Arabidopsis MPK3/MPK6 and the downstream ERF6 substrate promote the biosynthesis of indole glucosinolates and their derivatives in plant immunity.
Abstract
Antimicrobial compounds have critical roles in plant immunity; for example, Arabidopsis thaliana and other crucifers deploy phytoalexins and glucosinolate derivatives in defense against pathogens. The pathogen-responsive MITOGEN-ACTIVATED PROTEIN KINASE3 (MPK3) and MPK6 have essential functions in the induction of camalexin, the major phytoalexin in Arabidopsis. In search of cyanide, a coproduct of ethylene and camalexin biosynthesis, we found that MPK3 and MPK6 also affect the accumulation of extracellular thiocyanate ion derived from the indole glucosinolate (IGS) pathway. Botrytis cinerea infection activates MPK3/MPK6, which promote indole-3-yl-methylglucosinolate (I3G) biosynthesis and its conversion to 4-methoxyindole-3-yl-methylglucosinolate (4MI3G). Gain- and loss-of-function analyses demonstrated that MPK3/MPK6 regulate the expression of MYB51 and MYB122, two key regulators of IGS biosynthesis, as well as CYP81F2 and IGMT1/IGMT2, which encode enzymes in the conversion of I3G to 4MI3G, through ETHYLENE RESPONSE FACTOR6 (ERF6), a substrate of MPK3/MPK6. Under the action of PENETRATION2 (PEN2), an atypical myrosinase, and PEN3, an ATP binding cassette transporter, 4MI3G is converted to extracellular unstable antimicrobial compounds, possibly isothiocyanates that can react with nucleophiles and release the stable thiocyanate ion. Recent studies demonstrated the importance of PEN2/PEN3-dependent IGS derivatives in plant immunity. Here, we report that MPK3/MPK6 and their substrate ERF6 promote the biosynthesis of IGSs and the conversion of I3G to 4MI3G, a target of PEN2/PEN3-dependent chemical defenses in plant immunity.
INTRODUCTION
Glucosinolates (GSs), a group of thioglucosides, including tryptophan-derived indole glucosinolates (IGSs) and methionine-derived aliphatic glucosinolates (AGSs), are important secondary metabolites in Brassicaceous species. Together with myrosinases, they form a potent plant chemical defense system against insect herbivores (Agerbirk et al., 2009; Kissen et al., 2009; Burow et al., 2010; Sønderby et al., 2010a). Myrosinases are stored separately from glucosinolates but get mixed with glucosinolates upon tissue damage or insect feeding. Hydrolysis of the thioglucosidic bond of glucosinolates by myrosinases releases unstable aglucones, which can rapidly decompose to various bioactive compounds, including isothiocyanates, simple nitriles, organic thiocyanates, or epithionitriles depending on the presence of specifier proteins, thiocyanate-forming proteins, and certain environmental factors, such as Fe(II) ions, and pH (Wittstock and Burow, 2010; Bednarek, 2012). In Arabidopsis thaliana, the Col-0 ecotype produces predominantly isothiocyanates because of the absence of epithiospecifier protein activity (Lambrix et al., 2001; Burow and Wittstock, 2009). Among all types of the GS hydrolysis products, isothiocyanates have been demonstrated to have the highest chemical reactivity and are toxic to a wide range of organisms including microorganisms, nematodes, and insects (Bednarek, 2012; Fan and Doerner, 2012).
Besides their role in plant insect defense, GSs and their derivatives have been shown to be essential for defense against pathogens in plant innate immunity (Bednarek et al., 2009; Clay et al., 2009; Fan et al., 2011; Stotz et al., 2011). It was reported that sulforaphane (4-methylsulfinylbutyl isothiocyanate), a natural product derived from AGS, inhibits the growth of nonadapted Pseudomonas syringae bacteria in Arabidopsis (Fan et al., 2011). Besides AGSs and their derivatives, IGSs and their derivative are also critical to plant defense (Bednarek et al., 2009; Clay et al., 2009). PENETRATION2 (PEN2)-dependent breakdown products of a specific IGS, 4-methoxyindol-3-ylmethylglucosinolate (4MI3G), have been implicated in resistance against a variety of fungal and oomycetic pathogens (Schlaeppi et al., 2010; Schlaeppi and Mauch, 2010; Stotz et al., 2011; Buxdorf et al., 2013). Pathogen infection of Arabidopsis leads to accumulation of 4MI3G, which is dependent on CYP81F2, a cytochrome P450 monooxygenase. 4MI3G can be hydrolyzed by PEN2, an atypical myrosinase, into metabolites that are involved in defense against fungal pathogens (Bednarek et al., 2009). Recently, 4-O-β-d-glucosyl-indol-3-yl formamide was identified as a pathogen-inducible, tryptophan-derived compound whose biosynthesis is dependent on an intact PEN2 metabolic pathway. Based on the overaccumulation of 4-O-β-d-glucosyl-indol-3-yl formamide in the leaf tissues of pen3 mutants, it was proposed that its precursor is the cargo of PEN3, an ATP binding cassette transporter, in extracellular pathogen defense (Lu et al., 2015).
At present, the core biosynthetic pathway(s) of GSs are well understood (Halkier and Gershenzon, 2006; Sønderby et al., 2010a; Wittstock and Burow, 2010). In addition, the transcription factors directly upstream in regulating the expression of GS biosynthetic genes have been identified. They are a group of R2R3-MYB transcription factors. MYB28, MYB29, and MYB76 play prominent roles in controlling AGS biosynthesis (Hirai et al., 2007; Sønderby et al., 2007; Gigolashvili et al., 2008; Sønderby et al., 2010b; Li et al., 2013), whereas, MYB34, MYB51, and MYB122 act in transcriptional regulation of IGS biosynthesis genes (Celenza et al., 2005; Gigolashvili et al., 2007b; Frerigmann and Gigolashvili, 2014). Besides these, several basic helix-loop-helix transcription factors that act as interaction partners with these MYBs, including MYC2, MYC3, and MYC4, have been implicated in regulating GS levels in Arabidopsis (Schweizer et al., 2013; Frerigmann et al., 2014). However, the signaling components/pathways involved in regulating the biosynthesis of GSs are mostly unknown, which is a major gap in our understanding of the chemical defense of plants against pathogens.
Mitogen-activated protein kinase (MAPK) cascades are conserved signaling modules downstream of receptors/sensors that transduce extracellular stimuli into cellular responses and regulate various cellular activities in eukaryotes (Pedley and Martin, 2005; Pitzschke et al., 2009; Andreasson and Ellis, 2010; Rodriguez et al., 2010; Tena et al., 2011; Meng and Zhang, 2013; Xu and Zhang, 2015). A MAPK cascade comprises a MAPK, its upstream MAPK kinase and its upstream MAPK kinase kinase. Together, they play important roles in plant growth, development, and response to environment. There are at least three pathogen-responsive MAPKs in Arabidopsis, including MPK3, MPK6, and MPK4, of which MPK3 and MPK6 are mostly redundant (Meng et al., 2013). Three important genetic systems allowed us to demonstrate the diverse functions of MPK3 and MPK6. One is the conditional gain-of-function system that allows us to turn on the activation of MPK3/MPK6 in the absence of stress/pathogen stimuli (Yang et al., 2001; Ren et al., 2002). The second is the conditionally rescued mpk3 mpk6 double mutant (Wang et al., 2007). Recently, we generated another rescued mpk3 mpk6 double mutant line using a chemical genetic approach (Xu et al., 2014), which allowed us to overcome the developmental defects of the rescued double mutant system that we generated earlier (Wang et al., 2007).
In search of the underlying mechanisms of MPK3/MPK6 in plant immunity, we found that MPK3/MPK6 regulate the biosynthesis of ethylene, an important plant stress hormone (Kim et al., 2003; Liu and Zhang, 2004; Joo et al., 2008; Han et al., 2010; Li et al., 2012; Guan et al., 2015), and camalexin, the major phytoalexin in Arabidopsis (Ren et al., 2008; Mao et al., 2011). Recently, a metabolomic analysis demonstrated that gain-of-function activation of MPK3/MPK6 reprograms defense metabolism, including camalexin and IGS (Lassowskat et al., 2014; Lee et al., 2015). However, loss-of-functional evidence is lacking and the molecular mechanism underlying MPK3/MPK6-reprogramed IGS biosynthesis is unclear. In this report, we demonstrate a critical role of MPK3/MPK6 signaling pathway in reprogramming IGS biosynthesis in plant fungal immunity. MPK3/MPK6 activation in response to Botrytis cinerea inoculation or in the conditional gain-of-function GVG-NtMEK2DD (abbreviated as DD) plants triggers a major shift from I3G to 4MI3G biosynthesis. Loss of function of MPK3/MPK6 greatly inhibits the accumulation of 4MI3G induced by B. cinerea. Furthermore, gene expression analyses revealed that B. cinerea-induced activation of genes encoding CYP81F2 and IGMT1/IGMT2, two enzymes involved in the conversion of I3G to 4MI3G, and MYB51/MYB122, two MYB transcription factors involved in the regulation of IGS biosynthetic genes, is dependent on the MPK3/MPK6 signaling pathway. In addition, ERF6, a substrate of MPK3/MPK6 in fungal pathogen resistance, plays an important role in 4MI3G biosynthesis downstream of MPK3/MPK6 cascade. It regulates the expression of CYP81F2 and IGMT1/IGMT2 directly based on chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) and genetic analyses and therefore controls the synthesis of 4MI3G from I3G and production of IGS-derived antimicrobial compounds in plant immunity.
RESULTS
Accumulation of Thiocyanate in Response to Pathogen Infection or MPK3/MPK6 Activation Is Dependent on the IGS Biosynthetic Pathway
MPK3 and MPK6 play important roles in plant immunity by regulating the biosynthesis of ethylene, an important plant stress hormone (Kim et al., 2003; Liu and Zhang, 2004; Li et al., 2012), and camalexin, the major phytoalexin in Arabidopsis (Ren et al., 2008; Mao et al., 2011). We speculated that cyanide, a coproduct of ethylene and camalexin biosynthesis (Peiser et al., 1984; Böttcher et al., 2009), might accumulate to a level high enough to interrupt the normal activities in plants. Furthermore, cyanide might be able to inhibit pathogen growth directly due to its cytotoxicity and therefore play an important role in plant immunity. As the first step, we measured the levels of cyanide in the media of liquid-cultured seedlings after pathogen infection and in the conditional gain-of-function DD transgenic Arabidopsis after dexamethasone (DEX) treatment using the isonicotinic-barbituric acid method, a spectrophotometric assay for cyanide detection (Nagashima, 1981). We observed a dramatic increase in the level of “cyanide” (Figure 1A). However, knocking out of the majority of ethylene induction in the high-order acs mutants (Li et al., 2012) or blocking the camalexin accumulation in pad3 mutants (Ren et al., 2008) failed to prevent the induction of “cyanide” (Supplemental Figure 1). Surprisingly, mutation of CYP79B2 and CYP79B3, two redundant P450 monooxygenase isoforms, prohibited the accumulation of “cyanide” (Figure 1B). This observation puzzled us at first. A more extensive literature search revealed that the spectrophotometric assay we used to detect cyanide also detects thiocyanate ion (SCN−), which could be a product in the breakdown of the unstable isothiocyanates derived from CYP79B2/CYP79B3-dependent IGS biosynthesis (Agerbirk et al., 2009; Wittstock and Burow, 2010) (Figure 2).
Figure 1.
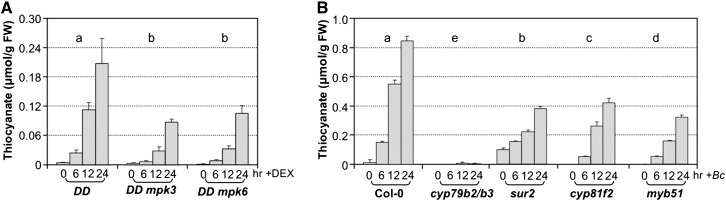
Induction of Extracellular Thiocyanate after MPK3/MPK6 Activation or B. cinerea Infection Is Dependent on IGS Biosynthetic Pathway.
(A) Activation of MPK3/MPK6 in the gain-of-function GVG-Nt-MEK2DD transgenic plants (DD) seedlings after DEX treatment induces the accumulation of thiocyanate in culture medium. Twelve-day-old DD, DD mpk3, and DD mpk6 seedlings were treated with DEX (1 μM). FW, fresh weight.
(B) B. cinerea-induced accumulation of thiocyanate is dependent on CYP79B2/CYP79B3 and other components in the IGS biosynthesis pathway. Twelve-day-old seedlings were inoculated with B. cinerea (Bc) spores (4.0 × 105 spores per vial). Medium samples were collected at indicated times. The levels of thiocyanate ion in culture medium were quantified using the isonicotinic-barbituric acid method. Error bars indicate sd (n = 3). Two-way ANOVA was performed to compare the levels of thiocyanate ions in different genotypes after treatment. Lowercase letters above the columns indicate statistically different groups (P < 0.05).
Figure 2.
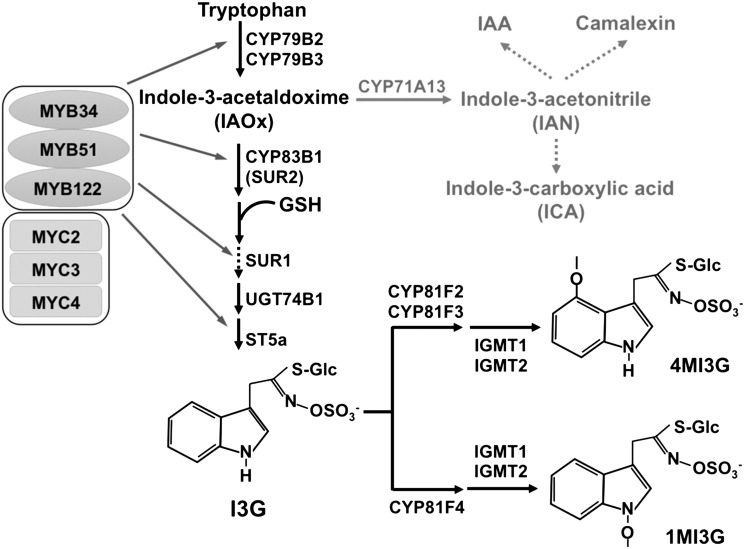
Key Enzymes, Regulators, and Major Intermediates in the IGS Biosynthetic Pathway.
Indole-3-yl-methyl-glucosinolate (I3G), 1MI3G, and 4MI3G are three major IGSs found in Arabidopsis. Their de novo biosynthesis is dependent on MYB34, MYB51, and MYB122, three key transcription factors that control the expression of IGS biosynthetic genes. Solid arrows indicate single enzymatic steps, whereas dashed lines stand for several enzymatic steps. IAOx is also the key intermediate leading to camalexin and auxin (adapted from Figure 8 in Frerigmann and Gigolashvili, 2014).
