Abstract
Cytochrome P450's (CYP's) constitute a diverse group of over 500 monooxygenase hemoproteins, catalyzing transformations that involve xenobiotic metabolism, steroidogenesis and other metabolic processes. Over-production of the steroid hormone cortisol is implicated in the progression of diseases such as diabetes, heart failure and hypertension, stroke, Cushing's syndrome, obesity and renal failure, among others. The biosynthesis of cortisol involves a cascade of cholesterol metabolizing reactions regulated through three major CYP proteins: 17α–hydroxylase-C17/20-lyase (CYP17), 21-hydroxylase (CYP21), and 11β-hydroxylase (CYP11B1). Excess activities of these enzymes are linked to the progression of malignancies including prostate, breast, ovarian, and uterine cancers. A series of novel functionalized dioxane analogs have been developed and recently patented as CYP17, CYP21, and CYP11B1 inhibitors, which lead to the modulation of cortisol production as a method for treating, delaying, slowing, and inhibiting the implicated diseases. The findings disclosed in this patent have been analyzed and compared with the literature data on inhibitors of CYP17, CYP21, and CYP11B1. The compiled data provide insight into the novel functionality of the compounds described in the patent. In this regard, an objective opinion on the effectiveness and novel biochemistry of these compounds in comparison to current CYP inhibitors used in the treatment of cortisol-related diseases is presented in this paper.
Keywords: cortisol, CYP11, CYP17, CYP21, cytochrome P450, P450 inhibition
1. Introduction
Cortisol is a glucocorticoid class steroid hormone that plays an important role in the regulation of carbohydrate, protein and lipid metabolism.[1] It is also known to assist in regulating blood pressure and cardiovascular maintenance, as well as inducing insulin resistance.[2,3] In humans, cortisol is biosynthesized through the androgen- and progestragen-catalyzed reactions during the metabolism of cholesterol. Cortisol dysregulation has been associated with diseases such as diabetes, heart failure, hypertension, stroke, obesity, renal failure, prostate cancer, breast cancer, uterine cancer and ovarian cancer, among others.[4–7]
In some cases, an excess amount of circulating cortisol is produced. This condition, sometimes classified as Cushing's syndrome, is known as hypercortisolemia. This excess production of cortisol may be due to a number of reasons including abnormalities due to hypothalamic, pituitary or adrenal axis impairment, or from the use of corticosteroid medications.[8] Another common disorder linked to the overproduction of cortisol is metabolic syndrome.[9] Also known as syndrome X or insulin-resistance syndrome, this condition involves abnormal energy storage and utilization issues, and is diagnosed by having a combination of the following risk factors: abdominal obesity, high blood pressure, elevated fasting plasma glucose, high serum triglycerides and low high-density lipoprotein (HDL) levels.[10]
Cortisol production is primarily regulated through cytochrome P450 (CYP) enzymes. These enzymes comprise a superfamily responsible for catalyzing numerous reactions including xenobiotic metabolism, hormone synthesis/break-down, bile acid synthesis, fatty acid metabolism and other important metabolic regulations.[11] The active site of CYP enzymes features a heme prosthetic group that uses the electron transport properties of NAD(P)H and molecular oxygen to generate important metabolites in the excretion and signaling pathways of exogenous and endogenous compounds.[12] Although the steroidogenic reactions use the same electron transport and oxidative functionality as xenobiotic metabolism, these reactions produce the progestragens and androgens, which are important hormones in metabolic and signaling pathways. These enzymes are prominently found in the inner mitochondrial membrane and endoplasmic reticulum of liver and adrenal cells as well as gonadal and surrounding tissue.[13]
