmentation using the deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay according to the manufacturer's recommendations. A total of 1 × 106/mL cells were pelleted by centrifugation, resuspended in 3.7% formaldehyde solution and incubated at room temperature for 10 minutes. The cells were collected by centrifugation, resuspended in PBS and incubated for 2 minutes at room temperature. Following another centrifugation, 100 µL of cytonin was added to the cell pellets, and pellets were incubated for 30 minutes. The cells were washed with TdT labeling buffer and incubated with labeling reaction mix for 1 hour at 37°C. The reaction was stopped, and the cells were incubated with streptavidin-FITC working solution for 10 minutes at room temperature. The cells were centrifuged, resuspended in 500 µL of PBS, and then submitted to flow cytometer analysis with an excitation wavelength of 488 nm (emission wavelength of 550 nm for FITC).
Synapse assessment by flow cytometry
The frontal cortex and hippocampus from the other hemisphere were homogenized with 0.32 M ice-cold glucose water. After the homogenate was centrifuged, the supernatants were collected and covered with 1.2 M ice-cold glucose water. The samples were then centrifuged at 4°C, and the middle layer was carefully collected. The surface was then covered with 0.8 M ice-cold glucose water and centrifuged. The sediments were stored for further analysis. For all specimens, four-color flow cytometer was performed on a BD FACSCanto II flow cytometer (BD BioSciences, NJ, USA) with commercially available reagents. The sediments were washed with 1 mL of Na-PSS buffer (145 mM NaCl, 2.6 mM KCl, 2.4 mM KH2PO4, 0.02 mM CaCl2, 1.2 mM MgCl2, 10 mM glucose, 10 mM HEPES, pH 7.4) and resuspended to the desired protein concentration in Na-PSS according to the protein concentration of the sediments assessed by the Bradford method (Ku et al., 2013). Next, 100 µL of the suspension was incubated with 50 µL of primary antibodies (anti-synaptophysin, dilution 1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1.5 hours at 4°C, washed with wash buffer, centrifuged and incubated with 50 µL of secondary antibodies (FITC-anti-mouse-antibodies, dilution 1:1,000, Santa Cruz Biotechnology) for 1.5 hours at 4°C. Then, the samples were washed twice with wash buffer, centrifuged, and resuspended with appropriate amounts of PBS. The number of synapses was analyzed by flow cytometry.
Membrane fluidity of synaptosomes detected by fluorescence polarization method
The same volume (20 µM) of fluorescence 1,6-dipheny-1,3,5-hexatriene work fluid was added to each of the 500-µL synapse suspensions. The final concentration was 10 µM. The control synapse suspensions did not contain 1,6-dipheny-1,3,5-hexatriene. The fluid was incubated for 15 minutes at 37°C in the dark and then centrifuged at 4°C. The supernatants were removed, and the remaining samples were resuspended with Na-PSS buffer and reacted for 10 minutes at 37°C. The fluorescence intensity was analyzed with a fluorescence spectrophotometer (Eppendorf, Hamburg, Germany). The stimulation light wavelength was 360 nm, and the emission light wavelength was 430 nm. The fluorescence intensity was analyzed using control fluid (I900, 00, I900, 900). The fluorescence polarization correction factor (G= I900, 00/I900, 900) was calculated. Then, each sample's fluorescence intensity of I900, 00 and I900, 900 was assessed. The fluorescence polarization degree [(P= (I900, 900-G I900, 00)/(I900, 900 +G I900, 00)] and membrane viscosity [(η=2P/(0.46-P)] were also calculated.
Immunohistochemistry staining
Fas, Bax, and Bcl-2 are related to apoptosis (Hyun et al., 2015), and BDNF plays a supporting role in synaptic plasticity and promoting the growth of neurons (Yang et al., 2015). Another five animals in each group were anesthetized with intraperitoneal sodium pentobarbital (40 mg/kg). Heart perfusion was performed with PBS and then with 4% paraformaldehyde in 0.1 M PBS. The brains were removed and hemispheres were post-fixed in 4% paraformaldehyde overnight at 4°C (the other hemispheres were used for real-time PCR examination of gene expression). The hemispheres were cut into 35-µm-thick sections and incubated overnight with mouse anti-rat fas/bax/bcl-2/BDNF antibody (Santa Cruz Biotechnology; 1:500 in Tris-buffered saline containing 3% bovine serum and 0.1% Triton X-100) at 4°C followed by biotinylated goat or rabbit anti-mouse IgG, 1:500; Santa Cruz Biotechnology). Then, 3,3′-diaminobenzidine tetrahydrochloride (DAB, Sigma) was used for immunohistochemistry staining. An irrelevant primary antibody served as a negative control. Assessments were independently performed by two pathologists who were blinded to the experimental conditions. Positive cells were counted in several hippocampal subregions and all images were acquired using a Nikon microscope (Nikon 80i, Tokyo, Japan).