It is believed that the highly reactive isothiocyanate derivatives from IGSs are responsible for the direct antimicrobial activity (Agerbirk et al., 2009). However, using cyclocondensation assay, a spectrophotometric assay for the detection of isothiocyanates, but not cyanide and thiocyanate (Zhang et al., 1992), we failed to detect the accumulation of isothiocyanates in the culture media. This is likely because isothiocyanates are highly unstable. To distinguish cyanide and thiocyanate, we treated the culture media with formaldehyde to mask cyanide (Meeussen et al., 1989) and then used the isonicotinic-barbituric acid method to quantify only the thiocyanate ion. We found that the total levels of cyanide/thiocyanate measured using the isonicotinic-barbituric acid method were comparable with and without formaldehyde treatment, indicating that the chemical we detected using the isonicotinic-barbituric acid method in the liquid media samples is thiocyanate ion, not cyanide. This is consistent with the previous finding that cyanide from the biosynthesis of ethylene can be rapidly removed (Miller and Conn, 1980; Yip and Yang, 1988; Murphy et al., 2014). It was reported that cyanide plays a role in rice resistance against blast fungus (Seo et al., 2011). The source of cyanide was believed to be from ethylene biosynthesis. Further research is needed to determine whether cyanide also plays a role in Arabidopsis immunity.
The dependency of extracellular thiocyanate accumulation on functional CYP79B2/CYP79B3 but not PAD3 (Figure 1; Supplemental Figure 1) suggests that the thiocyanate is derived from IGS biosynthetic pathway. Complete abolishment of thiocyanate accumulation in cyb79b2 cyb79b3 double mutant also excluded the involvement of AGS biosynthetic pathway. We also analyzed additional mutants of the IGS biosynthetic and regulatory pathways including sur2 (CYP83B1), cyp81f2, and myb51. SUR2 encodes a cytochrome P450 that catalyzes the first committed step in IGS biosynthesis as shown in Figure 2 (Barlier et al., 2000; Bak et al., 2001). CYP81F2 is a key cytochrome P450 monooxygenase that catalyzes the first step in the conversion from I3G to 4MI3G (Bednarek et al., 2009; Clay et al., 2009; Pfalz et al., 2009). MYB51 is a key transcription factor regulating IGS biosynthesis (Gigolashvili et al., 2007b; Frerigmann and Gigolashvili, 2014). As shown in Figure 1B, mutation of SUR2, CYP81F2, or MYB51 significantly inhibited thiocyanate accumulation. It is known that the isothiocyanates derived from IGSs are very unstable. They can readily react with various nucleophiles, releasing the functional group (-N=C=S) as the thiocyanate ion (SCN−) (Agerbirk et al., 2009). The accumulation of thiocyanate ion in response to B. cinerea infection or after MPK3/MPK6 activation indicates that MPK3/MPK6 activation in response to pathogen infection may induce IGS biosynthesis and the formation of isothiocyanate derivatives.
Reprogramming of the IGS Pathway to Increase 4MI3G Biosynthesis after B. cinerea Infection or MPK3/MPK6 Activation
Using HPLC, we measured cellular levels of three IGSs (I3G, 4MI3G, and 1-methoxy-3-indolylmethyl-GS [1MI3G]) and two AGSs (4-methysulfinylbutyl GS and 4-methythiobutyl GS). As shown in Figure 3A, the levels of two AGSs decreased slightly after B. cinerea infection and stayed at similar levels throughout the later infection process. The levels of 1MI3G, one of the three IGSs, changed slightly. In contrast, I3G gradually decreased, which was associated with a gradual increase in 4MI3G levels. A net increase in the ratio of 4MI3G versus I3G suggests a metabolic shift from I3G to 4MI3G biosynthesis. We found that the basal levels of AGSs in the liquid cultured seedlings were lower than those in the soil-grown seedlings/plants reported by other groups (Supplemental Figure 2) (Brown et al., 2003; Barth and Jander, 2006). In contrast, IGS contents before induction are comparable in both conditions.
Figure 3.
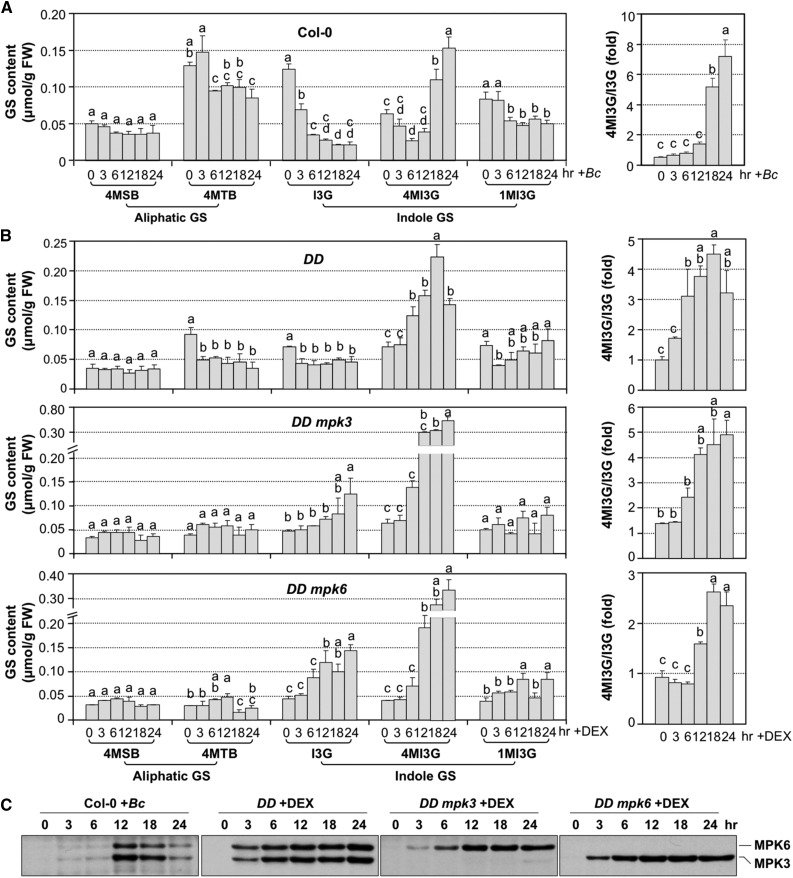
Accumulation of 4MI3G in Arabidopsis Seedlings Infected with B. cinerea and DD Seedlings after DEX Treatment.
(A) Accumulation of selected GSs in Col-0 seedlings after inoculation with B. cinerea (Bc) spores (left panel). Twelve-day-old Col-0 seedlings were inoculated with B. cinerea spores (4 × 105 spores/vial). Samples were collected at indicated times. Glucosinolates were measured using the HPLC assay. Ratios of steady state levels of 4MI3G and I3G were calculated and presented (right panel).
(B) Accumulation of selected glucosinolates in 12-d-old DD, DD mpk3, and DD mpk6 seedlings after DEX (1 μM) treatment. Glucosinolates were measured at indicated times. Ratios of steady state levels of 4MI3G and I3G were calculated and presented (right panels). Error bars indicate sd (n = 3). One-way ANOVA was performed to compare the levels of GSs at different time point after treatment. Lowercase letters above the columns indicate statistically different time points (P < 0.05). FW, fresh weight.
(C) Activation of MPK3/MPK6 in response to B. cinerea and in DD, DD mpk3, and DD mpk6 seedlings after DEX treatment. Activation of MPK6 and MPK3 was determined by immunoblot analysis using anti-pTEpY antibody.
In the conditional gain-of-function DD seedlings, DEX treatment induced a similar shift from I3G to 4MI3G (Figure 3B, upper panel), which is consistent with a recent metabolomic profiling using MKK5DD transgenic plants (Lassowskat et al., 2014). Again, the increase in 4MI3G was associated with a decrease in I3G, resulting in an increase in 4MI3G/I3G ratio. Loss of either MPK3 or MPK6 in the DD background slowed down the shift to 4MI3G (Figure 3B, middle and bottom panels). The higher accumulation of 4MI3G at 24 h in DD mpk3 and DD mpk6 seedlings in comparison to DD seedlings is likely a result of delayed cell death (Ren et al., 2008), which allows a more active metabolic capacity. It appears that the induction of 4MI3G is earlier in DEX-treated DD plants than in B. cinerea-infected plants (Figures 3A and 3B), which correlates well with the activation of MPK3/MPK6 (Figure 3C). MPK3/MPK6 activation was detectable 12 h after B. cinerea infection, while, in gain-of-function DD plants, MPK3/MPK6 activation was observable within 3 h after DEX treatment.
MPK3 and MPK6 Have Redundant Functions in Promoting 4MI3G Biosynthesis in Arabidopsis after B. cinerea Infection
To provide loss-of-function evidence to support the conclusion based on the gain-of-function DD seedlings (Figure 3B), we profiled the same set of AGSs and IGSs in Col-0, mpk3, and mpk6 seedlings after B. cinerea inoculation. We failed to observe a clear reduction in 4MI3G biosynthesis in mpk3 or mpk6 single mutant, likely a result of the functional redundancy of MPK3 and MPK6 in the process (Supplemental Figure 3).
MPK3 and MPK6 play essential roles in many growth and developmental processes including embryogenesis (Wang et al., 2007; Xu and Zhang, 2015). Recently, using a chemical genetic approach, we generated a rescued mpk3 mpk6 double mutant using a chemical (NA-PP1)-sensitized version of MPK6, MPK6YG (named MPK6SR plants; genotype: mpk3 mpk6 PMPK6:MPK6YG) (Xu et al., 2014). The kinase activity of MPK6YG can be specifically inhibited by NA-PP1, a derivative of PP1 kinase inhibitor with a bulky side chain, which therefore cannot enter the ATP binding pocket of a normal kinase (Bishop et al., 2000). Similarly, we also generated a rescued double mpk3 mpk6 mutant using a chemical-sensitized version of MPK3, MPK3TA, and the plants were named MPK3SR (genotype: mpk3 mpk6 PMPK3:MPK3TA). Using these two mpk3 mpk6 double mutant systems, we demonstrated that MPK3 and MPK6 are redundant in regulating the metabolic shift from I3G to 4MI3G production. As shown in Figure 4, pretreatment of MPK3SR and MPK6SR seedlings with NA-PP1 greatly reduced the steady state levels of 4MI3G. Without NA-PP1 treatment, MPK3SR and MPK6SR plants behaved similarly to the wild-type controls. Equally important, the Col-0 control with NA-PP1 pretreatment did not show reduced 4MI3G accumulation, demonstrating the specific inhibition of sensitized MPK3TA and MPK6YG by NA-PP1. Associated with the reduced 4MI3G accumulation, the 4MI3G/I3G ratios also reduced in mpk3 mpk6 double mutants. The levels of 1MI3G with and without NA-PP1 pretreatment were comparable (Supplemental Figure 4A), suggesting that its metabolism is not affected by MPK3/MPK6. Another observation is that I3G levels were lower in MPK3SR and MPK6SR seedlings pretreated with NA-PP1 at the later time points (Figure 4A). This is likely a result of reduced biosynthesis of I3G. As shown later, MPK3/MPK6 also promotes I3G synthesis.
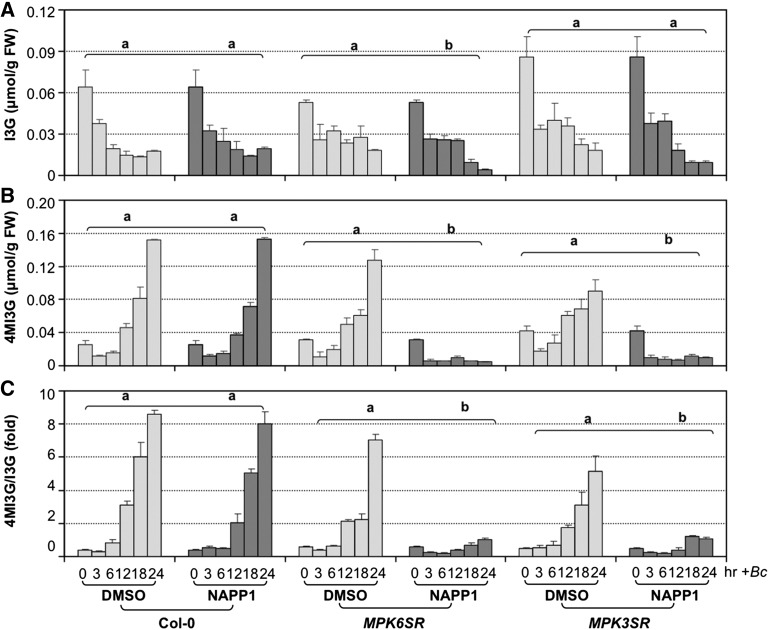
Figure 4.
B. cinerea-Induced 4MI3G Biosynthesis Is Compromised in mpk3 mpk6 Double Mutants.
(A) and (B) Steady state levels of I3G (A) and 4MI3G (B) in 12-d-old Col-0 and chemical-genetically rescued mpk3 mpk6 double mutant seedlings after B. cinerea (Bc) inoculation. Col-0, MPK6SR, and MPK3SR seedlings were pretreated with DMSO (solvent of NA-PP1 stock) or NA-PP1 (final concentration of 2.5 μM) for 30 min before Bc inoculation. Samples were collected at indicated times for GS assay.
(C) Ratios of steady state levels of 4MI3G and I3G in Col-0, MPK6SR, and MPK3SR seedlings after Bc inoculation. Error bars indicate sd (n = 3). Differences in IGS contents between DMSO and NA-PP1 pretreatment groups were analyzed by two-way ANOVA. Different lowercase letters above the brackets indicate statistically different groups (P < 0.01). MPK6SR genotype: mpk3 mpk6 PMPK6:MPK6YG line #58; MPK3SR genotype: mpk3 mpk6 PMPK3:MPK3TA line #17.