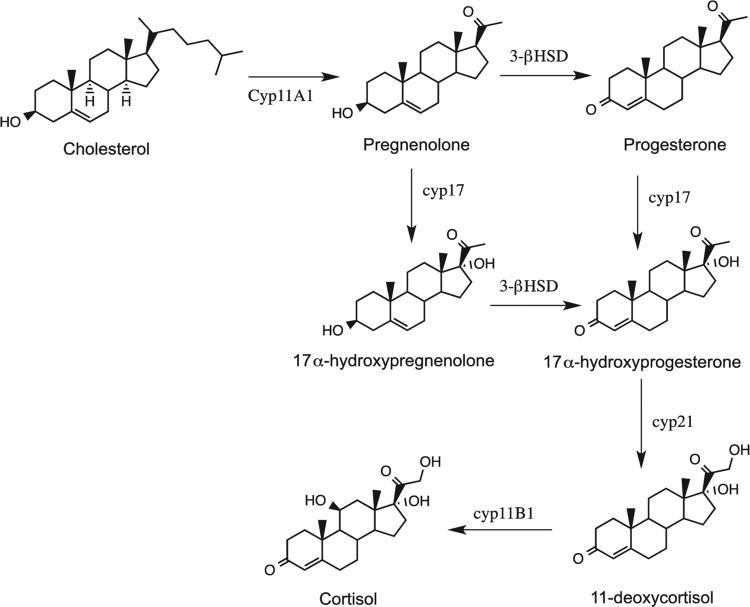
While steroid production occurs primarily in the adrenal cortex and gonads, cortisol production is localized mainly in the zona fasiculata region of the adrenal cortex. The production of cortisol is regulated through three major CYP proteins, 17α−hydroxylase-C-17/20-lyase (CYP17), 21-hydroxylase (CYP21) and 11β-hydroxylase (CYP11B1) in the zona glomurulosa and zona fasiculata (Figure 1).[14] The bifunctional CYP17 enzyme contains both C-17 hydroxylation and C-17/C-20 lyase functionality. The latter of the functions is responsible for the production of androgenic compounds and does not play a role in the production of cortisol.
Figure 1.
Cholesterol metabolizing reactions in the cortisol biosynthesis pathway.
The patent WO2015048311 describes the design, syntheses and formulations of the 5-phenoxymethyl-1,3-dioxane analogs.[15] The in vitro inhibition of CYP17, CYP21 and CYP11B1 is also described in this patent. The in vitro goal of this class of compounds is an IC50 value of <100 nM for CYP17, CYP21 and CYP11B1, with lower potency for off-target CYP19 and CYP3A4. The effect of these analogs on the liver was also estimated by analyzing the inhibition of bile acid synthesis followed by pharmacokinetic studies in the guinea pig.
2. Cortisol production
In the biosynthesis of cortisol, pregnenolone and progesterone are both hydroxylated at the C-17 position by CYP17 (hydroxylase) activity in the zona fasiculata producing 17α-hydroxypregnenolone and 17α-hydroxyprogesterone, respectively. Additionally, pregnenolone can be converted to progesterone through the non-P450-catalyzed oxido-reductase 3β-hydroxysteroid dehydrogenase (3β-HSD) enzyme. This enzyme also catalyzes the conversion of 17α-hydroxypregnenolone to 17α-hydroxyprogesterone, which is then hydroxylated to 11-deoxycortisol at the C-21 position by CYP21 activity. The last step in the biosynthesis of cortisol involves additional hydroxylation at the C-11 position, which is catalyzed by CYP11B1.
3. Cortisol inhibitors
Compounds shown to inhibit enzymatic activities of CYP17, CYP21 and CYP11B1 lead to a decreased amount of cortisol production and provide the most effective strategy in treating diseases caused by cortisol overproduction.[16] There are many CYP17 and selected CYP11 inhibitors, but the literature on CYP21 inhibitors is not as prevalent. Since many CYP inhibitors have been developed, the most widely known and used inhibitors are briefly reviewed below.
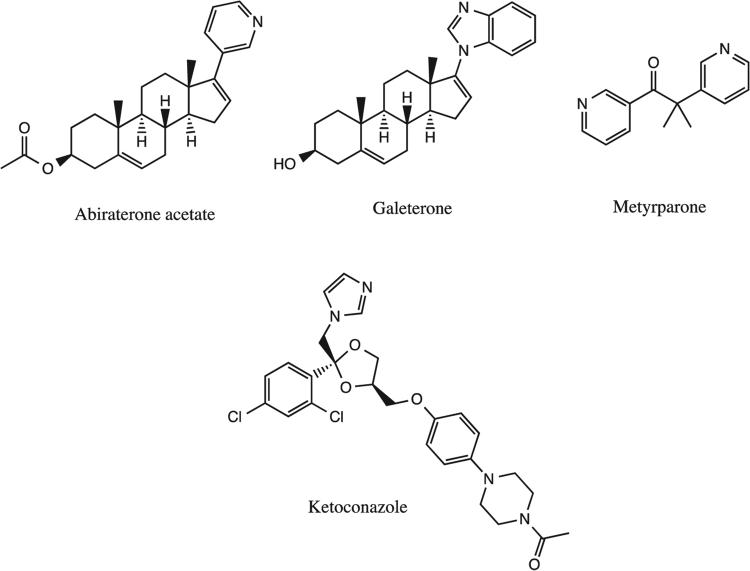
3.1. Abiraterone acetate (CB 7630)
Abiraterone acetate is a potent CYP17 inhibitor derived from naturally occurring endogenous substrates (Figure 2).[17] Because of its poor bioavailability, the acetylated pro-drug was developed and found to inhibit enzymatic activities of both CYP17 and CYP11, leading to noted antitumoral effects.[18] Abiraterone acetate irreversibly binds to the iron heme complex through N-pyridyl binding at the C-23 position, causing a decreased production of cortisol and upregulation of adrenocorticotropic hormone production (ACTH). [19,20] Abiraterone acetate has also demonstrated increased survival rates in patients with metastatic castration-resistant prostate cancer (mCRPC) and is currently undergoing Phase II trials.[21]
Figure 2.