Glucose transporter-1 (GLUT1) and glucose transporter-4 (GLUT4) mRNA expression measurement by real-time PCR
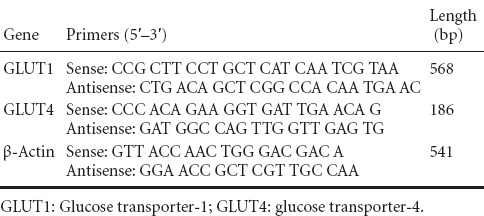
Previous studies indicated that glucose tolerance can impact cognitive performance (Nilsson et al., 2013). Poor glucose tolerance appears to be strongly associated with detrimental verbal memory, logical memory, and spatial memory (Soares et al., 2013). Total RNA was extracted from a 100-mg tissue sample (50 mg cortex and hippocampus each) using the RNeasy kit (Qiagen AG, Hilden, Germany) according to the manufacturer's instructions. Two micrograms of total RNA was reverse transcribed using the T-primed first strand kit (Amersham, Piscataway, NJ, USA). Oligonucleotide primers and MGB fluorescent probes were purchased from Applied Biosystems. cDNA (2 µL) of GLUT1 and GLUT4 was amplified using primer pairs (Table 1). Real-time PCR was performed at 95°C for 3 minutes, followed by 40 cycles of denaturation at 94°C for 45 seconds, annealing at 60°C for 45 seconds, and extension at 72°C for 1 minute. For each PCR run, a standard curve was produced with four consecutive 1:10 dilutions of a positive sample. All samples were performed in triplicate. An Applied Biosystems Prism 7300 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) was used for detection and quantification.
Table 1.
Primer sequences and the size of amplification products
Statistical analysis
The data were presented as the mean ± SEM derived from a minimum of two separate cell preparations. One-way analysis of variance (ANOVA) or unpaired Student's t-test was used for statistical analyses between experimental groups. A value of P < 0.05 was considered significant. The data were analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA).
Results
Effect of moderate exercise on the body weight of D-galactose-induced aging mice
As expected, the mice with exercise weighed less than sedentary mice, but there was no significant difference between groups (P > 0.05).
Moderate exercise improved learning in D-galactose-induced aging mice
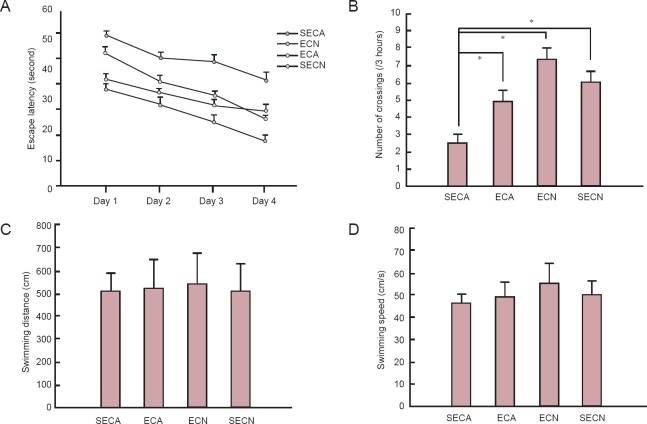
As shown in Figure 2A, the escape latency gradually shortened in all groups. SECA mice had a longer latency to find the hidden platform compared with the other groups (P < 0.05), but the prolonged escape time was significantly reduced in ECA mice compared with the SECA group (P < 0.05). There were no significant differences among the other groups except for the SECA group (P > 0.05). The SECA group performed significantly fewer platform crossings than the other three groups (P < 0.05; Figure 2B). The spatial memory curve for swimming distance and speed did not show the same pattern as the latency curve. There were no differences in swimming pattern or speed between groups (P > 0.05), but the swimming pattern and speed of the ECN group had a tendency to be longer and faster (Figure 2C and D).
Figure 2.
Effects of exercise on the performance of mice in the Morris water maze test.
(A) Mean latency in the hidden platform test after training. (B) The number of platform crossings in 3 hours. (C) The mean swimming distance on day 6 in each group. (D) The mean swimming speed on day 6. All data are reported as the mean ± SEM. One-way analysis of variance or unpaired Student's t-test was used for statistical analyses. *P < 0.05. ECA: Aging model groups with exercise; SECA: aging model group without exercise; ECN: normal control group with exercise; SECN: normal control group without exercise.