Upregulation of 4MI3G Biosynthetic Genes by MPK3/MPK6 Cascade
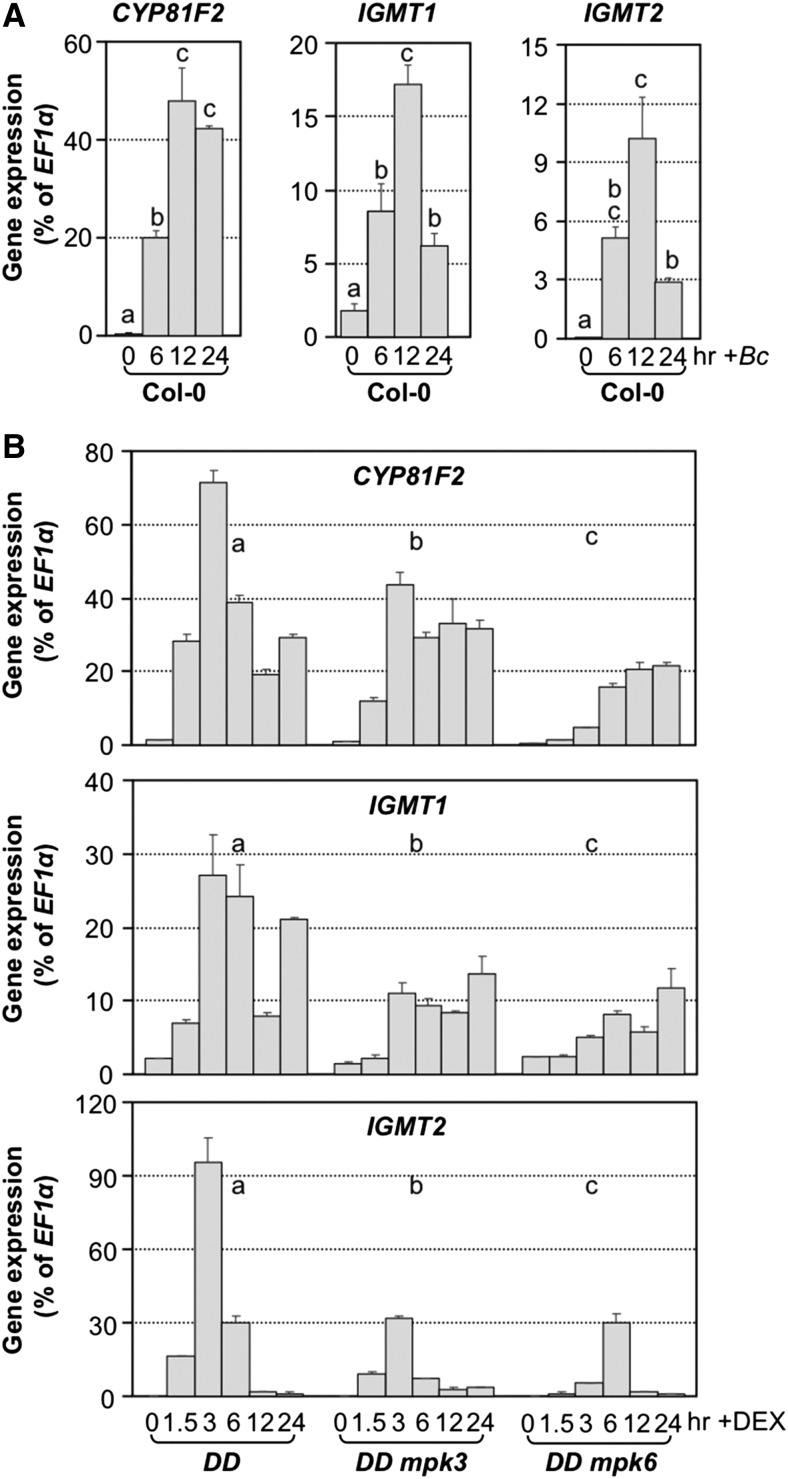
I3G can be modified to form 4MI3G or 1MI3G through the action of two enzymes (Figure 2). The first step is catalyzed by cytochrome P450 monooxygenases encoded by CYP81Fs, which add a hydroxyl (OH) group to the 4th position (CYP81F2 and CYP81F3) or 1st (CYP81F4) position of the indole ring (Bednarek et al., 2009; Pfalz et al., 2009). The second step is catalyzed by a pair of methyl transferases encoded by IGMT1 and IGMT2 (Pfalz et al., 2011). We found that CYP81F2, IGMT1, and IGMT2, but not CYP81F3 and CYP81F4, were highly induced in Arabidopsis seedlings after B. cinerea inoculation (Figure 5A; Supplemental Figures 5A and 5B). Between CYP81F2 and CYP81F3, the expression of CYP81F3 was much lower, only ∼0.007% of the EF1α transcript level. In contrast, the expression of CYP81F2 could reach ∼50% of the EF1α transcript level. Furthermore, in the gain-of-function DD seedlings, CYP81F2, IGMT1, and IGMT2 were also highly induced after DEX treatment (Figure 5B). In either the mpk3 or mpk6 mutant background, DD-induced activation of these three genes was partially blocked.
Figure 5.
Activation of 4MI3G Biosynthetic Genes in Response to B. cinerea and Gain-of-Function Activation of MPK3/MPK6 Cascade.
(A) Expression of CYP81F2, IGMT1, and IGMT2, genes encoding the two key enzymes in the conversion of I3G to 4MI3G, was highly induced in response to B. cinerea (Bc) inoculation. One-way ANOVA was used to compare gene expression at different time points (P < 0.05).
(B) Activation of 4MI3G biosynthetic genes in DEX-treated DD seedlings was compromised in either mpk3 or mpk6 mutant background. Gene expression was quantified by RT-qPCR and calculated as percentages of the EF1α transcript. Error bars indicate sd (n = 3). Gene expression in seedlings of different genotypes was analyzed by two-way ANOVA. Different lowercase letters above the columns indicate statistically different groups (P < 0.01).
In addition to the gain-of-function evidence, we also obtained loss-of-function evidence to support the role of MPK3/MPK6 in regulating B. cinerea-induced activation of CYP81F2, IGMT1, and IGMT2. In the chemical genetically rescued MPK3SR and MPK6SR seedlings, pretreatment with NA-PP1 blocked the B. cinerea-induced CYP81F2, IGMT1, and IGMT2 gene expression (Figure 6). In the absence of NA-PP1 inhibitor, the induction of these genes was much higher. Furthermore, Col-0 control seedlings with and without NA-PP1 pretreatment showed no significant difference in the induction of CYP81F2, IGMT1, and IGMT2 gene expression, demonstrating the specific inhibition of MPK3TA or MPK6YG variants by NA-PP1. The varying gene activation levels in MPK3SR and MPK6SR seedlings in the absence of NA-PP1 inhibitor are likely a result of different expression levels of MPK3TA or MPK6YG variants. Although MPK3TA and MPK6YG transgenes were driven by their respective native promoters, they did show varying expression levels due to position effect of the transgenes (Supplemental Figure 6). In summary, the chemical genetically rescued mpk3 mpk6 double mutants allow us to demonstrate the essential role of MPK3 and MPK6 in the pathogen-induced expression of CYP81F2, IGMT1, and IGMT2 during the induction of 4MI3G.
Figure 6.
Loss of Function of MPK3 and MPK6 Compromises B. cinerea-Induced Activation of 4MI3G Biosynthetic Genes.
Col-0, MPK6SR, and MPK3SR seedlings were pretreated with DMSO (solvent of NA-PP1 inhibitor) or NA-PP1 (2.5 μM) for 30 min before B. cinerea (Bc) inoculation. Samples were collected at indicated times for total RNA preparation. CYP81F2, IGMT1, and IGMT2 gene expression was determined by RT-qPCR and calculated as percentages of the EF1α transcript. Error bars indicate sd (n = 3). Differences in gene expression between DMSO and NA-PP1 pretreatment groups were analyzed by two-way ANOVA. Different lowercase letters above the brackets indicate statistically different groups (P < 0.01). MPK6SR: mpk3 mpk6 PMPK6:MPK6YG line #58; MPK3SR: mpk3 mpk6 PMPK3:MPK3TA line #17.
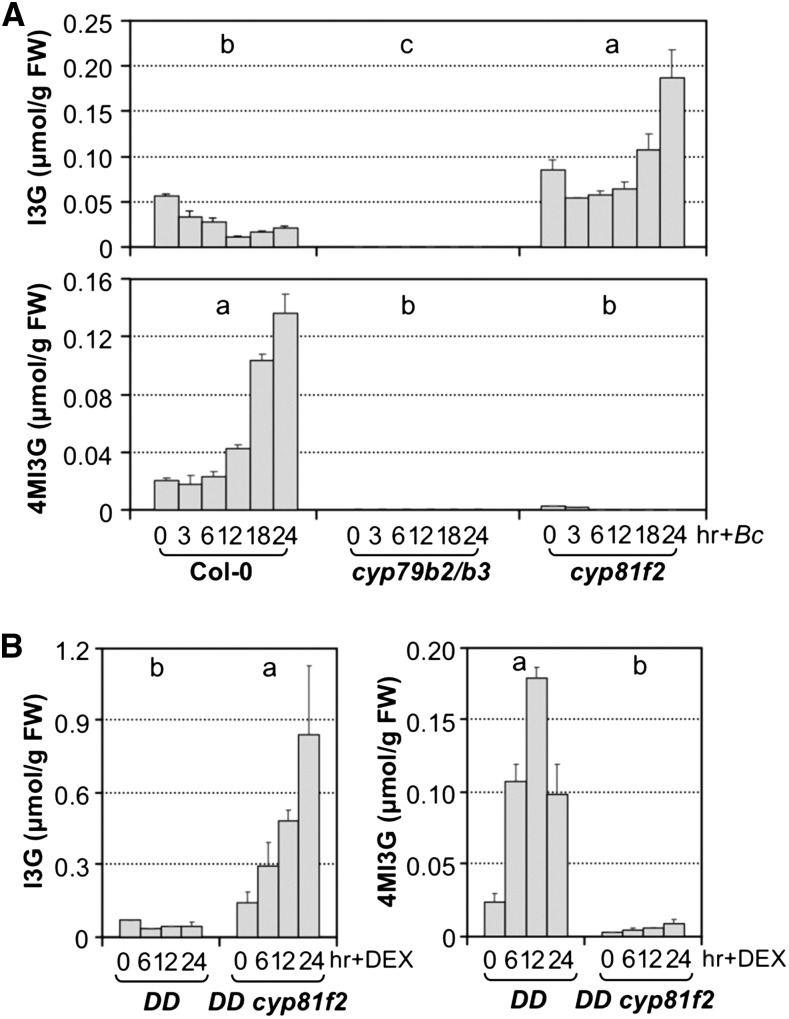
CYB79B2 and CYB79B3 together are essential to the synthesis of several groups of indole derivatives in Arabidopsis. In addition to their essential function in pathogen-induced camalexin biosynthesis (Glawischnig et al., 2004; Ren et al., 2008), they are also essential for IGS biosynthesis. As shown in Figure 7A, neither I3G nor 4MI3G was detectable in cyp79b2 cyp79b3 double mutant before and after B. cinerea inoculation. In cyp81f2 mutants, only 4MI3G biosynthesis was blocked (Figure 7B). In response to B. cinerea infection, I3G accumulated to higher levels than those in wild-type (Col-0) control, a result of elevated overall CYB79B2/CYB79B3-dependent IGS biosynthesis in combination with the blockage of conversion of I3G to 4MI3G. A similar effect was observed in the gain-of-function DD plants (Figure 7B). In DD cyp81f2 seedlings, DEX treatment failed to induce the accumulation of 4MI3G. Associated with this, there was a significant accumulation of I3G. These results demonstrate that pathogen-induced MPK3/MPK6 activation also promote the biosynthesis of I3G, despite the observation of an apparent decrease in the steady state levels of I3G (Figures 3A and 3B), a result of a higher rate of its conversion to 4MI3G and other derivatives (more information in Discussion). Almost complete blockage of 4MI3G biosynthesis in cyp81f2 single mutant suggests CYP81F3 contributes very little to the biosynthesis of 4MI3G, possibly a result of very low level expression of CYP81F3 in comparison to CYP81F2 (Figure 5A; Supplemental Figures 5A and 5B).
Figure 7.
I3G Biosynthesis Is Highly Induced in Response to B. cinerea Infection or MPK3/MPK6 Activation.
(A) Steady state levels of I3G and 4MI3G in 12-d-old Col-0, cyp79b2/b3, and cyp81f2 seedlings after inoculation with B. cinerea (Bc) spores.
(B) Steady state levels of I3G and 4MI3G in 12-d-old DD, DD cyp81f2 seedlings after DEX treatment. Samples were collected at indicated times after treatment, and IGS levels were determined by HPLC assay. Error bars indicate sd (n = 3). Differences in IGS contents between different genotypes were analyzed by two-way ANOVA. Different lowercase letters above the columns indicate statistically different groups (P < 0.01).
MYB51 and MYB122 Are Downstream of MPK3/MPK6 in Pathogen-Induced IGS Biosynthesis
MYB transcription factors play important roles in regulating GS biosynthesis, with MYB28/MYB29/MYB76 involved in AGS biosynthesis and MYB51/MYB122/MYB34 in IGS biosynthesis (Gigolashvili et al., 2007a, 2007b, 2008; Hirai et al., 2007; Li et al., 2013; Frerigmann and Gigolashvili, 2014). Consistent with our observation that MPK3/MPK6 only activate IGS biosynthetic genes (Figure 5; Supplemental Figure 7), the expression of MYB122 and MYB51, but not MYB28 and MYB29, was highly induced after B. cinerea inoculation or in the gain-of-function DD seedlings after DEX treatment (Figure 8; Supplemental Figures 8A to 8D). Interestingly, MYB34, another important regulator of IGS biosynthesis, was down-regulated upon B. cinerea infection or activation of MPK3/MPK6 (Supplemental Figure 8E and 8F), suggesting that MYB34 is differentially regulated in comparison to MYB51 and MYB122 in this process.
Figure 8.