Widely known CYP inhibitors known to decrease cortisol production.
3.2. Galeterone (VN/124-1,TOK-001)
Galeterone is an azolyl steroid inhibitor shown to inhibit CYP17 hydroxylase activity with increased 17,20 lyase selectivity.[22] It was also shown to competitively inhibit androgen receptor activity.[23] While galeterone exhibited decreased cortisol suppression efficacy compared to other drugs, its anti-tumoral effects were significant in CYP17 overexpressed malignancies.[23] Contrary to other CYP17 inhibitors, patients given galeterone show no signs of hepatotoxicity, which has aided galeterone in entering Phase III trials.[24]
3.3. Ketoconazole
Ketoconazole is a broad-spectrum imidazole antifungal drug that inhibits steroidogensesis through CYP17 hydroxylase and CYP11A1 cholesterol side chain cleavage inhibition. [25,26] High doses have shown to exhibit selective CYP17 hydroxylase inhibition.[27] In patients with androgen-independent diseases such as metastatic prostatic carcinoma, ketoconazole exhibited response rates in the range of 11 – 14%.[28] However, alternatives to ketoconazole have been investigated due to the development of resistances and adverse side effects such as hepatotoxicity, as well as neurological and respiratory associated complications.[26,29]
3.4. Metyrapone
Metyrapone is a pyridyl derivative that suppresses steroidogenesis through selective inhibition of CYP11B activity, causing a reduction of both cortisol and aldosterone.[30,31] Although frequently used for the treatment of Cushing's disease, inhibition using metyrapone is reversible and induces a negative feedback response due to low levels of cortisol.[31] This response drives the production of ACTH and allows for continued steroidogenic function.[32] It has been suggested for use as a combinatorial inhibitor in conjunction with other drugs such as ketoconazole and mitotane to increase efficacy and limit associated toxicities.[33] Adverse effects are mild, but in rare cases hypokalemia may occur.[34]
4. 5-(phenoxymethy)-1,3-dioxane analogs described in the patent WO2015048311
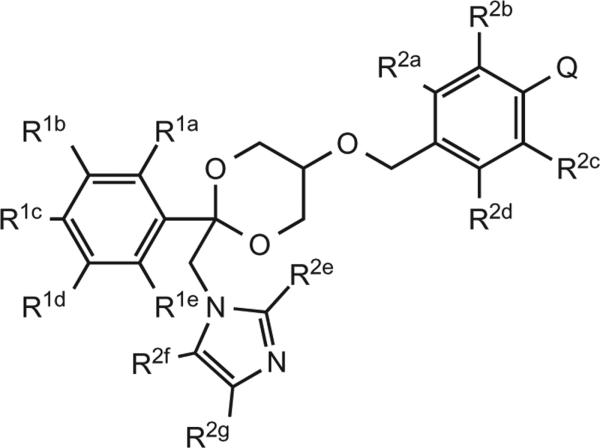
The patented compounds are novel dioxane inhibitors showing enzymatic inhibition of CYP17, CYP11 and CYP21 (Figure 3).[15] Target compounds were considered highly active if they met the in vitro target potency activity of IC50 < 100 nM (CYP17, CYP11 and CYP21), as well as decreased selectivity for specified off-target enzymatic activity and bile acid synthesis inhibition. By selecting these off-target enzymes, it was estimated that no potential harmful liver effects would occur in vivo as a result.