Effect of moderate exercise on neuronal apoptosis in the hippocampus of D-galactose-induced aging mice
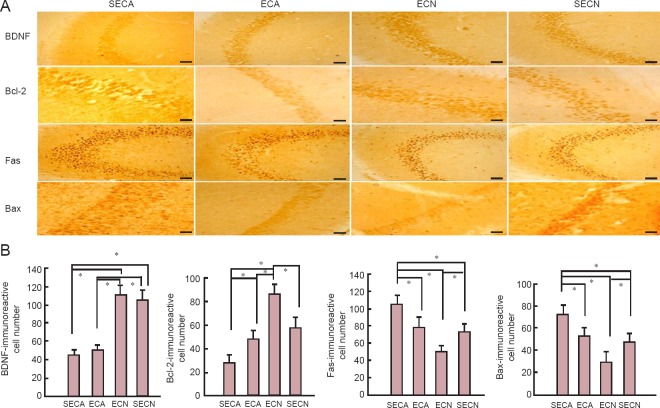
The results of immunohistochemistry showed significantly greater amounts of Fas and Bax positive neurons in the mouse hippocampus of the SECA group compared with the SECN and ECA groups (P < 0.05). The number of Fas and Bax positive neurons in the mouse hippocampus of the ECN group was significantly lower than in the SECN group (P < 0.05). The number of BDNF positive neurons in the mouse hippocampus of the SECA and ECA groups was significantly lower than in the SECN and ECN groups (P < 0.05). The number of Bcl-2 positive neurons in the mouse hippocampus of ECA group was significantly greater than in the SECA group (P < 0.05). The number of Bcl-2 positive neurons in the mouse hippocampus of the ECN group was significantly greater than in the SECN group (P < 0.05). There were no significant differences in the number of BDNF positive neurons in the mouse hippocampus of the ECA and SECA groups (P > 0.05), or the ECN and SECN groups (P > 0.05; Figure 3).
Figure 3.
Effect of moderate exercise on BDNF, Bcl-2, Fas, and Bax immunoreactivity in the hippocampus of D-galactose-induced aging mice.
(A) The images show BDNF, Bcl-2, Fas, and Bax immunoreactivity in the hippocampus of mice (immunohistochemistry). Scale bars: 50 μm. (B) Quantitation of BDNF, Bcl-2, Fas, and Bax immunoreactive cells (200-fold field) in the hippocampus of mice. Data are presented as the mean ± SEM. One-way analysis of variance or unpaired Student's t-test was used for statistical analyses. *P < 0.05. BDNF: Brain-derived neurotrophic factor; ECA: aging model groups with exercise; SECA: aging model group without exercise; ECN: normal control group with exercise; SECN: normal control group without exercise.
The TUNEL assay showed that the neuronal apoptosis ratio in the hippocampus of the ECA group was much lower than in the SECA group (P < 0.05). There were no significant differences in the neuronal apoptosis ratio among the ECA, SECN, and ECN groups (P > 0.05; Figure 4).
Figure 4.
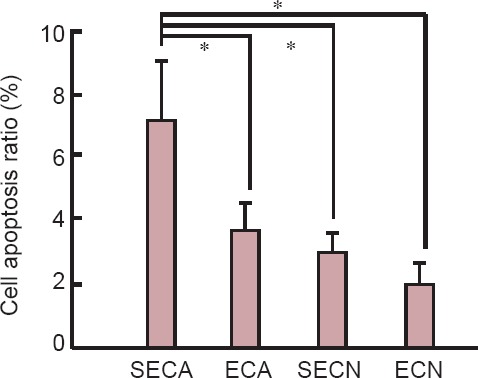
Effect of moderate exercise on cell apoptosis in the hippocampus of D-galactose-induced aging mice (TUNEL assay).
Data are presented as the mean ± SEM, derived from a minimum of two separate cell preparations. One-way analysis of variance or unpaired Student's t-test was used for the statistical analyses. *P < 0.05. ECA: Aging model group with exercise; SECA: aging model group without exercise; ECN: normal control group with exercise; SECN: normal control group without exercise.
Effect of moderate exercise on hippocampal synapse number and membrane fluidity of synaptosomes in the D-galactose-induced aging mice
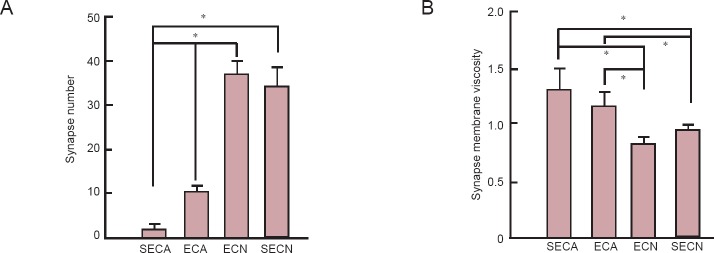
The number of hippocampal synapses in aged mice (SECA group) was significantly lower than that in the SECN group (P < 0.05). The number of hippocampal synapses in the mice in the ECA group was greater than those in the SECA group (P < 0.05), but there was no significant difference in the number of hippocampal synapses between the ECN and SECN groups (P > 0.05). The membrane viscosity of synaptosomes in the mice of SECA and ECA groups was significantly higher than that in the SECN and ECN groups (P < 0.05), but there was no difference in the membrane viscosity of synaptosomes in the mice between the ECA and SECA groups (P > 0.05; Figure 5).