B. cinerea-Induced MYB51/MYB122 Gene Activation Is Dependent on MPK3/MPK6.
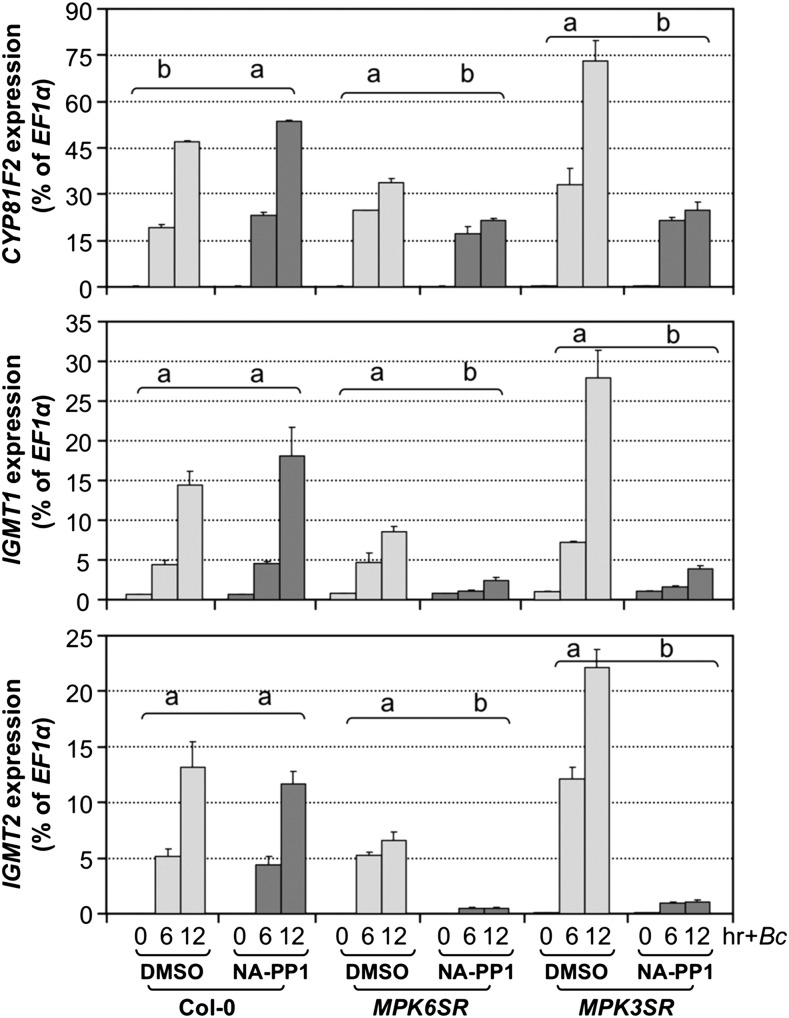
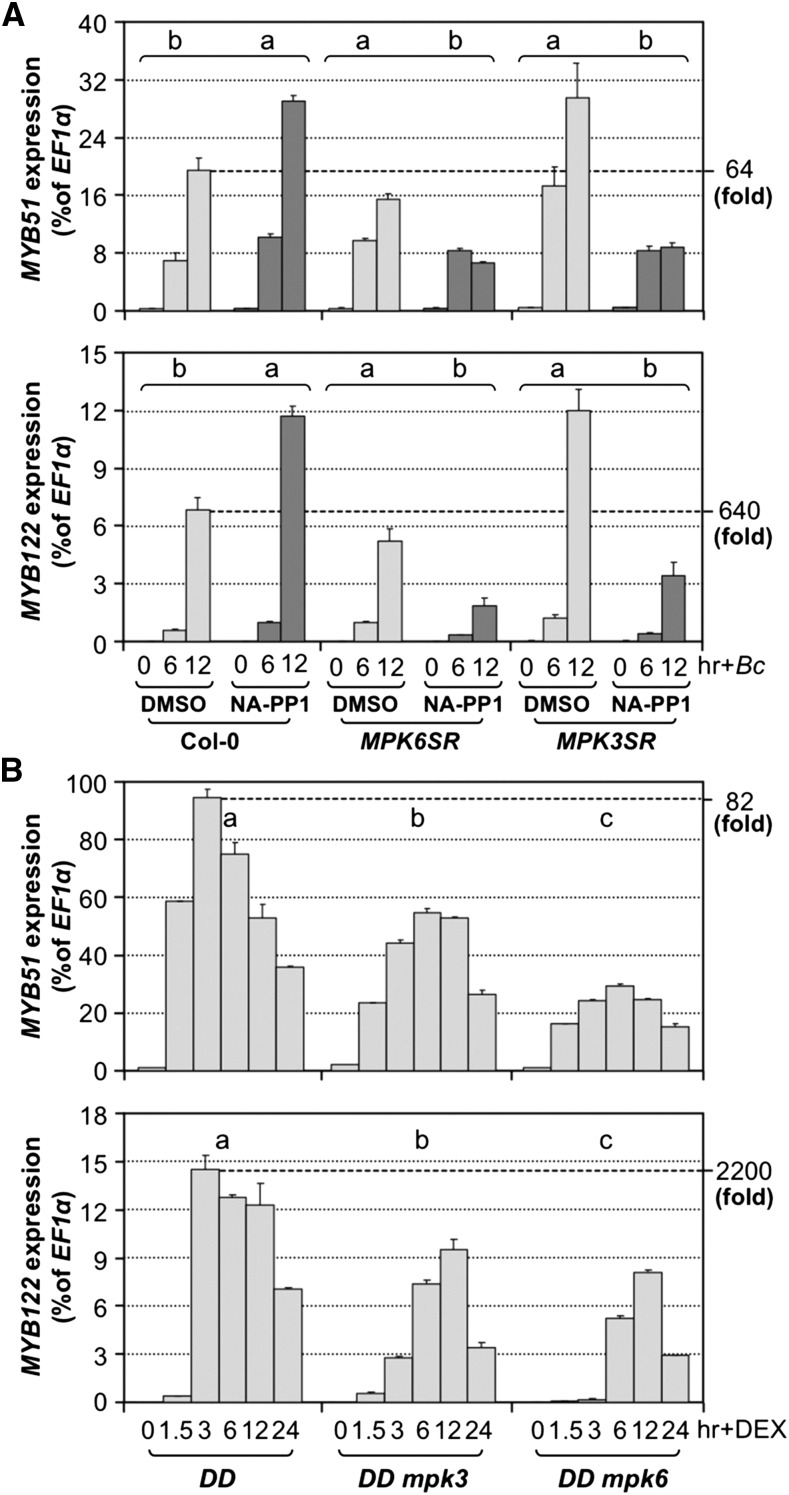
(A) Induction of MYB51 and MYB122 gene expression by B. cinerea (Bc) infection is dependent on MPK3/MPK6. Col-0, MPK6SR, and MPK3SR seedlings were pretreated with DMSO or NA-PP1 (2.5 μM) for 30 min before Bc inoculation. Differences in gene expression between DMSO and NA-PP1 pretreatment groups were analyzed by two-way ANOVA. Different lowercase letters above the brackets indicate statistically different groups (P < 0.01).
(B) Activation of MYB51 and MYB122 expression in DEX-treated DD seedlings was compromised in mpk3 or mpk6 mutant background. Samples were collected at indicated times. Gene expression was quantified by RT-qPCR and calculated as percentages of the EF1α transcript. Gene expression in seedlings of different genotypes after treatment was analyzed by two-way ANOVA. Different lowercase letters above the columns indicate statistically different groups (P < 0.01). Numbers on the right indicate the induction folds relative to 0 h. Error bars indicate sd (n = 3).
The rapid and high-level induction of MYB51 and MYB122 in DD seedlings is dependent on functional MPK3 and MPK6 genes. In either mpk3 or mpk6 mutant background, gain-of-function activation of MYB51 and MYB122 gene expression was compromised (Figure 8B). In support of the gain-of-function evidence, B. cinerea-induced MYB51 and MYB122 expression was inhibited in the MPK3SR and MPK6SR seedling in the presence of NA-PP1 inhibitor (Figure 8A).
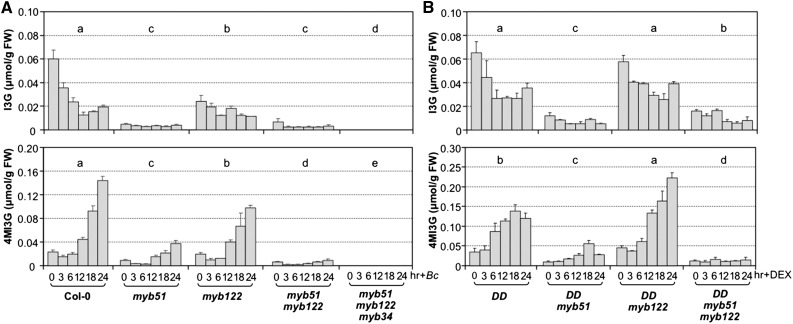
MYB51 played a more important role than MYB122 in the induction of IGS biosynthesis based on mutant analysis, despite a higher fold of induction of MYB122 in response to pathogen infection (64-fold versus 640-fold for MYB51 and MYB122, respectively, in Figure 8A) or MPK3/MPK6 activation in the gain-of-function DD plants (82-fold versus 2200-fold for MYB51 and MYB122, respectively, in Figure 8B). This is likely because of the higher basal level expression of MYB51, and as a result, the absolute transcript level of MYB51 is higher even with a lower fold of induction (Figure 8). In myb122 single mutant, there was a decrease in I3G levels, which was associated with a reduction in B. cinerea-induced biosynthesis of 4MI3G (Figure 9A). The reduction in I3G and 4MI3G levels was more significant in myb51 single mutant, suggesting MYB51 plays a more important role than MYB122 in the process. In the myb51 myb122 double mutant, the levels of I3G and the induction of 4MI3G were very low. The residual levels of I3G and the induction of 4MI3G were completely abolished in the myb51 myb122 myb34 triple mutant (Figure 9A), consistent with the previous finding that these three MYBs are essential to IGS biosynthesis in Arabidopsis (Frerigmann and Gigolashvili, 2014). Similarly, gain-of-function induction of 4MI3G in DD plants was also compromised in myb51 single and myb51 myb122 double mutant backgrounds (Figure 9B). These results suggest that MYB51/MYB122/MYB34 regulate both the biosynthesis of I3G and its conversion to 4MI3G in response to pathogen. Alternatively, the reduction of 4MI3G induction is simply a result of the reduction of I3G precursor, and these MYBs do not control the conversion of I3G to 4MI3G directly. To distinguish these two possibilities, we examined the expression of CYP81F2, IGMT1, and IGMT2 in myb51 myb122 and myb51 myb122 myb34 after B. cinerea infection and DD myb51 myb122 after DEX treatment. As shown in Supplemental Figure 9, there was no reduction in the expression these biosynthetic genes, suggesting that the MYBs mainly control the de novo synthesis of I3G but not its modification, i.e., these MYBs do not directly control the conversion of I3G to 4MI3G.
Figure 9.
MYB51 and MYB122 Are Essential for I3G and 4MI3G Biosynthesis in Response to B. cinerea and Activation of MPK3/MPK6 Cascades.
(A) Steady state levels of I3G (upper panel) and 4MI3G (lower panel) in 12-d-old Col-0, myb51, myb122, and myb51 myb122 single/double mutant seedlings after B. cinerea (Bc) inoculation.
(B) Steady state levels of I3G (upper panel) and 4MI3G (lower panel) in 12-d-old DD, DD myb51, DD myb122, and DD myb51 myb122 seedlings after DEX treatment. Samples were collected at indicated times, and IGS levels were determined by HPLC assay. Error bars indicate sd (n = 3). Differences in IGS contents between different genotypes were analyzed by two-way ANOVA. Different lowercase letters above the columns indicate statistically different groups (P < 0.01).
ERF6, a Substrate of MPK3/MPK6, Is Involved in Regulating the Conversion of I3G to 4MI3G
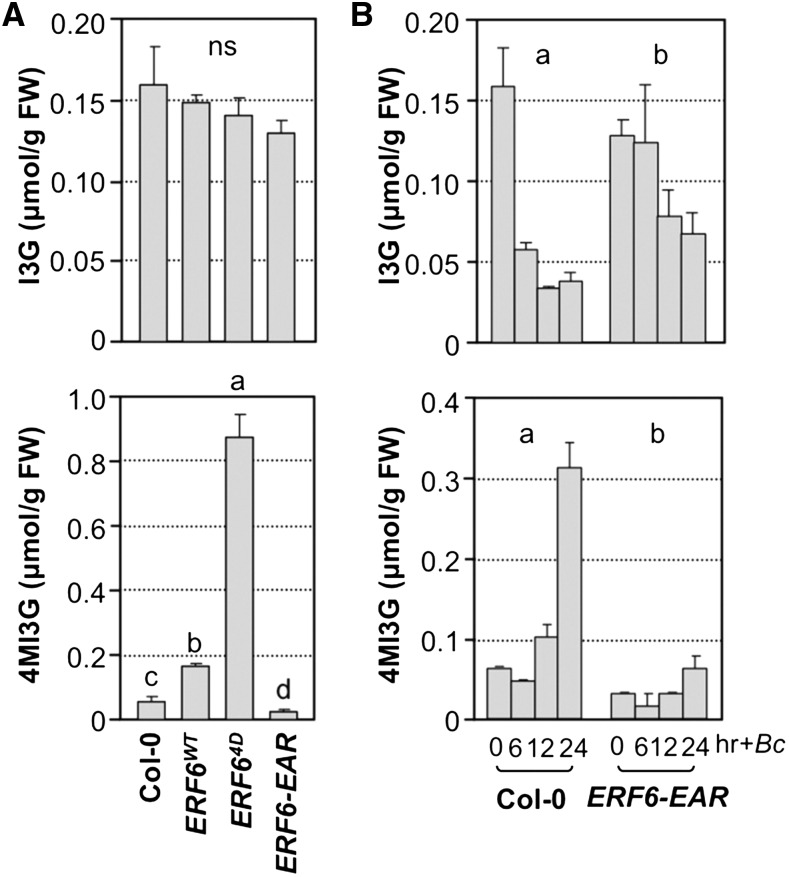
Recently, we identified an ERF transcription factor, ERF6, as a key regulator downstream of MPK3/MPK6 in plant defense against fungal pathogens (Meng et al., 2013). Expression of phospho-mimicking ERF64D significantly enhances plant resistance to B. cinerea, while expression of dominant-negative ERF6-EAR results in opposite phenotypes. Because of the important role of ERF6 in fungal pathogen resistance, we wondered if IGS biosynthesis is one of its downstream targets and measured the levels of I3G, 4MI3G, and 1MI3G in ERF6WT, ERF64D, and ERF6-EAR plants. As shown in Figure 10A, while the steady state levels of I3G were comparable, 4MI3G accumulated to a slightly higher level in ERF6WT transgenic seedlings in comparison to the wild type. A much higher level of 4MI3G was detected in the phospho-mimicking ERF64D transgenic seedlings. Conversely, a lower level of 4MI3G was found in ERF6-EAR transgenic seedlings. In contrast, 1MI3G did not show a similar trend of changes as 4MI3G in the gain- and loss-of-function ERF6 transgenic plants (Supplemental Figure 4D). In support of this, the expression of CYP81F4 did not show a similar trend as CYP81F2 either (Supplemental Figures 5C and 5D).
Figure 10.
ERF6, a Substrate of MPK3/MPK6, Plays an Important Role in the B. cinerea-Induced Biosynthesis of 4MI3G from I3G.
(A) Steady state levels of I3G and 4MI3G in 12-d-old Col-0, 35S:ERF6WT, 35S:ERF64D, and 35S:ERF6-EAR seedlings. One-way ANOVA was used to compare the levels of IGSs in different genotypes (P < 0.05).
(B) Intracellular levels of I3G and 4MI3G in 12-d-old Col-0 and 35S:ERF6-EAR seedlings after B. cinerea (Bc) inoculation.
Samples were collected without treatment (A) or at indicated times after treatment (B), and IGS levels were determined by HPLC assay. IGS contents in different genotypes were analyzed by two-way ANOVA. Different lowercase letters above the columns indicate statistically different groups (P < 0.01). Error bars indicate sd (n = 3). ns, not significant.