Figure 3.
Novel dioxane analogs claimed in patent.
Of the compounds tested in vitro, 13 showed CYP17 IC50 values < 100 nM, 11 showed CYP11 IC50 values < 100 nM and only 1 met the targeted potency activity for CYP21, exhibiting an IC50 value < 100 nM (Table 1).
Table 1.
Compounds meeting at least one of the targeted potency criteria (IC50 < 100 nM).
| Compound | Structure | IC50 (nM) |
||
|---|---|---|---|---|
| CYP17 | CYP11 | CYP21 | ||
| COR-500012 |
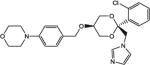
|
12 | 100 | 1520 |
| COR-510013 |
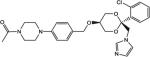
|
27 | 70 | 800 |
| COR-510014 |
|
20 | 140 | 570 |
| COR-510017 |
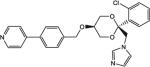
|
42 | 440 | 391 |
| COR-510018 |
|
40 | 107 | 2800 |
| COR-500015 |
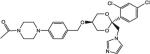
|
8 | 12 | 208 |
| COR-500016 |
|
75 | 37 | 415 |
| COR-500017 |
|
106 | 27 | 1100 |
| COR-500018 |
|
11 | 6 | 262 |
| COR-500019 |
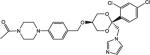
|
1100 | 12 | 10,000 |
| COR-500020 |
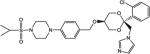
|
11 | 25 | 110 |
| COR-500021 |
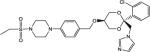
|
9 | 200 | 130 |
| COR-500024 |
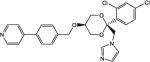
|
33 | 23 | 68 |
| COR-500025 |
|
13 | 12 | 273 |
| COR-500022 |
|
101 | 13 | 170 |
| COR-500023 |
|
67 | 14 | 426 |
4.1. In vivo pharmacokinetic studies
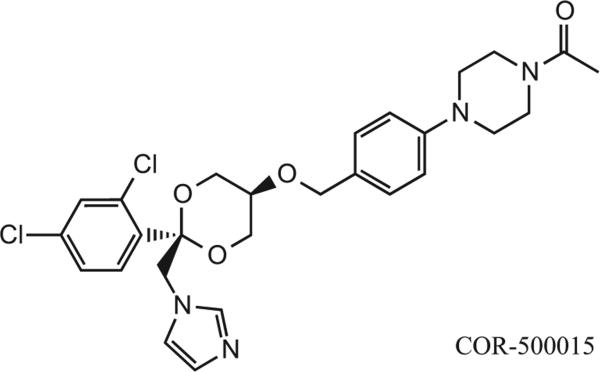
Over 200 compounds were initially screened for in vitro inhibitory activity. Thirteen compounds showed an inhibition potency of <100 nM for CYP17. The patent describes the achieved target goals for the representative compound COR-500015, the most potent inhibitor (Figure 4). COR-500015 showed high enzymatic activity in CYP17 (IC50 = 8 nM) and CYP11 (IC50 = 12 nM), with moderate activity in CYP21 (IC50 = 208 nM) (Table 2). This compound was chosen as the lead compound for in vivo studies where pharmacokinetic evaluations were performed using the guinea pig with a 1 mg/kg IV dosing (20% dimethacrylate (DMA), 40% triethylene glycol (TEG), 40% water) and 10 mg/kg oral dosing (2% Tween-80, 97% hydroxypropyl methylcellulose, 1% water). Maximum drug serum concentration was 1018 ng/ml (Cmax) at 3.0 h (Tmax), and the half-life was determined at 6.0 h (t1/2). Total drug exposure over time was 14,891 ng.h/ml (AUC0-inf).
Figure 4.
Compound COR-500015 used for in vivo studies.
Table 2.