Figure 5.
Effect of moderate exercise on hippocampal synapse number and membrane fluidity of D-galactose-induced aging mice.
Synapse number and membrane fluidity of synaptosomes analyzed by flow cytometry (A) and fluorescence polarization (B). Higher synapse membrane viscosity indicates lower synapse membrane fluidity. Data are presented as the mean ± SEM, derived from a minimum of two separate cell preparations. One-way analysis of variance or unpaired Student's t-test was used for statistical analyses. *P < 0.05. ECA: Aging model group with exercise; SECA: aging model group without exercise; ECN: normal control group with exercise; SECN: normal control group without exercise.
Effect of moderate exercise on GLUT1 and GLUT4 gene expression in the brain of D-galactose-induced aging mice
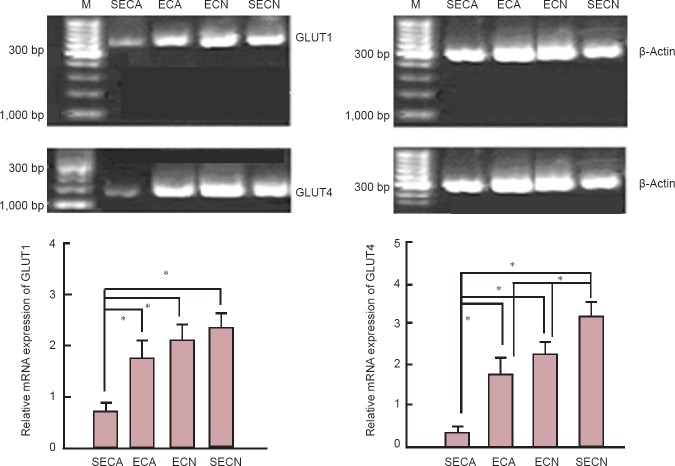
The expression levels of GLUT1 and GLUT4 mRNA in the mouse brains of the SECA group were significantly decreased compared with the other three groups (P < 0.05). There was no significant difference in the expression levels of GLUT1 mRNA in the mouse brain among the ECA, ECN and SECN groups (P > 0.05). The expression level of GLUT4 mRNA in the mouse brain of the ECA group was significantly decreased compared with the ECN and SECN groups (P < 0.05). The level of GLUT4 mRNA in the mouse brain of the ECN group was significantly higher than in the SECN group (P < 0.05; Figure 6).
Figure 6.
Influence of exercise on GLUT1 and GLUT4 mRNA expression in the aging mouse brain (quantitative real-time PCR).
The expression of GLUT1 and GLUT4 mRNA was expressed as the optical density ratio of GLUT1 and GLUT4 mRNA to β-actin. Data are presented as the mean ± SEM, derived from a minimum of two separate cell preparations. One-way analysis of variance or unpaired Student's t-test was used for statistical analyses. *P < 0.05. ECA: Aging model group with exercise; SECA: aging model group without exercise; ECN: normal control group with exercise; SECN: normal control group without exercise; GLUT1: glucose transporter-1; GLUT4: glucose transporter-4; M: marker.
Discussion
D-galactose is a natural substance found in the body, but excessive levels of D-galactose can occur by the overexpression of superoxide anions and free radicals, resulting in neurodegeneration and cognitive dysfunction (Lu et al., 2006). Previous studies revealed that D-galactose increased apoptosis in the hippocampal neurons of mice, causing a decline in spatial learning and memory and ultimately resulting in brain aging (Lipman et al., 1999; Lu et al., 2007; Wu et al., 2008; Yu et al., 2013). D-galactose-induced neurobiological and behavioral changes imitate the characteristics of the natural aging brain, and D-galactose-induced rodents have been used as an animal model for the study of brain aging (Wei et al., 2005; Cui et al., 2006; Kumar et al., 2009). Here, our findings show the results of an intervention with exercise training in an animal model of D-galactose-induced aging mice.