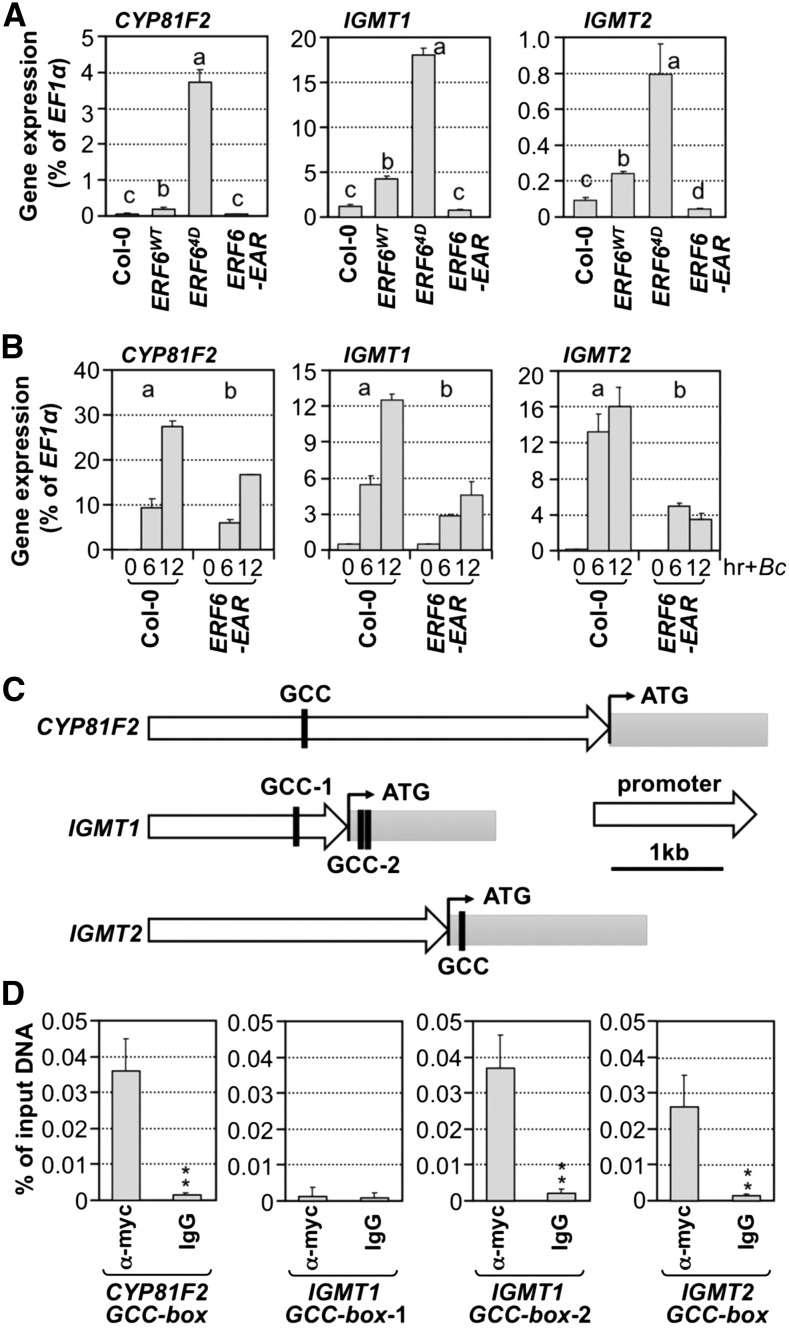
Consistent with higher levels of 4MI3G content, expression of CYP81F2, IGMT1, and IGMT2 was higher in ERF6WT and ERF64D transgenic seedlings (Figure 11A). More importantly, B. cinerea-induced conversion of I3G to 4MI3G was almost completely blocked in the dominant-negative ERF6EAR transgenic seedlings (Figure 10B, lower panel), which is associated with a reduced induction of CYP81F2, IGMT1, and IGMT2 gene expression (Figure 11B). Besides, MYB51 and MYB122 showed a similar trend of changes in their expression in these ERF6 transgenic lines (Supplemental Figure 10), indicating that I3G biosynthesis is also regulated by ERF6. Based on these results, we conclude that ERF6 and possibly its close homolog ERF5, ERF104, and ERF105 (Nakano et al., 2006; Bethke et al., 2009; Meng et al., 2013) play an important role in reprogramming IGS biosynthesis.
Figure 11.
ERF6 Regulates the Expression of CYP81F2, IGMT1, and IGMT2 by Directly Interacting with Their Promoters.
(A) Gene expression of CYP81F2, IGMT1, and IGMT2 in 12-d-old Col-0, 35S:ERF6WT, 35S:ERF64D, and 35S:ERF6-EAR seedlings was quantified by RT-qPCR and calculated as percentages of the EF1α transcript. One-way ANOVA was used to compare gene expression in different genotypes (P < 0.05).
(B) Gene expression of CYP81F2, IGMT1, and IGMT2 in 12-d-old Col-0 and 35S:ERF6-EAR seedlings after B. cinerea (Bc) inoculation. Samples were collected without treatment (A) or at indicated times after treatment (B) for total RNA preparation. The transcript levels were determined by RT-qPCR and calculated as percentages of the EF1α transcript. Error bars indicate sd (n = 3). Gene expression in different genotypes was analyzed by two-way ANOVA. Different lowercase letters above the columns indicate statistically different groups (P < 0.01).
(C) Diagrams showing the GCC boxes in the genomic sequences of CYP81F2, IGMT1, and IGMT2 genes.
(D) ERF64D binds to the GCC-boxes in CYP81F2, IGMT1, and IGMT2 genes in vivo. ChIP-qPCR was performed using 12-d-old 35S:ERF64D seedlings. Tagged 4myc-ERF64D protein-chromatin complex was immunoprecipitated with an anti-myc antibody. A control reaction was processed side-by-side using mouse IgG. ChIP- and input-DNA samples were quantified by real-time qPCR using primers specific to the GCC-box-containing regions (C). The ChIP results are presented as a percentage of input DNA. Differences in DNA abundance of anti-myc and IgG antibody samples were analyzed by Student’s t test (*P < 0.05 and **P < 0.01). Error bars indicate sd (n = 3).
Expression of phospho-mimicking ERF64D constitutively activates fungal resistance-related genes, including those involved in the IGS biosynthetic pathway (Meng et al., 2013). It is possible that ERF6 may reprogram IGS biosynthesis by directly targeting the biosynthetic genes. A search of genomic sequences of IGS biosynthetic genes revealed GCC-boxes in the promoters and/or coding regions of CYP81F2, IGMT1, and IGMT2 (Figure 11C), but not in MYB51, MYB122, SUR2, UGT74B1, or ST5a. We then performed ChIP-qPCR assays to determine whether CYP81F2, IGMT1, and IGMT2 are direct targets of ERF6 in vivo. As shown in Figure 11D and Supplemental Figure 11, anti-myc antibody could coimmunoprecipitate 4myc-ERF64D, 4myc-ERF6-EAR, and 4myc-ERF6WT proteins with all the GCC-box-containing regions of CYP81F2, IGMT1, and IGMT2 with the exception of GCC box-1 of IGMT1. In addition, the abundance of coimmunoprecipitated GCC-box-containing DNA fragments was positively correlated with the expression levels of ERF64D, ERF6-EAR, and ERF6WT proteins, suggesting that ERF6WT and its variants have similar DNA binding activity. This result provides in vivo evidence to support a key role of ERF6 in regulating the conversion of I3G to 4MI3G by directly binding to the promoters of CYP81F2, IGMT1, and IGMT2.
PEN2/PEN3-Dependent and -Independent Accumulation of Extracellular IGS Derivatives in Plant Immunity
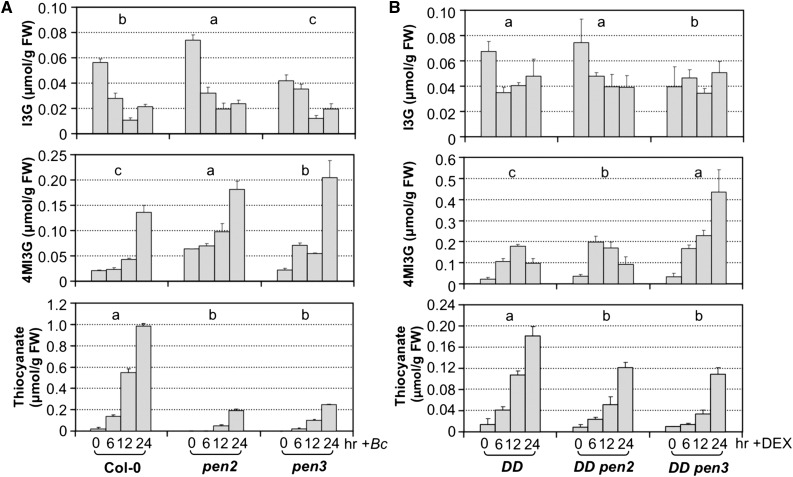
PEN2, a thioglucosidase with β-glucosidase activity similar to the canonical myrosinases, and the plasma membrane-associated PEN3 ATP binding cassette transporter have been implicated in Arabidopsis immunity against pathogens (Bednarek et al., 2009; Clay et al., 2009; Johansson et al., 2014; Lu et al., 2015; Campe et al., 2016). They are likely to be involved in the cytoplasmic synthesis and transport, respectively, of unknown small molecules derived from IGSs. We investigated their roles in the accumulation of extracellular thiocyanate ion. As shown in Figure 12A, both PEN2 and PEN3 were required for the full accumulation of extracellular thiocyanate ion. PEN2 mutation caused an ∼80% reduction in the accumulation of extracellular thiocyanate ion, suggesting that PEN2 is the major enzyme involved in the hydrolysis of 4MI3G in this process, and other thioglucosidases/myrosinases also contributed. In Arabidopsis seedlings, TGG1 and TGG2 are abundant myrosinases. As a result, we tested whether they are involved in the accumulation of extracellular thiocyanate ion in response to B. cinerea infection. As shown in Supplemental Figure 12, the levels of I3G, 4MI3G, and extracellular thiocyanate ion in B. cinerea-infected tgg1 tgg2 double mutant seedlings were comparable with those in Col-0 seedlings, suggesting that TGG1 and TGG2 are not required in the process. Similar to pen2 mutant, mutation of PEN3 also resulted in ∼80% reduction in thiocyanate ion. Other transporters are likely to be responsible for the residual activity and play a minor role in the export of IGS derivatives.
Figure 12.
PEN2 and PEN3 Are Involved in the Accumulation of Extracellular Thiocyanate Ion in Response to B. cinerea Infection.
Intracellular levels of I3G and 4MI3G and extracellular levels of thiocyanate in B. cinerea-inoculated Col-0, pen2-1, and pen3-1 mutant seedlings (A) and DEX-treated DD, DD pen2, and DD pen3 seedlings (B). Twelve-day-old seedlings were treated with B. cinerea spores (4.0 × 105 spores per vial) or DEX (1 µM). Samples were collected at indicated times, and IGSs were measured using HPLC assay. Thiocyanate levels were quantified using the isonicotinic-barbituric acid method. Error bars indicate sd (n = 3). IGS or thiocyanate ion levels in seedlings of different genotypes were analyzed by two-way ANOVA. Different lowercase letters above the columns indicate statistically different groups (P < 0.01).
In pen2 mutant seedlings, B. cinerea-induced 4MI3G accumulation was slightly higher (Figure 12A), possibly because of a reduced conversion of 4MI3G to its derivatives (such as isothiocyanates). In pen3 mutant seedlings, the accumulation of 4MI3G was also higher than that in wide-type seedlings (Figure 12A), a likely result of reduced efflux of 4MI3G. It is also important to notice that mutation of CYP81F2 completely blocked the biosynthesis of 4MI3G (Figure 7), but only partially block the accumulation of extracellular thiocyanate ion (Figure 1B), suggesting that IGSs other than 4MI3G could also be targeted by PEN2 (or other myrosinases) and PEN3 (or other transporters) for the accumulation of stable extracellular thiocyanate ion during Arabidopsis immunity.
DISCUSSION
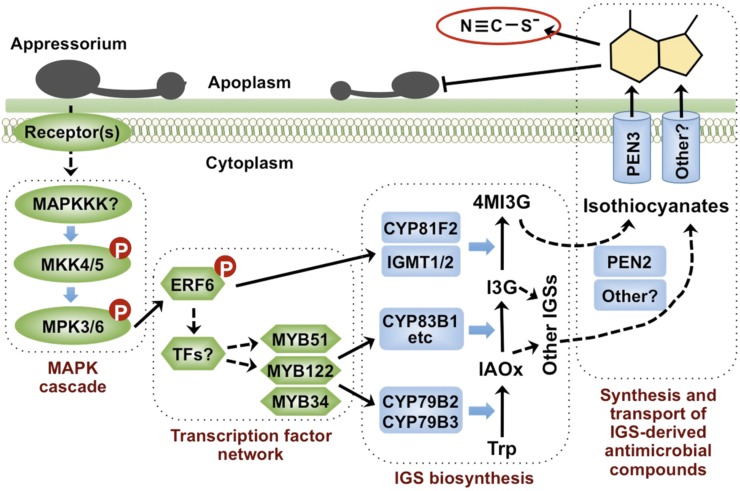
In recent years, the function of IGSs in plant immunity has been recognized (Bednarek et al., 2009; Clay et al., 2009; Hiruma et al., 2010; Schlaeppi and Mauch, 2010; Stotz et al., 2011). Genes encoding key enzymes in the biosynthetic pathways and transcriptional factors directly involved in the activation of these biosynthetic genes have been characterized (Sønderby et al., 2010a). However, the signaling pathway(s) remain unclear. In this report, we demonstrate that MPK3 and MPK6, two pathogen-responsive MAPKs, promote the biosynthesis of I3G and its conversion to 4MI3G in response to B. cinerea infection, as depicted in our working model (Figure 13). Genetic evidence demonstrated that ERF6, one of the known MPK3/MPK6 substrates (Meng et al., 2013), regulates the expression of CYP81F2, IGMT1, and IGMT2, genes encoding the enzymes in the conversion of I3G to 4MI3G. ChIP-qPCR assay further revealed that ERF6 directly targets these genes (Figure 11). In addition, ERF6 is also involved in the activation of MYB51 and MYB122, two MYB transcription factors controlling the biosynthesis of I3G (Figure 9), possibly indirectly via additional unknown transcription factors. Under the action of PEN2 and PEN3, 4M13G derivatives (possibly the active antimicrobial compounds) are transported into the apoplastic space, where they actively inhibit the pathogen growth and result in the accumulation of stable extracellular thiocyanate ion.
Figure 13.
A Model Depicting the Function of the MPK3/MPK6 Cascade in the Induction of IGS Biosynthesis and Their Derivatives in Arabidopsis Challenged by B. cinerea.
In response to fungal pathogen infection, activation of the MPK3/MPK6 cascade promotes the biosynthesis of I3G and the conversion of I3G to 4MI3G. ERF6, one of the substrates of MPK3 and MPK6, is able to activate gene expression of its direct target genes including CYP81F2, IGMT1, and IGMT2 to drive the biosynthesis of 4MI3G from I3G. ERF6 is also involved in the activation of MYB51/MYB122 expression through unidentified factors, which promotes the biosynthesis of I3G, the precursor for 4MI3G synthesis. After hydrolyzing by PEN2 and/or other myrosinases, IGSs are converted to unidentified unstable compounds, possibly isothiocyanates, which release SCN− in their breakdown process.