Targeted inhibitor potency and profile goals observed for the selected representative compound, COR-500015, for in vivo trials.
| Target activity | Activity of selected compound | |
|---|---|---|
| CYP17 | IC50 < 100 nM | IC50 = 8 nM |
| CYP11B1 | IC50 < 100 nM | IC50 =12 nM |
| CYP21 | IC50 < 100 nM | IC50 = 208 nM |
| Off-target counter screens | ||
| CYP19 | 50× CYP17 | IC50 = 350 nM |
| Bile Acid (CYP7A surrogate) | 100× CYP17 | IC50 = 880 nM |
| CYP450 34A > 50% @ 1 mM | IC50 (nM) | IC50 = 109 nM |
| CYP450 2D6 > 50% @ 1 mM | >50% @ 1 μM | Data not given |
| CYP450 2C9 > 50% @ 1 mM | >50% @ 1 μM | Data not given |
| Pharmaceutical profiling/ADME | ||
| Solubility >5 μM | μMolar | Data not given |
| Molecular Weight | <500 AMU | 545 AMU |
| ClogP | <5 | 4.2 (logP) |
| TPSA | <140 | 69 |
| Cytotoxicity in HerG2 | 10% @1 μM | Data not given |
| Caco-2-permeability (A-B, B-A) | >20 (10–6 cm/s) | Data not given |
| Guinea pig liver microsomal stability | >30 min | Data not given |
| Plasma stability | >30 min | Data not given |
| Plasma protein binding | >30 min | Data not given |
| Guinea pig bioavailability | >25% | Data not given |
| Guinea pig t1/2 | <4 h | 6 h |
5. Conclusion
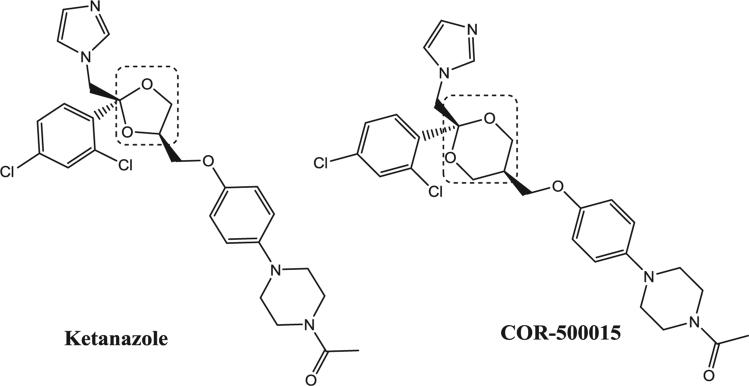
The most potent inhibitors contain a 2,4-dichlorophenyl group in the C-2 position. Some less active inhibitors, which contain a 2-chlorophenyl group in the C-2 position, showed moderate activity in CYP17, but much higher, or even no activity in some cases in CYP11 and CYP21. A few of the compounds showed some selectivity toward CYP17 or CYP11, but very few compounds exhibited high inhibition potency for CYP21. It is important to note the structural similarities between ketoconazole and the lead compound used for in vivo studies. The compounds share the same scaffold and stereochemical features with the exception of dioxane (patent compound) in place of the dioxolane (ketoconazole) and an oxygen substitution on the adjacent carbon position, shown in box (Figure 5). The most remarkable feature of these compounds is that the stereocenter at C-2 of the dioxane ring seems to have enormous impact on the potency of these compounds against CYP17, CYP11B and CYP21. For example, COR-500015 and COR-500019 are enatiomers. While COR-500015 has an inhibition profile of 8, 12 and 208 nM for CYP17, CYP11B and CYP21, COR-500019 exhibits a profile of 10,000, 5800 and 10,000 nM for the same enzymes. Glucocorticoids are locally synthesized by enzymes such as CYP17 (androgens, estrogens and cortisone), CYP21 (11-deoxycorticosterone) and CYP11B (corticosterone and aldosterone) and these regulate metabolic activity and immune function with high spatial specificity. Inhibition of CYP17 alone can lead to elevated levels of corticosterone. Since COR-500015 is an equipotent inhibitor of CYP11B and CYP17 and a less potent inhibitor CYP21, it will also inhibit the synthesis of corticosterone. Inhibition of all three enzymes (CYP17, CYP21 and CYP11B) will potentially result in elevated levels of pregnolone. This can lead to lower levels of the glucocorticoids and an accumulation of pregnolone which can lead to other side effects as pregnolone interacts with progestin, testosterone and estrogen.
Figure 5.
Structural differences (shown in box) between COR-500015 and ketoconazole.