We first investigated the expression of GLUT1 and GLUT4 in the brain of aging mice and the influence of exercise on their expression. Many studies have suggested that cell energy metabolism supports brain function and survival (Attwell and Laughlin, 2001). Glucose transporters (GLUTs) accelerate glucose transport across the blood-brain barrier and the entry of glucose into neurons (Brown, 2000; McEwen and Reagan, 2004; Simpson et al., 2007). Brain GLUTs display a cell type and region-specific distribution, indicating the transfer of glucose across the blood-brain barrier is tightly compartmentalized. GLUT1 is widely expressed in the brain and is responsible for the majority of its glucose uptake and utilization. Insulin-sensitive GLUT4 (Temofonte et al., 2009; Ong et al., 2012; Osorio-Fuentealba et al., 2013) is mainly distributed in neurons, especially those with somatodendritic expression, suggesting it may be involved in the central actions of insulin and highly specialized activities in the central nervous system. We found that the expression of GLUT1 and GLUT4 in aging brains was reduced. The expression level of GLUT1 and GLUT4 was increased by exercise. These results indicate exercise increased glucose uptake by elevating insulin sensitivity, which in turn improved the effects of GLUT-4 by trafficking to the cell membrane and elevating GLUT4 expression levels. Exercise also elevated GLUT1 expression levels. These results are consistent with the findings reported by Kivelä and Henriksen et al. (Henriksen, 2002; Kivelä et al., 2006). This experiment also indicated that cognitive function is partially associated with insulin sensitivity and brain glucose metabolization, which are impaired in the elderly, causing increased or reduced cognitive impairment, respectively. Biessels et al. (1998) and Magariños et al. (2001) reported that insulin replacement reversed impaired cognitive function and hippocampal synaptic plasticity in streptozotocin-treated rats. In summary, impaired glucose utilization, gluco-regulatory activities and transport of GLUTs have a negative impact on hippocampal cognitive functions. Exercise may partially reverse these effects.
In addition, we measured BDNF expression levels in the hippocampus. BDNF promoted the survival and differentiation of neurons, protected neurons against neurodegeneration and neural damage, and inhibited age-related decline in memory and cognition (Mu et al., 1999; Tyler et al., 2002; Yamada et al., 2002). Exercise regulated the expression of BDNF with age (Oliff et al., 1998). Previous studies showed that exercise alone did not improve spatial memory or only had mild positive effects. However, complicated environmental enrichment paradigms improved and increased neurogenesis and growth factor levels in the hippocampus (Frick and Benoit, 2010). In this study, we observed that BDNF in the ECA group was not significantly improved compared with the SECA group. Only the ECN group displayed a slight elevation compared with the SECN group. Normal mice with or without exercise had significantly different BDNF levels from the D-galactose-induced aging mice. This result is similar to a previous study reported by Adlard et al. (Adlard et al., 2005). This indicates that BDNF is decreased in the hippocampus of aging mice and is in agreement with Kuhn et al. (Kuhn et al., 1996). Exercise may not effectively reverse the BDNF levels of aging mice. This suggests that improved learning and memory ability in aging mice might not be induced by BDNF. Although BDNF has a crucial role in the maintenance of neuron survival and normal cognitive functions, it is not the only factor involved in these processes. Other factors, such as basic fibroblast growth factor, can also regulate neural cell survival, differentiation, learning, and memory processes (Benito and Barco, 2010). Other growth factors should therefore be observed in future studies.
In addition to the above-mentioned neurotrophic factors, other molecules, such as anti-apoptotic Bcl-2 family members, promote neural-protective actions (Akhtar et al., 2004). In this study, we investigated Bcl-2 and the main pro-apoptosis protein Bax, which forms heterodimers with Bcl-2 to prevent the protective functions of Bcl-2 (Kuwana and Newmeyer, 2003). The balance between Bax/Bcl-2 is an important factor in determining whether cells undergo apoptosis, and this Bax/Bcl-2 balance can be altered during aging (Savory et al., 1999; Almeida et al., 2000). In the present study, it was observed that Bcl-2-immunopositive cells were enhanced after training in all exercise groups regardless of whether they were injected with D-galactose with respect to the sedentary groups, and Bax showed the opposite result. Furthermore, the other indicator of apoptosis, Fas, displayed the same results as Bax. To confirm that apoptosis was increased in the SECA group, we determined the neuronal apoptosis ratio in the hippocampus of each group by flow cytometry. The results verified that the SECA group had the highest neuronal apoptosis ratio among the four groups. Treadmill exercise suppressed D-galactose-induced apoptosis in the hippocampus, which indicated that treadmill exercise inhibits the effect of D-galactose-induced apoptotic neuronal cell death and aging. Consistent with other studies (Kuhn et al., 2005; Chae and Kim, 2009; Kim et al., 2010), forced, moderate-intensity treadmill exercise also ameliorated apoptotic neuronal cell death caused by D-galactose-induced brain aging. The current explanation is that a loss of memory occurs through decreased neurogenesis and increased apoptosis in the hippocampal dentate gyrus. Treadmill exercise improves the spatial memory by increasing neurogenesis and suppressing apoptosis in the hippocampus of D-galactose-induced aging mice.
The decline in spatial memory is caused by structural and physiological alterations in the hippocampus (Kuhn et al., 1996; Mattson and Magnus, 2006; Jacobson et al., 2008). We also investigated the numbers of synapses and membrane fluidity of synaptosomes, termed “synaptic plasticity” (Mattson, 2012). We found that the membrane fluidity was decreased in the SECA and ECA groups, and was not significantly improved by exercise. The number of synapses was significantly decreased in aged mice. Exercise prevented the aging-related decline in the number of synapses. These results are consistent with that of Patten et al. (2013). Other studies indicated that higher synaptic numbers have a beneficial effect on the performance of the central nervous system (deToledo-Morrell et al., 1988). Tamakoshi et al. (2014) reported that motor skill training enhanced the neural activity and synaptic plasticity of the exercise group compared with the no exercise group. The synapse membrane fluidity in the ECA group was not significantly improved compared with the SECA group, which may be explained by the short-term training periods. These results suggest that long-term training is required to enhance hippocampal functions.