The MPK3/MPK6 Cascade Regulates the Biosynthesis of Camalexin and IGSs in Plant Immunity
Previously, we demonstrated the critical role of MPK3 and MPK6 in the induction of camalexin, the major phytoalexin in Arabidopsis (Ren et al., 2008; Mao et al., 2011). Here, we show that MPK3/MPK6 activation in response to B. cinerea infection also leads to a significant change in the IGS profile, i.e., a decrease in I3G content and the associated increase in 4MI3G (Figure 3). It appears that a transient activation of MPK3 and MPK6 is not sufficient to drive the conversion from I3G to 4MI3G. In response to wounding, which transiently activates MPK3/MPK6, there was no major shift from I3G to 4MI3G in the IGS profile (Supplemental Figure 13). Similar relationship exists in the activation of MPK3/MPK6 and the induction of camalexin. Plants elicited with flg22, which causes only transient MPK3/MPK6 activation (Liu and Zhang, 2004; Suarez-Rodriguez et al., 2007), accumulate little camalexin (Millet et al., 2010; Schenke et al., 2011). Long-lasting activation of MPK3/MPK6, such as in plants infected by B. cinerea, is required to achieve a high-level and long-lasting activation of camalexin and IGS biosynthetic genes and the reprogramming of plant metabolism to the synthesis of camalexin and IGSs (Ren et al., 2008; Mao et al., 2011; this report). In support of this conclusion, recent metabolomic analyses also found that long-lasting activation of MPK3/MPK6 in MKK5DD transgenic plants leads to reprogramming of plant metabolism including the induction of 4MI3G (Lassowskat et al., 2014; Lee et al., 2015). In addition, phospho-proteomics analysis identified change of PEN2/PEN3 phosphorylation status. Since the identified phosphorylation sites in PEN2/PEN3 do not match the typical MAPK phosphorylation motif, suggesting the involvement of additional kinases downstream of MPK3/MPK6. Further research is needed to determine the role of phosphorylation regulation of PEN2/PEN3.
Camalexin and IGSs are both derived from tryptophan (Zhao et al., 1998; Hiruma et al., 2013). In recent years, it has been recognized that accumulation of both camalexin and IGSs contributes to the chemical defense of plants against fungal pathogen infection. To restrict nonadapted powdery mildew fungi infection in Arabidopsis, sequential actions of PEN2-dependent breakdown of 4MI3G and camalexin are required (Bednarek et al., 2009; Schlaeppi et al., 2010; Schlaeppi and Mauch, 2010). It is likely that IGS derivatives prevent the entry of pathogens into epidermal cells, while camalexin restricts the development and spread of invasive pathogens. The critical role of the pathogen-responsive MPK3/MPK6 cascade in regulating both camalexin and IGS biosynthesis highlights the importance of this signaling pathway in plant immune response.
MPK3/MPK6 Activation Promotes Both I3G Biosynthesis and Its Conversion to 4MI3G
In response to pathogen infection, IGSs or their derivatives are actively and continuously secreted into apoplastic spaces or the extracellular medium in the liquid cultured seedlings system via membrane-localized transporters including PEN3. Measurement of cellular IGSs using the HPLC assay only quantifies the steady state concentrations of IGSs, which are determined by the rates of both biosynthesis and consumption. B. cinerea infection- or MPK3/MPK6 activation-induced 4MI3G elevation is associated with an apparent decrease in I3G levels (Figure 3). This is not a result of the reduction in I3G biosynthesis, but rather a result of rapid conversion of I3G to 4MI3G, i.e., higher consumption rate than the synthesis rate. Based on the activation of genes in I3G biosynthetic pathway (Supplemental Figure 14), the rate of I3G biosynthesis is likely to be elevated in the process as well, which feeds into the induction of 4MI3G and their derivatives in plant immunity.
Genes encoding all known enzymes involved in the I3G biosynthesis including SUR2, UGT74B1, and ST5a and the transcription factors MYB51 and MYB122 that control the expression of these biosynthetic genes were highly induced in Arabidopsis inoculated with B. cinerea (Supplemental Figure 14B) and gain-of-function DD plants after DEX treatment (Supplemental Figure 14A). Together with the induction of CYP79B2/CYP79B3, two genes encoding P450 enzymes involved in the conversion of Trp to IAOx (Glawischnig et al., 2004; Ren et al., 2008), we expect that the rate of I3G biosynthesis was also elevated after B. cinerea infection or gain-of-function MPK3/MPK6 activation. Indeed, in B. cinerea-infected cyp81f2 or in DD cyp81f2 seedlings, in which the conversion of I3G to 4MI3G was blocked, I3G accumulated to higher levels (Figure 7). This conclusion is also consistent with the findings in the loss-of-functional mutants of key activators of IGS biosynthesis. In mpk3 mpk6 double mutant (Figure 4A), myb51 single, myb51 myb122 double, and myb51 myb122 myb34 triple mutants (Figure 9A) inoculated with B. cinerea, I3G levels were even lower than those in the wild-type controls, suggesting that MPK3/MPK6 and MYB51/MYB122/MYB34 are important in maintaining a higher rate of I3G biosynthesis.
A Network of Transcription Factors Functions Downstream of MPK3/MPK6 in Controlling the Biosynthesis of IGSs
Three MYB transcription factors, MYB34, MYB51, and MYB122, are important regulators of de novo biosynthesis of IGS (Celenza et al., 2005; Gigolashvili et al., 2007b; Frerigmann and Gigolashvili, 2014). Despite sharing over 80% amino acid sequence identity in their conserved DNA binding domains, they are differentially regulated at the transcriptional level in shoots and roots and in response to distinct hormones (Frerigmann and Gigolashvili, 2014). Here, we show that in response to B. cinerea infection or MPK3/MPK6 activation, only MYB51 and MYB122 are highly induced, while the expression of MYB34 is downregulated (Figure 8; Supplemental Figures 8E and 8F). Consistent with their expression levels, MYB51 contributes the most to the IGS induction, and MYB122 the second. MYB34 is responsible for the low basal-level IGS biosynthesis in the myb51 myb122 double mutant (Figure 9). In addition, we found that MYB51, MYB122, and MYB34 are not required for the induction of CYP81F2, IGMT1, and IGMT2 genes (Supplemental Figure 9), suggesting the involvement of additional transcription factors. This is consistent with the recent report that concluded additional unknown transcription factors besides MYB51/MYB122 are involved in the biosynthesis of modified IGSs (Frerigmann and Gigolashvili, 2014).
In this study, we identified ERF6, a previously characterized MPK3/MPK6 substrate (Meng et al., 2013), as a positive regulator in promoting the conversion of I3G to 4MI3G by directly targeting CYP81F2, IGMT1, and IGMT2 biosynthetic genes (Figures 10 and 11). In the phospho-mimicking ERF64D transgenic seedlings, 4MI3G is accumulated to a high level in comparison to that in Col-0 seedlings. In contrast, in the dominant-negative ERF6-EAR seedlings, B. cinerea-induced 4MI3G accumulation is blocked. ChIP-qPCR analysis revealed that ERF6 directly binds to CYP81F2, IGMT1, and IGMT2, but not other IGS biosynthetic genes, indicating a specific role of ERF6 in promoting 4MI3G biosynthesis. Besides a direct role of ERF6 in controlling the expression of CYP81F2, IGMT1, and IGMT2 genes, it is also indirectly involved in the expression of MYB51 and MYB122 (Supplemental Figure 10). MYB51/MYB122 do not contain GCC box in their promoters, suggesting the involvement of additional transcription factors downstream of ERF6. Additional research is needed to fully elucidate this multilayered complex network of transcription factors downstream of MPK3/MPK6 in regulating the expression of these IGS biosynthetic genes (Figure 13).
Redundant Components Leading to the Accumulation of Extracellular Thiocyanate Ion
The high-level accumulation of thiocyanate ion in the medium of DEX-treated DD seedlings and B. cinerea-infected plants is partially dependent on functional PEN2 and PNE3 (Figures 1C and 11), suggesting the involvement of additional parallel pathways/components. Based on the fact that the accumulation of extracellular thiocyanate ion is completely blocked in cyp79b2 cyp79b3 double mutant (Figure 1B), we can also conclude that the extracellular thiocyanate is derived from IGSs, but not AGSs, in response to MPK3/MPK6 activation after B. cinerea infection. CYP81F2 is a key enzyme in the conversion of I3G to 4MI3G, and 4MI3G biosynthesis is completely blocked in the cyp81f2 mutant (Figure 7). The accumulation of reduced levels of thiocyanate ion in the cyp81f2 mutant (Figure 1B) also suggests that additional unidentified IGSs are involved in the process. Similarly, partial inhibition of thiocyanate accumulation in pen2 and pen3 mutant also suggests the involvement of additional myrosinases other than PEN2 in the deglycosylation of IGSs and additional transporters other than PEN3 to transport IGS derivatives to the extracellular space.
A number of recent studies demonstrated the importance of IGSs including 4MI3G in plant immunity (Bednarek et al., 2009; Hiruma et al., 2010; Sanchez-Vallet et al., 2010; Schlaeppi et al., 2010; Schlaeppi and Mauch, 2010; Buxdorf et al., 2013). At this stage, the exact identities of the active antimicrobial compounds derived from IGSs are still unknown because of their high reactivity and unstable nature but believed to be isothiocyanate derivatives (Bednarek et al., 2011). We detected the accumulation of thiocyanate ion, a stable compound released from the putative isothiocyanates derived from I3G and, more importantly, 4MI3G (Agerbirk et al., 2009) (Supplemental Figure 15A). Besides thiocyanate ion, we quantified additional IGS derivatives using liquid chromatography-mass spectrometry analysis. As shown in Supplemental Figure 15B, the levels of I3M-ascorbate (also known as ascorbigen) and I3M-glutathione, two derivatives from I3G (Kim et al., 2008), decreased in response to B. cinerea infection. In contrast, the levels of 4MI3M-ascorbate and 4MI3M-glutathione, two derivatives from 4MI3G, increased. The opposite changes in I3G and 4MI3G derivatives might result from the decrease in I3G and the increase in 4MI3G. Since thiocyanate ion is produced at equal molar ratio during the formation of these derivatives, we can also conclude that the accumulated thiocyanate ion is mostly from 4MI3G. In addition, we detected the accumulation of raphanusamic acid in response to B. cinerea infection (Supplemental Figure 15B). However, its level was much lower than thiocyanate ion, 4MI3M-ascorbate, or 4MI3M-glutathione, suggesting that it is not a major derivative of IGSs in response to B. cinerea infection.
After B. cinerea infection, thiocyanate ion accumulates to high levels, several times of the steady state levels of IGSs (Figures 1B and 12, bottom panels). This is because thiocyanate ion is relatively stable. In the culture media, we did not detect any decrease in its level after 24 h (Supplemental Figure 16). In the presence of Arabidopsis seedlings, there was only a ∼20% decrease after 24 h. In vitro assay revealed that thiocyanate ion does not have direct antimicrobial activity at physiological concentrations (Supplemental Figure 17). Nonetheless, its high accumulation is an indication of the presence of antimicrobial IGS derivatives, likely isothiocyanates, in the apoplastic space and culture medium. Compromised B. cinerea resistance in MAPK mutants has been recognized for years (Ren et al., 2008). The identification of MPK3/MPK6 cascade in controlling I3G biosynthesis and the conversion of I3G to 4MI3G in response to pathogen invasion revealed a new potential mechanism underlying the function of MPK3/MPK6 in plant immunity. This research also helps us to understand how plant sensing of invading pathogens reprograms plant cellular secondary metabolism to fight off pathogen infection.
METHODS
Plant Maintenance and Treatment
Arabidopsis thaliana seedlings were grown in 20-mL gas chromatography vials with 6 mL of half-strength Murashige and Skoog liquid medium in a growth chamber under continuous light as described before (Ren et al., 2008; Han et al., 2010). Twelve-day-old seedlings were used for experiments. Seedlings were collected at various time points after the addition of DEX (1 µM final concentration) or inoculation of Botrytis cinerea spores (4.0 × 105 spores per vial).
Statistical Analyses
At least two independent repetitions were performed for experiments with multiple time points. For single time point experiments, at least three independent repetitions were done. Results from one of the independent repeats that gave similar results were shown. Statistical analysis was performed using GraphPad Prism 6.0 (http://www.graphpad.com/). Student’s t test was used to determine whether the difference between two groups of data at a specific time point is statistically significant. Single and double asterisks above the columns indicate differences that are statistically significant (*P < 0.05) or very significant (**P < 0.01), respectively. When more than two samples are compared, one-way ANOVA with Tukey’s post hoc test was performed (P < 0.05). Two-way ANOVA analysis with Tukey’s post hoc test was performed when time-course data of different mutants were compared (Brady et al., 2015). Different letters above the data points are used to indicate differences that are statistically significant.
Mutants and Transgenic Lines
Arabidopsis mutants and transgenic lines used in this study are all in Col-0 ecotype background. Steroid-inducible promoter-driven tobacco MEK2DD transgenic Arabidopsis line (DD), mpk3-1, mpk6-2, DD mpk3, DD mpk6 (Liu and Zhang, 2004; Wang et al., 2007), pad3, cyp79b2 cyp79b3 (Zhou et al., 1999; Glawischnig et al., 2004; Ren et al., 2008), and high-order acs mutants (Tsuchisaka et al., 2009; Li et al., 2012) were described previously.
T-DNA insertion mutant alleles of sur2 (CS16401), cyp81f2 (SALK_073776), myb51 (CS306630), myb122 (SALK_022993), and pen3 (SALK_000578) were obtained from the ABRC (Alonso et al., 2003). EMS-induced pen2-1 mutant was kindly supplied by Paul Schulze-Lefert (Lipka et al., 2005). myb51 myb122 myb34 triple and tgg1 tgg2 double mutants were kindly supplied by Tamara Gigolashvili (Frerigmann and Gigolashvili, 2014) and Georg Jander (Barth and Jander, 2006). DD pen2, DD pen3, DD cyp81f2, DD myb51, DD myb122, myb51myb122, and DD myb51 myb122 double or triple mutants were generated by genetic cross, and homozygous lines were used for experiments.
Generation of the Conditional MPK3SR Mutant Using a Chemical Genetic Approach
Chemical-sensitized MPK3-rescued mpk3 mpk6 double mutant (MPK3SR plants) was generated similarly as previously described for MPK6SR (Xu et al., 2014). Thr-144 residue in the ATP binding pocket of a MPK3 genomic clone was first mutated to alanine (MPK3TA). This genomic clone in pCambia3300 vector (PMPK3:MPK3TA) was transformed into mpk3 mpk6/+ plants. Multiple independent single-insertion lines with MPK3TA expressed at a similar level as the endogenous MPK3 gene were identified by immunoblot analysis using an anti-MPK3 antibody. T3 homozygous PMPK3:MPK3TA transgenic plants in mpk3 mpk6 background (mpk3 mpk6 PMPK3:MPK3TA), also called inhibitor-sensitized MPK3 variant-rescued plants (MPK3SR), were used for experiments. Two independent transgenic lines were followed and the same results were obtained.