6. Expert opinion
Abiraterone acetate is a more potent inhibitor of CYP17 than ketoconazole. When the effectiveness of these agents was compared in docetaxel refractory, metastatic castrate-resistant prostate cancer (mCRPC), abiraterone treatment was found to be much more effective.[35,36] Ketoconazole also inhibits CYP3A and CYP24A1, while abiraterone is a highly selective inhibitor of CYP17. This may reduce the many adverse effects associated with ketoconazole treatment. The illustrated compound COR-500015 in the patent WO2015048311 is inhibiting CYP3A4 with equal potency and CYP19 with slightly lower potency. This might cause these agents to have similar adverse side effects as ketoconazole. There are three isoenzymes of CYP11: (1) CYP11A1 which converts cholesterol to pregnenolone, is the first and rate-limiting enzyme in all steroid biosynthesis and its deficiency leads to congenital adrenal hyperplasia (CAH); (2) CYP11B1 which is the enzyme that makes cortisol from 11-deoxycortisol, and its deficiency leads to CAH and hypertension; and (3) CYP11B2 which is a aldosterone synthase and hydroxylates corticosterone is known to have angiogenic effects. The CYP11B2 (−344 C) allele in the promoter region greatly increases the risk of nonfatal myocardial infarction in those with smoking and dyslipidemia. The present patent describes the biological effect of the inhibition of CYP11B1. While describing the CYP11 inhibition assays, the isozyme of CYP11 analyzed is not specified. If the authors have used a mixture of CYP11 isozymes, then the side effects of inhibiting the other isozymes of CYP11 need to be considered. These compounds have a huge therapeutic potential for treating diseases associated with overproduction of cortisol given their high inhibition potency for CYP17, CYP11 and CYP21. These compounds are capable of exhibiting therapeutic potential that is comparable and possibly better than the existing drug candidates such as ketoconazole, metyrapone and abiraterone to treat such disease conditions.
Article highlights.
● The biosynthesis of cortisol involves several steps regulated by many enzymes including the three major CYP proteins, 17α–hydroxylase-C17/20-lyase (CYP17), 21-hydroxylase (CYP21) and 11β-hydroxylase (CYP11B1).
● Cortisol dysregulation has been associated with diseases such as diabetes, heart failure, hypertension, stroke, obesity, renal failure, prostate cancer, breast cancer, uterine cancer, ovarian cancer and so on.
● The patent evaluated in this article WO2015048311 describes the development of ~200 analogs of 5-(phenoxymethyl)-1,3-dioxane that inhibit the CYP proteins CYP17, CYP21 and CYP11B1 with IC50 values of <100 nM.
● Of the compounds tested in vitro, 13 showed CYP17 IC50 values < 100 nM, 11 showed CYP11 IC50 values < 100 nM and only 1 met the targeted potency activity for CYP21, exhibiting an IC50 value <100 nM.
● Pharmacokinetic studies were performed in vivo using the guinea pig and COR-500015 satisfied all required criteria.
This box summarizes key points contained in the article.
Footnotes
Declaration of interest
R Schroeder, J Sridhar, J Liu and M Foroozesh are supported by the Louisiana Cancer Research Consortium. R Schroeder and J Sridhar are supported by the National Institute on Minority Health and Health Disparities under their Research Centers in Minority Institutions Program, grant number 2G12MD007595. P Tram is supported by the Center for Undergraduate Research at Xavier University of Louisiana in the form of stipend. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the Louisiana Cancer Research Consortium, or the National Institutes of Health (NIH). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as either of interest
(•) or of considerable interest
(••) to readers.