Based on the results of the current study, D-galactose-induced apoptosis and cognitive deficiency in the hippocampus can be prevented by exercise training. Moreover, exercise is a simple, low-cost intervention easily performed by the young and old under many conditions.
Footnotes
Conflicts of interest: None declared.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81373020; Beijing Natural Science Foundation of China, No. 7112014; a grant from the Science and Technology Development Project of Beijing Municipal Education Commission of China, No. KM201110025014; and a grant from the Beijing Municipal Science and Technology Project of China, No. Z131107002213071.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Croxford L, Pack M, Yu J, Wang J, Li CH, Song LP, Zhao M
References
- Adams MM, Shi L, Linville MC, Forbes ME, Long AB, Bennett C, Newton IG, Carter CS, Sonntag WE, Riddle DR, Brunso-Bechtold JK. Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp Neurol. 2008;211:141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol Aging. 2005;26:511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Akhtar RS, Ness JM, Roth KA. Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim Biophys Acta. 2004;1644:189–203. doi: 10.1016/j.bbamcr.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Almeida OF, Condé GL, Crochemore C, Demeneix BA, Fischer D, Hassan AH, Meyer M, Holsboer F, Michaelidis TM. Subtle shifts in the ratio between pro- and antiapoptotic molecules after activation of corticosteroid receptors decide neuronal fate. FASEB J. 2000;14:779–790. doi: 10.1096/fasebj.14.5.779. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Benito E, Barco A. CREB's control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci. 2010;33:230–240. doi: 10.1016/j.tins.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Kamal A, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH. Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment. Brain Res. 1998;800:125–135. doi: 10.1016/s0006-8993(98)00510-1. [DOI] [PubMed] [Google Scholar]
- Brown GK. Glucose transporters: structure, function and consequences of deficiency. J Inherit Metab Dis. 2000;23:237–246. doi: 10.1023/a:1005632012591. [DOI] [PubMed] [Google Scholar]
- Chae CH, Kim HT. Forced moderate-intensity treadmill exercise suppresses apoptosis by increasing the level of NGF and stimulating phosphatidylinositol 3-kinase signaling in the hippocampus of induced aging rats. Neurochem Int. 2009;55:208–213. doi: 10.1016/j.neuint.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Cui X, Zuo P, Zhang Q, Li X, Hu Y, Long J, Packer L, Liu J. Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: Protective effects of R-α-lipoic acid. J Neurosci Res. 2006;83:1584–1590. doi: 10.1002/jnr.20845. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, Geinisman Y, Morrell F. Age-dependent alterations in hippocampal synaptic plasticity: relation to memory disorders. Neurobiol Aging. 1988;9:581–590. doi: 10.1016/s0197-4580(88)80117-9. [DOI] [PubMed] [Google Scholar]
- Duman RS. Neurotrophic factors and regulation of mood: Role of exercise, diet and metabolism. Neurobiol Aging. 2005;26:88–93. doi: 10.1016/j.neurobiolaging.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Ferrari CK. Functional foods and physical activities in health promotion of aging people. Maturitas. 2007;58:327–339. doi: 10.1016/j.maturitas.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Frick KM, Benoit JD. Use it or lose it: environmental enrichment as a means to promote successful cognitive aging. The Scientific World Journal. 2010;10:1129–1141. doi: 10.1100/tsw.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, He H, Jiang W, Chang X, Zhu L, Luo F, Zhou R, Ma C, Yan T. Salidroside ameliorates cognitive impairment in a d-galactose-induced rat model of Alzheimer's disease. Behav Brain Res. 2015;293:27–33. doi: 10.1016/j.bbr.2015.06.045. [DOI] [PubMed] [Google Scholar]
- Głombik K, Stachowicz A, Ślusarczyk J, Trojan E, Budziszewska B, Suski M, Kubera M, Lasoń W, Wędzony K, Olszanecki R, Basta-Kaim A. Maternal stress predicts altered biogenesis and the profile of mitochondrial proteins in the frontal cortex and hippocampus of adult offspring rats? Psychoneuroendocrinology. 2015;60:151–162. doi: 10.1016/j.psyneuen.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ. Invited review: Effects of acute exercise and exercise training on insulin resistance. J Appl Physiol (1985) 2002;93:788–796. doi: 10.1152/japplphysiol.01219.2001. [DOI] [PubMed] [Google Scholar]