Isonicotinic-Barbituric Acid Assay of Cyanide and Thiocyanate Ion
Cyanide and thiocyanate ion were measured using the standard spectrophotometric assay based on the isonicotinic-barbituric acid method (Nagashima, 1981). Briefly, 200 μL of potassium phosphate buffer (1 M, pH 7.3), 13.3 μL of sodium hypochlorite (0.5%, w/v), and 333 μL of isonicotinic-barbituric acid reagent (12 g/L NaOH, 20 g/L isonicotinic acid, and 10 g/L barbituric acid) were added to 666-μL samples sequentially, with mixing after the addition of each solution. After being incubated at room temperature for 15 min, A600 of the mixture was determined using a spectrophotometer. Standard curves were generated using different concentrations of KCN or KSCN. To measure the levels of thiocyanate ion in the medium without the interference from cyanide, free cyanide was first masked by the addition of 5 μL formaldehyde (3.7%) to 1 mL of liquid sample. After incubation at room temperature for 30 min, the levels of thiocyanate ion were determined using the isonicotinic-barbituric acid method (Meeussen et al., 1989).
Cyclocondensation Assay of Isothiocyanates
Isothiocyanates in the culture media were detected as previously described (Zhang et al., 1992). Liquid media samples (100 mL) were quick frozen in liquid N2 and lyophilized to dryness. The lyophilized samples were then extracted with 1 mL methanol, and 50 μL extracts were used for isothiocyanates assay. The reaction mixture has a total volume of 200 μL containing 50% methanol (v/v), 25 mM potassium phosphate (pH 8.5), and 20 mM 1.2-benzenedithiol. The reaction mixture was incubated at 65°C for 1 h and then cooled to 25°C. Absorbance was determined at 365 nm.
HPLC Assay of Glucosinolates
GSs were extracted and analyzed as previously described (Miao et al., 2013). Twelve-day-old seedlings were harvested at indicated times after treatment. GSs were extracted from freshly collected tissues (100 to 200 mg) by boiling in water (1 mL) for 10 min. A second extraction was done. After heating, the internal standard sinigrin (Sigma-Aldrich) was added to the mixture. The combined aqueous extract was applied to a DEAE-Sephadex A-25 column (pyridine acetate form, equivalent of 30 mg dry weight). After the column was washed three times with 20 mM pyridine acetate and twice with 1 mL of water, the bound glucosinolates were converted to their desulpho analogs by reacting with 100 μL of 0.1% (1.4 units) aryl sulphatase overnight. The desulphoglucosinolates were eluted with 1 mL of water.
The HPLC analysis was performed using an Agilent 1260 HPLC system. A Hypersil C18 column (5-µm particle size, 4.6 mm × 250 mm; Elite Analytical Instruments) was used with a mobile phase of acetonitrile and water at a flow rate of 1.0 mL min–1. The procedure employed isocratic elution with 1.5% acetonitrile for the first 5 min, a linear gradient to 20% acetonitrile over the next 15 min, followed by isocratic elution with 20% acetonitrile for the final 13 min. A 20-μL sample was injected into the column by an autosampler. Absorbance was detected at 226 nm. Sinigrin was used as an internal standard for calculation of molar concentrations of individual glucosinolates. The selected glucosinolates were quantified on the basis of their peak areas relative to that of the internal standard, and relative response factors were applied to correct absorbance differences between the standard and other glucosinolates (Brown et al., 2003). Data were given as nmol mg–1 fresh weight.
Quantification of Transcript Levels by Real-Time PCR Analysis
Total RNA was extracted using TRIzol reagent (Invitrogen). After DNase treatment, 1 µg of total RNA was used for reverse transcription, and real-time quantitative PCR analysis was performed using an Eppendorf real-time PCR machine as previously described (Ren et al., 2008). The levels of gene expression were calculated as percentages of the EF-1α transcript. The primer pairs used for real-time PCR are listed in Supplemental Table 1.
ChIP-qPCR Analysis
ChIP-qPCR assay was performed as previously described (Mao et al., 2011; Meng et al., 2013). Twelve-day-old 35S:ERF64D seedlings were first cross-linked and processed using a standard procedure (Kaufmann et al., 2010). Chromatin was then isolated from 1 g of tissue and sheared using an enzymatic shearing kit (Active Motif). Immunoprecipitation was performed by incubating the chromatin samples with 2 µg of anti-myc antibody (Millipore) or mouse IgG (negative control) for 1 h at 4°C. The protein-chromatin immunocomplexes were captured using Protein G-Dynal magnetic beads (Invitrogen). After Proteinase K digestion, the immunoprecipitated DNA was purified using a ChIP DNA clean and concentrator kit (Zymo Research). Immunoprecipitated DNA and input DNA samples were analyzed by qPCR using primers specific for the GCC-box regions of CYP81F2, IGMT1, and IGMT2 (Supplemental Table 1). The ChIP-qPCR results are presented as percentage of input DNA.
Protein Extraction and Immunoblot Analysis
Protein was extracted from seedlings and stored at −80°C as previously described (Liu and Zhang, 2004). The concentration of protein extracts was determined using the Bio-Rad protein assay kit with BSA as the standard. Immunoblot detection of MAPKs was performed as previously described (Xu et al., 2014). MAPK activation was detected by immunoblot analysis using anti-pTEpY (anti-phospho-p44/42-ERK; http://www.cellsignal.com/) as described (Tsuda et al., 2009).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: MPK3 (At3g45640), MPK6 (At2g43790), CYP79B2 (At4g39950), CYP79B3 (At2g22330), EF1α (At5g60390), SUR2 (At4g31500), CYP81F2 (At5g57220), IGMT1 (At1g21100), IGMT2 (At1g21120), MYB51 (At1g18570), MYB122 (At1g74080), ERF6 (At4g17490), PEN2 (At2g44490), and PEN3 (At1g59870).
Supplemental Data
Supplemental Figure 1. Accumulation of extracellular thiocyanate remains unaltered in acs or pad3 mutants.
Supplemental Figure 2. Relative contents of AGSs and IGSs in liquid-cultured and soil-grown seedlings and older soil-grown plants.
Supplemental Figure 3. B. cinerea-induced IGS biosynthesis is comparable in wild type (Col-0) and mpk3 or mpk6 single mutant.
Supplemental Figure 4. IMI3G contents in various mutant backgrounds after B. cinerea infection or gain-of-function activation of MPK3 and MPK6 in DD plants.
Supplemental Figure 5. Expression of CYP81F3 and CYP81F4 in response to B. cinerea infection in Col-0 or in ERF6 transgenic mutant seedlings.
Supplemental Figure 6. Protein levels of the chemical-sensitized MPK6YG and MPK3TA in MPK6SR and MPK3SR seedlings.
Supplemental Figure 7. Expression of AGS biosynthetic genes is downregulated in response to B. cinerea infection or gain-of-function activation of MPK3 and MPK6.
Supplemental Figure 8. Downregulation of MYB28, MYB29, and MYB34 gene expression in response to B. cinerea infection or activation of MPK3 and MPK6.
Supplemental Figure 9. Induction of CYP81F2, IGMT1, and IGMT2 gene expression in response to B. cinerea infection or MPK3/MPK6 activation is not dependent on MYB51 and MYB122.
Supplemental Figure 10. ERF6 is involved in the induction of MYB51 and MYB122 gene expression.
Supplemental Figure 11. ERF6-EAR and ERF6WT directly interact with the GCC-box-containing regions of CYP81F2, IGMT1, and IGMT2.
Supplemental Figure 12. TGG1 and TGG2 are not involved in the accumulation of extracellular thiocyanate ion in response to B. cinerea infection.
Supplemental Figure 13. Transient activation of MPK3/MPK6 induced by wounding fails to induce accumulation of 4MI3G.
Supplemental Figure 14. B. cinerea-induced expression of SUR2, UGT74B1, and ST5a is not fully dependent on MPK3/MPK6 activation, although gain-of-function MPK3/MPK6 activation is sufficient to activate their expression.
Supplemental Figure 15. IGS derivatives in Arabidopsis seedlings after B. cinerea infection.
Supplemental Figure 16. Stability of thiocyanate ion in culture medium.
Supplemental Figure 17. Thiocyanate ion does not have direct antimicrobial activity at physiological concentrations.
Supplementary Material
Acknowledgments
We thank Paul Schulze-Lefert, Tamara Gigolashvili, Georg Jander, and the Arabidopsis Biological Resource Center for mutant seeds. This work was supported by grants from the Natural Science Foundation of China (31570297), Zhejiang University Special Fund for Young Researchers (2015QNA6002) and 985 Project (118000-193411801), and the National Science Foundation (IOS-0743957) to J.X. and S.Z.
AUTHOR CONTRIBUTIONS
J.X. and S.Z. designed the research. J.X., J.M., X.M., J.L., T.S., and Y.L. performed research. Y.Z. and Q.W. contributed new analytic tools. J.X. and S.Z. analyzed data and wrote the article.
Glossary
- GS
glucosinolate
- AGS
aliphatic glucosinolate
- IGS
indole glucosinolate
- 4MI3G
4-methoxyindol-3-ylmethylglucosinolate
- MAPK
mitogen-activated protein kinase
- ChIP-qPCR
chromatin immunoprecipitation-quantitative PCR
- 1MI3G
1-methoxy-3-indolylmethyl-GS
- DEX
dexamethasone
Footnotes
Articles can be viewed without a subscription.
References
- Agerbirk N., De Vos M., Kim J.H., Jander G. (2009). Indole glucosinolate breakdown and its biological effects. Phytochem. Rev. 8: 101–120. [Google Scholar]
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. [DOI] [PubMed] [Google Scholar]
- Andreasson E., Ellis B. (2010). Convergence and specificity in the Arabidopsis MAPK nexus. Trends Plant Sci. 15: 106–113. [DOI] [PubMed] [Google Scholar]
- Böttcher C., Westphal L., Schmotz C., Prade E., Scheel D., Glawischnig E. (2009). The multifunctional enzyme CYP71B15 (PHYTOALEXIN DEFICIENT3) converts cysteine-indole-3-acetonitrile to camalexin in the indole-3-acetonitrile metabolic network of Arabidopsis thaliana. Plant Cell 21: 1830–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak S., Tax F.E., Feldmann K.A., Galbraith D.W., Feyereisen R. (2001). CYP83B1, a cytochrome P450 at the metabolic branch point in auxin and indole glucosinolate biosynthesis in Arabidopsis. Plant Cell 13: 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlier I., Kowalczyk M., Marchant A., Ljung K., Bhalerao R., Bennett M., Sandberg G., Bellini C. (2000). The SUR2 gene of Arabidopsis thaliana encodes the cytochrome P450 CYP83B1, a modulator of auxin homeostasis. Proc. Natl. Acad. Sci. USA 97: 14819–14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C., Jander G. (2006). Arabidopsis myrosinases TGG1 and TGG2 have redundant function in glucosinolate breakdown and insect defense. Plant J. 46: 549–562. [DOI] [PubMed] [Google Scholar]
- Bednarek P. (2012). Chemical warfare or modulators of defence responses - the function of secondary metabolites in plant immunity. Curr. Opin. Plant Biol. 15: 407–414. [DOI] [PubMed] [Google Scholar]
- Bednarek P., Piślewska-Bednarek M., Ver Loren van Themaat E., Maddula R.K., Svatoš A., Schulze-Lefert P. (2011). Conservation and clade-specific diversification of pathogen-inducible tryptophan and indole glucosinolate metabolism in Arabidopsis thaliana relatives. New Phytol. 192: 713–726. [DOI] [PubMed] [Google Scholar]
- Bednarek P., Pislewska-Bednarek M., Svatos A., Schneider B., Doubsky J., Mansurova M., Humphry M., Consonni C., Panstruga R., Sanchez-Vallet A., Molina A., Schulze-Lefert P. (2009). A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323: 101–106. [DOI] [PubMed] [Google Scholar]
- Bethke G., Unthan T., Uhrig J.F., Pöschl Y., Gust A.A., Scheel D., Lee J. (2009). Flg22 regulates the release of an ethylene response factor substrate from MAP kinase 6 in Arabidopsis thaliana via ethylene signaling. Proc. Natl. Acad. Sci. USA 106: 8067–8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop A.C., et al. (2000). A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407: 395–401. [DOI] [PubMed] [Google Scholar]
- Brady S.M., Burow M., Busch W., Carlborg Ö., Denby K.J., Glazebrook J., Hamilton E.S., Harmer S.L., Haswell E.S., Maloof J.N., Springer N.M., Kliebenstein D.J. (2015). Reassess the t test: interact with all your data via ANOVA. Plant Cell 27: 2088–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P.D., Tokuhisa J.G., Reichelt M., Gershenzon J. (2003). Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 62: 471–481. [DOI] [PubMed] [Google Scholar]
- Burow M., Wittstock U. (2009). Regulation and function of specifier proteins in plants. Phytochem. Rev. 8: 87–99. [Google Scholar]
- Burow M., Halkier B.A., Kliebenstein D.J. (2010). Regulatory networks of glucosinolates shape Arabidopsis thaliana fitness. Curr. Opin. Plant Biol. 13: 348–353. [DOI] [PubMed] [Google Scholar]
- Buxdorf K., Yaffe H., Barda O., Levy M. (2013). The effects of glucosinolates and their breakdown products on necrotrophic fungi. PLoS One 8: e70771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campe R., Langenbach C., Leissing F., Popescu G.V., Popescu S.C., Goellner K., Beckers G.J., Conrath U. (2016). ABC transporter PEN3/PDR8/ABCG36 interacts with calmodulin that, like PEN3, is required for Arabidopsis nonhost resistance. New Phytol. 209: 294–306. [DOI] [PubMed] [Google Scholar]
- Celenza J.L., Quiel J.A., Smolen G.A., Merrikh H., Silvestro A.R., Normanly J., Bender J. (2005). The Arabidopsis ATR1 Myb transcription factor controls indolic glucosinolate homeostasis. Plant Physiol. 137: 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay N.K., Adio A.M., Denoux C., Jander G., Ausubel F.M. (2009). Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Doerner P. (2012). Genetic and molecular basis of nonhost disease resistance: complex, yes; silver bullet, no. Curr. Opin. Plant Biol. 15: 400–406. [DOI] [PubMed] [Google Scholar]
- Fan J., Crooks C., Creissen G., Hill L., Fairhurst S., Doerner P., Lamb C. (2011). Pseudomonas sax genes overcome aliphatic isothiocyanate-mediated non-host resistance in Arabidopsis. Science 331: 1185–1188. [DOI] [PubMed] [Google Scholar]
- Frerigmann H., Gigolashvili T. (2014). MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol. Plant 7: 814–828. [DOI] [PubMed] [Google Scholar]
- Frerigmann H., Berger B., Gigolashvili T. (2014). bHLH05 is an interaction partner of MYB51 and a novel regulator of glucosinolate biosynthesis in Arabidopsis. Plant Physiol. 166: 349–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigolashvili T., Yatusevich R., Berger B., Müller C., Flügge U.I. (2007a). The R2R3-MYB transcription factor HAG1/MYB28 is a regulator of methionine-derived glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 51: 247–261. [DOI] [PubMed] [Google Scholar]