- 1.Christiansen JJ, Djurhuus CB, Gravholt CH, et al. Effects of cortisol on carbohydrate, lipid, and protein metabolism: studies of acute cortisol withdrawal in adrenocortical failure. J Clin Endocrinol Metab. 2007;92(9):3553–3559. doi: 10.1210/jc.2007-0445. [DOI] [PubMed] [Google Scholar]
- 2.Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor defect of insulin action. J Clin Endocrinol Metab. 1982;54(1):131–138. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- 3.Fraser R, Ingram MC, Anderson NH, et al. Cortisol effects on body mass, blood pressure, and cholesterol in the general population. Hypertens. 1999;33(6):1364–1368. doi: 10.1161/01.hyp.33.6.1364. [DOI] [PubMed] [Google Scholar]
- 4•.Connolly CK, Wills MR. Plasma cortisol levels in heart failure. Br Med J. 1967;2(5543):25–26. doi: 10.1136/bmj.2.5543.25. [One of the initial reports linking cortisol secretion rate to Cushing's syndrome, and congestive cardiac failure.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Chiodini I, Adda G, Scillitani A, et al. Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care. 2007;30(1):83–88. doi: 10.2337/dc06-1267. [Demonstrates the direct association between cortisol levels, insulin resistance and complications in type 2 diabetic subjects. Hypothalmic-pituitary-adrenal (HPA) activity was shown to be enhanced only in diabetic patients with chronic complications.] [DOI] [PubMed] [Google Scholar]
- 6•.Barugh A, Gray P, Shenkin S, et al. Cortisol levels and the severity and outcomes of acute stroke: a systematic review. J Neurol. 2014;261(3):533–545. doi: 10.1007/s00415-013-7231-5. [This review gives an extensive overview of the patient studies that link high cortisol levels after stroke that is associated with dependency, morbidity and mortality.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Mitrunen K, Jourenkova N, Kataja V, et al. Steroid metabolism gene CYP17 polymorphism and the development of breast cancer. Cancer Epidemiol Biomarkers Prev. 2000;9(12):1343–1348. [Analyzed the role of the A2 variant of CYP17 with incidence of advanced breast cancer. The study was conducted in 483 homogenous Finnish origin breast cancer patients and compared to earlier studies.] [PubMed] [Google Scholar]
- 8.Boscaro M, Barzon L, Sonino N. The diagnosis of Cushing's syndrome: atypical presentations and laboratory shortcomings. Arch Intern Med. 2000;160(20):3045–3053. doi: 10.1001/archinte.160.20.3045. [DOI] [PubMed] [Google Scholar]
- 9•.Walker BR. Cortisol—cause and cure for metabolic syndrome? Diabet Med. 2006;23(12):1281–1288. doi: 10.1111/j.1464-5491.2006.01998.x. [This review takes an in-depth look into the role of cortisol in the pathophysiology of metabolic syndrome and Cushing's disease suggesting that reducing cortisol levels will positively influence the outcome of type 2 diabetes and cardiovascular disease.] [DOI] [PubMed] [Google Scholar]
- 10.Hjemdahl P. Stress and the metabolic syndrome: an interesting but enigmatic association. Circ. 2002;106(21):2634–2636. doi: 10.1161/01.cir.0000041502.43564.79. [DOI] [PubMed] [Google Scholar]
- 11.Lorbek G, Lewinska M, Rozman D. Cytochrome P450s in the synthesis of cholesterol and bile acids—from mouse models to human diseases. FEBS J. 2012;279(9):1516–1533. doi: 10.1111/j.1742-4658.2011.08432.x. [DOI] [PubMed] [Google Scholar]
- 12.Ortiz de Montellano PR, Lyubimov AV. Structure and function of cytochrome P450 enzymes. In: Lyubimov AV, editor. Encyclopedia of drug metabolism and interactions. John Wiley & Sons Inc; Hoboken (NJ): 2011. [Google Scholar]
- 13.Seliskar M, Rozman D. Mammalian cytochromes P450-importance of tissue specificity. Biochim Biophys Acta—Gen Subj. 2007;1770(3):458–466. doi: 10.1016/j.bbagen.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Akhtar MK, Kelly SL, Kaderbhai MA. Cytochrome b(5) modulation of 17 {alpha} hydroxylase and 17-20 lyase (CYP17) activities in steroidogenesis. J Endocrinol. 2005;187(2):267–274. doi: 10.1677/joe.1.06375. [DOI] [PubMed] [Google Scholar]
- 15.Cortendo AB. Novel functionalized 5-(phenoxymethyl)-1,3-dioxane analogs exhibiting cytochrome P450 inhibition. 2015 Apr 2; WO048311 A1.