- Hyun HB, Lee WS, Go SI, Nagappan A, Park C, Han MH, Hong SH, Kim G, Kim GY, Cheong J, Ryu CH, Shin SC, Choi YH. The flavonoid morin from Moraceae induces apoptosis by modulation of Bcl-2 family members and Fas receptor in HCT 116 cells. Int J Oncol. 2015;46:2670–2678. doi: 10.3892/ijo.2015.2967. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Zhang R, Elliffe D, Chen KF, Mathai S, McCarthy D, Waldvogel H, Guan J. Correlation of cellular changes and spatial memory during aging in rats. Exp Gerontol. 2008;43:929–938. doi: 10.1016/j.exger.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Jeong JH, Park HG, Lee YR, Lee WL. Moderate exercise training is more effective than resveratrol supplementation for ameliorating lipid metabolic complication in skeletal muscle of high fat diet-induced obese mice. J Exerc Nutrition Biochem. 2015;19:131–137. doi: 10.5717/jenb.2015.15062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YS. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 2010;45:357–365. doi: 10.1016/j.exger.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Linden DJ. Ubiquitous plasticity and memory storage. Neuron. 2007;56:582–592. doi: 10.1016/j.neuron.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Kivelä R, Silvennoinen M, Touvra AM, Lehti TM, Kainulainen H, Vihko V. Effects of experimental type 1 diabetes and exercise training on angiogenic gene expression and capillarization in skeletal muscle. FASEB J. 2006;20:1570–1572. doi: 10.1096/fj.05-4780fje. [DOI] [PubMed] [Google Scholar]
- Ku HK, Lim HM, Oh KH, Yang HJ, Jeong JS, Kim SK. Interpretation of protein quantitation using the Bradford assay: Comparison with two calculation models. Anal Biochem. 2013;434:178–180. doi: 10.1016/j.ab.2012.10.045. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Biebl M, Wilhelm D, Li M, Friedlander RM, Winkler J. Increased generation of granule cells in adult Bcl-2-overexpressing mice: a role for cell death during continued hippocampal neurogenesis. Eur J Neurosci. 2005;22:1907–1915. doi: 10.1111/j.1460-9568.2005.04377.x. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dogra S, Prakash A. Effect of carvedilol on behavioral, mitochondrial dysfunction, and oxidative damage against d-galactose induced senescence in mice. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:431–441. doi: 10.1007/s00210-009-0442-8. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003;15:691–699. doi: 10.1016/j.ceb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Li JJ, Zhu Q, Lu YP, Zhao P, Feng ZB, Qian ZM, Zhu L. Ligustilide prevents cognitive impairment and attenuates neurotoxicity in d-galactose induced aging mice brain. Brain Res. 2015;1595:19–28. doi: 10.1016/j.brainres.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Lipman RD, Dallal GE, Bronson RT. Lesion biomarkers of aging in B6C3F1 hybrid mice. J Gerontol A Biol Sci Med Sci. 1999;54:B466–477. doi: 10.1093/gerona/54.11.b466. [DOI] [PubMed] [Google Scholar]
- Lu J, Zheng YL, Luo L, Wu DM, Sun DX, Feng YJ. Quercetin reverses D-galactose induced neurotoxicity in mouse brain. Behav Brain Res. 2006;171:251–260. doi: 10.1016/j.bbr.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Lu J, Zheng YL, Wu DM, Luo L, Sun DX, Shan Q. Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by d-galactose. Biochem Pharmacol. 2007;74:1078–1090. doi: 10.1016/j.bcp.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Magariños AMa, Jain K, Blount ED, Reagan L, Smith BH, McEwen BS. Peritoneal implantation of macroencapsulated porcine pancreatic islets in diabetic rats ameliorates severe hyperglycemia and prevents retraction and simplification of hippocampal dendrites. Brain Res. 2001;902:282–287. doi: 10.1016/s0006-8993(01)02400-3. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol. 2004;490:13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- Mora F, Segovia G, del Arco A. Aging plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res Rev. 2007;55:78–88. doi: 10.1016/j.brainresrev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Mu JS, Li WP, Yao ZB, Zhou XF. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 1999;835:259–265. doi: 10.1016/s0006-8993(99)01592-9. [DOI] [PubMed] [Google Scholar]
- Nilsson A, Tovar J, Johansson M, Radeborg K, Björck I. A diet based on multiple functional concepts improves cognitive performance in healthy subjects. Nutr Metab (Lond) 2013;10:49. doi: 10.1186/1743-7075-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff HS, Berchtold NC, Isackson P, Cotman CW. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res Mol Brain Res. 1998;61:147–153. doi: 10.1016/s0169-328x(98)00222-8. [DOI] [PubMed] [Google Scholar]