- Gigolashvili T., Engqvist M., Yatusevich R., Müller C., Flügge U.I. (2008). HAG2/MYB76 and HAG3/MYB29 exert a specific and coordinated control on the regulation of aliphatic glucosinolate biosynthesis in Arabidopsis thaliana. New Phytol. 177: 627–642. [DOI] [PubMed] [Google Scholar]
- Gigolashvili T., Berger B., Mock H.P., Müller C., Weisshaar B., Flügge U.I. (2007b). The transcription factor HIG1/MYB51 regulates indolic glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 50: 886–901. [DOI] [PubMed] [Google Scholar]
- Glawischnig E., Hansen B.G., Olsen C.E., Halkier B.A. (2004). Camalexin is synthesized from indole-3-acetaldoxime, a key branching point between primary and secondary metabolism in Arabidopsis. Proc. Natl. Acad. Sci. USA 101: 8245–8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R., Su J., Meng X., Li S., Liu Y., Xu J., Zhang S (2015). Multilayered regulation of ethylene induction plays a positive role in Arabidopsis resistance against Pseudomonas syringae. Plant Physiol. 169: 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkier B.A., Gershenzon J. (2006). Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 57: 303–333. [DOI] [PubMed] [Google Scholar]
- Han L., Li G.J., Yang K.Y., Mao G., Wang R., Liu Y., Zhang S. (2010). Mitogen-activated protein kinase 3 and 6 regulate Botrytis cinerea-induced ethylene production in Arabidopsis. Plant J. 64: 114–127. [DOI] [PubMed] [Google Scholar]
- Hirai M.Y., et al. (2007). Omics-based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Proc. Natl. Acad. Sci. USA 104: 6478–6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma K., Onozawa-Komori M., Takahashi F., Asakura M., Bednarek P., Okuno T., Schulze-Lefert P., Takano Y. (2010). Entry mode-dependent function of an indole glucosinolate pathway in Arabidopsis for nonhost resistance against anthracnose pathogens. Plant Cell 22: 2429–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma K., Fukunaga S., Bednarek P., Piślewska-Bednarek M., Watanabe S., Narusaka Y., Shirasu K., Takano Y. (2013). Glutathione and tryptophan metabolism are required for Arabidopsis immunity during the hypersensitive response to hemibiotrophs. Proc. Natl. Acad. Sci. USA 110: 9589–9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson O.N., Fantozzi E., Fahlberg P., Nilsson A.K., Buhot N., Tör M., Andersson M.X. (2014). Role of the penetration-resistance genes PEN1, PEN2 and PEN3 in the hypersensitive response and race-specific resistance in Arabidopsis thaliana. Plant J. 79: 466–476. [DOI] [PubMed] [Google Scholar]
- Joo S., Liu Y., Lueth A., Zhang S. (2008). MAPK phosphorylation-induced stabilization of ACS6 protein is mediated by the non-catalytic C-terminal domain, which also contains the cis-determinant for rapid degradation by the 26S proteasome pathway. Plant J. 54: 129–140. [DOI] [PubMed] [Google Scholar]
- Kaufmann K., Muiño J.M., Østerås M., Farinelli L., Krajewski P., Angenent G.C. (2010). Chromatin immunoprecipitation (ChIP) of plant transcription factors followed by sequencing (ChIP-SEQ) or hybridization to whole genome arrays (ChIP-CHIP). Nat. Protoc. 5: 457–472. [DOI] [PubMed] [Google Scholar]
- Kim C.Y., Liu Y., Thorne E.T., Yang H., Fukushige H., Gassmann W., Hildebrand D., Sharp R.E., Zhang S. (2003). Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. Plant Cell 15: 2707–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Lee B.W., Schroeder F.C., Jander G. (2008). Identification of indole glucosinolate breakdown products with antifeedant effects on Myzus persicae (green peach aphid). Plant J. 54: 1015–1026. [DOI] [PubMed] [Google Scholar]
- Kissen R., Rossiter J., Bones A. (2009). The ‘mustard oil bomb’: not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem. Rev. 8: 69–86. [Google Scholar]
- Lambrix V., Reichelt M., Mitchell-Olds T., Kliebenstein D.J., Gershenzon J. (2001). The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell 13: 2793–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassowskat I., Böttcher C., Eschen-Lippold L., Scheel D., Lee J. (2014). Sustained mitogen-activated protein kinase activation reprograms defense metabolism and phosphoprotein profile in Arabidopsis thaliana. Front. Plant Sci. 5: 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Eschen-Lippold L., Lassowskat I., Böttcher C., Scheel D. (2015). Cellular reprogramming through mitogen-activated protein kinases. Front. Plant Sci. 6: 940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Meng X., Wang R., Mao G., Han L., Liu Y., Zhang S. (2012). Dual-level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis. PLoS Genet. 8: e1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Sawada Y., Hirai A., Sato M., Kuwahara A., Yan X., Hirai M.Y. (2013). Novel insights into the function of Arabidopsis R2R3-MYB transcription factors regulating aliphatic glucosinolate biosynthesis. Plant Cell Physiol. 54: 1335–1344. [DOI] [PubMed] [Google Scholar]
- Lipka V., et al. (2005). Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310: 1180–1183. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang S. (2004). Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16: 3386–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Dittgen J., Piślewska-Bednarek M., Molina A., Schneider B., Svatoš A., Doubský J., Schneeberger K., Weigel D., Bednarek P., Schulze-Lefert P. (2015). Mutant allele-specific uncoupling of PENETRATION3 functions reveals engagement of the ATP-binding cassette transporter in distinct tryptophan metabolic pathways. Plant Physiol. 168: 814–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G., Meng X., Liu Y., Zheng Z., Chen Z., Zhang S. (2011). Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 23: 1639–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeussen J.C.L., Temminghoff E.J.M., Keizer M.G., Novozamsky I. (1989). Spectrophotometric determination of total cyanide, iron-cyanide complexes, free cyanide and thiocyanate in water by a continuous-flow system. Analyst (Lond.) 114: 959–963. [Google Scholar]
- Meng X., Zhang S. (2013). MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 51: 245–266. [DOI] [PubMed] [Google Scholar]
- Meng X., Xu J., He Y., Yang K.-Y., Mordorski B., Liu Y., Zhang S. (2013). Phosphorylation of an ERF transcription factor by Arabidopsis MPK3/MPK6 regulates plant defense gene induction and fungal resistance. Plant Cell 25: 1126–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H., Wei J., Zhao Y., Yan H., Sun B., Huang J., Wang Q. (2013). Glucose signalling positively regulates aliphatic glucosinolate biosynthesis. J. Exp. Bot. 64: 1097–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.M., Conn E.E. (1980). Metabolism of hydrogen cyanide by higher plants. Plant Physiol. 65: 1199–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet Y.A., Danna C.H., Clay N.K., Songnuan W., Simon M.D., Werck-Reichhart D., Ausubel F.M. (2010). Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell 22: 973–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy L.J., Robertson K.N., Harroun S.G., Brosseau C.L., Werner-Zwanziger U., Moilanen J., Tuononen H.M., Clyburne J.A.C. (2014). A simple complex on the verge of breakdown: isolation of the elusive cyanoformate ion. Science 344: 75–78. [DOI] [PubMed] [Google Scholar]
- Nagashima S. (1981). Spectrophotometric determination of cyanide with isonicotinic acid and barbituric acid. Environ. Anal. Chem. 10: 99–106. [Google Scholar]
- Nakano T., Suzuki K., Fujimura T., Shinshi H. (2006). Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol. 140: 411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley K.F., Martin G.B. (2005). Role of mitogen-activated protein kinases in plant immunity. Curr. Opin. Plant Biol. 8: 541–547. [DOI] [PubMed] [Google Scholar]
- Peiser G.D., Wang T.-T., Hoffman N.E., Yang S.F., Liu H.W., Walsh C.T. (1984). Formation of cyanide from carbon 1 of 1-aminocyclopropane-1-carboxylic acid during its conversion to ethylene. Proc. Natl. Acad. Sci. USA 81: 3059–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz M., Vogel H., Kroymann J. (2009). The gene controlling the indole glucosinolate modifier1 quantitative trait locus alters indole glucosinolate structures and aphid resistance in Arabidopsis. Plant Cell 21: 985–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz M., Mikkelsen M.D., Bednarek P., Olsen C.E., Halkier B.A., Kroymann J. (2011). Metabolic engineering in Nicotiana benthamiana reveals key enzyme functions in Arabidopsis indole glucosinolate modification. Plant Cell 23: 716–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzschke A., Schikora A., Hirt H. (2009). MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 12: 421–426. [DOI] [PubMed] [Google Scholar]
- Ren D., Yang H., Zhang S. (2002). Cell death mediated by mitogen-activated protein kinase pathway is associated with the generation of hydrogen peroxide in Arabidopsis. J. Biol. Chem. 277: 559–565. [DOI] [PubMed] [Google Scholar]
- Ren D., Liu Y., Yang K.-Y., Han L., Mao G., Glazebrook J., Zhang S. (2008). A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 105: 5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M.C.S., Petersen M., Mundy J. (2010). Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 61: 621–649. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vallet A., Ramos B., Bednarek P., López G., Piślewska-Bednarek M., Schulze-Lefert P., Molina A. (2010). Tryptophan-derived secondary metabolites in Arabidopsis thaliana confer non-host resistance to necrotrophic Plectosphaerella cucumerina fungi. Plant J. 63: 115–127. [DOI] [PubMed] [Google Scholar]
- Schenke D., Böttcher C., Scheel D. (2011). Crosstalk between abiotic ultraviolet-B stress and biotic (flg22) stress signalling in Arabidopsis prevents flavonol accumulation in favor of pathogen defence compound production. Plant Cell Environ. 34: 1849–1864. [DOI] [PubMed] [Google Scholar]
- Schlaeppi K., Mauch F. (2010). Indolic secondary metabolites protect Arabidopsis from the oomycete pathogen Phytophthora brassicae. Plant Signal. Behav. 5: 1099–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaeppi K., Abou-Mansour E., Buchala A., Mauch F. (2010). Disease resistance of Arabidopsis to Phytophthora brassicae is established by the sequential action of indole glucosinolates and camalexin. Plant J. 62: 840–851. [DOI] [PubMed] [Google Scholar]
- Schweizer F., Fernández-Calvo P., Zander M., Diez-Diaz M., Fonseca S., Glauser G., Lewsey M.G., Ecker J.R., Solano R., Reymond P. (2013). Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 25: 3117–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S., Mitsuhara I., Feng J., Iwai T., Hasegawa M., Ohashi Y. (2011). Cyanide, a coproduct of plant hormone ethylene biosynthesis, contributes to the resistance of rice to blast fungus. Plant Physiol. 155: 502–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sønderby I.E., Geu-Flores F., Halkier B.A. (2010a). Biosynthesis of glucosinolates--gene discovery and beyond. Trends Plant Sci. 15: 283–290. [DOI] [PubMed] [Google Scholar]
- Sønderby I.E., Burow M., Rowe H.C., Kliebenstein D.J., Halkier B.A. (2010b). A complex interplay of three R2R3 MYB transcription factors determines the profile of aliphatic glucosinolates in Arabidopsis. Plant Physiol. 153: 348–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sønderby I.E., Hansen B.G., Bjarnholt N., Ticconi C., Halkier B.A., Kliebenstein D.J. (2007). A systems biology approach identifies a R2R3 MYB gene subfamily with distinct and overlapping functions in regulation of aliphatic glucosinolates. PLoS One 2: e1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz H.U., Sawada Y., Shimada Y., Hirai M.Y., Sasaki E., Krischke M., Brown P.D., Saito K., Kamiya Y. (2011). Role of camalexin, indole glucosinolates, and side chain modification of glucosinolate-derived isothiocyanates in defense of Arabidopsis against Sclerotinia sclerotiorum. Plant J. 67: 81–93. [DOI] [PubMed] [Google Scholar]
- Suarez-Rodriguez M.C., Adams-Phillips L., Liu Y., Wang H., Su S.-H., Jester P.J., Zhang S., Bent A.F., Krysan P.J. (2007). MEKK1 is required for flg22-induced MPK4 activation in Arabidopsis plants. Plant Physiol. 143: 661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tena G., Boudsocq M., Sheen J. (2011). Protein kinase signaling networks in plant innate immunity. Curr. Opin. Plant Biol. 14: 519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchisaka A., Yu G., Jin H., Alonso J.M., Ecker J.R., Zhang X., Gao S., Theologis A. (2009). A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics 183: 979–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K., Sato M., Stoddard T., Glazebrook J., Katagiri F. (2009). Network properties of robust immunity in plants. PLoS Genet. 5: e1000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Ngwenyama N., Liu Y., Walker J.C., Zhang S. (2007). Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 19: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittstock U., Burow M. (2010). Glucosinolate breakdown in Arabidopsis: mechanism, regulation and biological significance. The Arabidopsis Book 8: e0134, doi/10.1199/tab.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhang S. (2015). Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci. 20: 56–64. [DOI] [PubMed] [Google Scholar]
- Xu J., Xie J., Yan C., Zou X., Ren D., Zhang S. (2014). A chemical genetic approach demonstrates that MPK3/MPK6 activation and NADPH oxidase-mediated oxidative burst are two independent signaling events in plant immunity. Plant J. 77: 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K.-Y., Liu Y., Zhang S. (2001). Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc. Natl. Acad. Sci. USA 98: 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip W.-K., Yang S.F. (1988). Cyanide metabolism in relation to ethylene production in plant tissues. Plant Physiol. 88: 473–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Cho C.-G., Posner G.H., Talalay P. (1992). Spectroscopic quantitation of organic isothiocyanates by cyclocondensation with vicinal dithiols. Anal. Biochem. 205: 100–107. [DOI] [PubMed] [Google Scholar]
- Zhao J., Williams C.C., Last R.L. (1998). Induction of Arabidopsis tryptophan pathway enzymes and camalexin by amino acid starvation, oxidative stress, and an abiotic elicitor. Plant Cell 10: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N., Tootle T.L., Glazebrook J. (1999). Arabidopsis PAD3, a gene required for camalexin biosynthesis, encodes a putative cytochrome P450 monooxygenase. Plant Cell 11: 2419–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.