- Ong KW, Hsu A, Tan BK. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: a contributor to the beneficial effects of coffee on diabetes. PLoS One. 2012;7:e32718. doi: 10.1371/journal.pone.0032718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio-Fuentealba C, Contreras-Ferrat AE, Altamirano F, Espinosa A, Li Q, Niu W, Lavandero S, Klip A, Jaimovich E. Electrical stimuli release ATP to increase GLUT4 translocation and glucose uptake via PI3Kγ-Akt-AS160 in skeletal muscle cells. Diabetes. 2013;62:1519–1526. doi: 10.2337/db12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten AR, Sickmann H, Hryciw BN, Kucharsky T, Parton R, Kernick A, Christie BR. Long-term exercise is needed to enhance synaptic plasticity in the hippocampus. Learn Mem. 2013;20:642–647. doi: 10.1101/lm.030635.113. [DOI] [PubMed] [Google Scholar]
- Savory J, Rao JK, Huang Y, Letada PR, Herman MM. Age-related hippocampal changes in Bcl-2:Bax ratio, oxidative stress, redox-active iron and apoptosis associated with aluminum-induced neurodegeneration: increased susceptibility with aging. Neurotoxicology. 1999;20:805–817. [PubMed] [Google Scholar]
- Shibuya T, Kaburagi T, Nagai R, Oshiro S. The effects of moderate exercise on secretory IgA production in mice depends on dietary carbohydrate intake. J Clin Biochem Nutr. 2015;57:44–49. doi: 10.3164/jcbn.15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares E, Prediger RD, Nunes S, Castro AA, Viana SD, Lemos C, De Souza CM, Agostinho P, Cunha RA, Carvalho E, Fontes Ribeiro CA, Reis F, Pereira FC. Spatial memory impairments in a prediabetic rat model. Neuroscience. 2013;250:565–577. doi: 10.1016/j.neuroscience.2013.07.055. [DOI] [PubMed] [Google Scholar]
- Tamakoshi K, Ishida A, Takamatsu Y, Hamakawa M, Nakashima H, Shimada H, Ishida K. Motor skills training promotes motor functional recovery and induces synaptogenesis in the motor cortex and striatum after intracerebral hemorrhage in rats. Behav Brain Res. 2014;260:34–43. doi: 10.1016/j.bbr.2013.11.034. [DOI] [PubMed] [Google Scholar]
- Temofonte N, Sajan MP, Nimal S, Pastoor T, Fumero C, Casaubon L, Powe JL, Standaert ML, Farese RV. Combined thiazolidinedione-metformin treatment synergistically improves insulin signalling to insulin receptor substrate-1-dependent phosphatidylinositol 3-kinase, atypical protein kinase C and protein kinase B/Akt in human diabetic muscle. Diabetologia. 2009;52:60–64. doi: 10.1007/s00125-008-1180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Li L, Song Q, Ai H, Chu J, Li W. Behavioural study of the d-galactose induced aging model in C57BL/6J mice. Behav Brain Res. 2005;157:245–251. doi: 10.1016/j.bbr.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Wu DM, Lu J, Zheng YL, Zhou Z, Shan Q, Ma DF. Purple sweet potato color repairs d-galactose-induced spatial learning and memory impairment by regulating the expression of synaptic proteins. Neurobiol Learn Mem. 2008;90:19–27. doi: 10.1016/j.nlm.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Yamada K, Mizuno M, Nabeshima T. Role for brain-derived neurotrophic factor in learning and memory. Life Sci. 2002;70:735–744. doi: 10.1016/s0024-3205(01)01461-8. [DOI] [PubMed] [Google Scholar]
- Yang JW, Ru J, Ma W, Gao Y, Liang Z, Liu J, Guo JH, Li LY. BDNF promotes the growth of human neurons through crosstalk with the Wnt/β-catenin signaling pathway via GSK-3β. Neuropeptides. 2015 doi: 10.1016/j.npep.2015.08.005. doi:10.1016/j.npep.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Yu F, Xu B, Song C, Ji L, Zhang X. Treadmill exercise slows cognitive deficits in aging rats by antioxidation and inhibition of amyloid production. Neuroreport. 2013;24:342–347. doi: 10.1097/WNR.0b013e3283606c5e. [DOI] [PubMed] [Google Scholar]
- Yu Y, Bai F, Wang W, Liu Y, Yuan Q, Qu S, Zhang T, Tian G, Li S, Li D, Ren G. Fibroblast growth factor 21 protects mouse brain against d-galactose induced aging via suppression of oxidative stress response and advanced glycation end products formation. Pharmacol Biochem Behav. 2015;133:122–131. doi: 10.1016/j.pbb.2015.03.020. [DOI] [PubMed] [Google Scholar]
- Zhou YY, Ji XF, Fu JP, Zhu XJ, Li RH, Mu CK, Wang CL, Song WW. Gene transcriptional and metabolic profile changes in mimetic aging mice induced by D-galactose. PLoS One. 2015;10:e0132088. doi: 10.1371/journal.pone.0132088. [DOI] [PMC free article] [PubMed] [Google Scholar]