- 16••.Hille UE, Zimmer C, Vock CA, et al. First selective CYP11B1 inhibitors for the treatment of cortisol-dependent diseases. ACS Med Chem Lett. 2011;2(1):2–6. doi: 10.1021/ml100071j. [Describes the development of chiral etomidate derivatives as CYP11B1 selective inhibitors for the treatment of cortisol-dependent diseases such as Cushing's syndrome and related disease.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Hartmann RW, Hector M, Wachall BG, et al. Synthesis and evaluation of 17-aliphatic heterocycle-substituted steroidal inhibitors of 17α-hydroxylase/C17−20-lyase (P450 17). J Med Chem. 2000;43(23):4437–4445. doi: 10.1021/jm991070n. [Outlines the synthesis of a series of steroidal inhibitors of CYP17 that decreased the plasma testosterone concentration in rats by 81 – 84% after 2h.] [DOI] [PubMed] [Google Scholar]
- 18.Yin L, Hu Q. CYP17 inhibitors-abiraterone, C17,20-lyase inhibitors and multi-targeting agents. Nat Rev Urol. 2014;11(1):32–42. doi: 10.1038/nrurol.2013.274. [DOI] [PubMed] [Google Scholar]
- 19.Rowlands MG, Barrie SE, Chan F, et al. Esters of 3-pyridylacetic acid that combine potent inhibition of 17.alpha.-hydroxylase/C17,20-lyase (cytochrome P45017.alpha.) with resistance to esterase hydrolysis. J Med Chem. 1995;38(21):4191–4197. doi: 10.1021/jm00021a008. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann RW, Hector M, Haidar S, et al. Synthesis and evaluation of novel steroidal oxime inhibitors of P450 17 (17α-hydro-xylase/C17−20-lyase) and 5α-reductase types 1 and 2. J Med Chem. 2000;43(22):4266–4277. doi: 10.1021/jm001008m. [DOI] [PubMed] [Google Scholar]
- 21.De Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22••.Stein MN, Patel N, Bershadskiy A, et al. Androgen synthesis inhibitors in the treatment of castration-resistant prostate cancer. Asian J Androl. 2014;16(3):387–400. doi: 10.4103/1008-682X.129133. [An exhaustive review on the development of abiraterone and other CYP17 inhibitors for treatment of metastatic prostate cancer. The clinical parameters of abiraterone administration and the results of post-translational studies are described.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasaitis T, Belosay A, Schayowitz A, et al. Androgen receptor inactivation contributes to antitumor efficacy of 17α-hydroxylase/ 17,20-lyase inhibitor 3β-hydroxy-17-(1H-benzimidazole-1-Yl)androsta-5,16-diene in prostate cancer. Mol Cancer Ther. 2008;7(8):2348–2357. doi: 10.1158/1535-7163.MCT-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta E, Guthrie T, Tan W. Changing paradigms in management of metastatic castration resistant prostate cancer (mCRPC). BMC Urol. 2014;14(1):55. doi: 10.1186/1471-2490-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Ang JE, Olmos D, de Bono JS. CYP17 blockade by abiraterone: further evidence for frequent continued hormone-dependence in castration-resistant prostate cancer. Br J Cancer. 2009;100(5):671–675. doi: 10.1038/sj.bjc.6604904. [The efficacy of abiraterone acetate in the treatment of castration-resistant prostate cancer is evaluated. The studies have shown successful reversal of resistance to abiraterone acetate using low-dose dexamethasone in some patients.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loose DS, Kan PB, Hirst MA, et al. Ketoconazole blocks adrenal steroidogenesis by inhibiting cytochrome P450-dependent enzymes. J Clin Invest. 1983;71(5):1495–1499. doi: 10.1172/JCI110903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Coster R, Caers I, Coene M-C, et al. Effects of high dose ketoconazole therapy on the main plasma testicular and adrenal steroids in previously untreated prostatic cancer patients. Clin Endocrinol (Oxf) 1986;24(6):657–664. doi: 10.1111/j.1365-2265.1986.tb01662.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnson DE, Babaian RJ, Von Eschenbach AC, et al. Ketoconazole therapy for hormonally refractive meta-static prostate cancer. Urology. 2015;31(2):132–134. doi: 10.1016/0090-4295(88)90036-2. [DOI] [PubMed] [Google Scholar]
- 29.Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a Phase III trial (CALGB 9583). J Clin Oncol. 2004;22(6):1025–1033. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 30••.Thorén M, Adamson U, Sjöberg HE. Aminoglutethimide and metyrapone in the management of Cushing's syndrome. Acta Endocrinol. 1985;109(4):451–457. doi: 10.1530/acta.0.1090451. [Treatment of patients with endogenous Cushing's syndrome with metyrapone and/or aminoglutethimide is reported. The report shows marked clinical improvement for a majority of the patients.] [DOI] [PubMed] [Google Scholar]
- 31.Coppage WS, Island D, Smith M, et al. Inhibition of aldosterone secretion and modification of electrolyte excretion in man by a chemical inhibitor of 11β-hydroxylation. J Clin Invest. 1959;38(12):2101–2110. doi: 10.1172/JCI103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strott CA, West CD, Nakagawa K, et al. Plasma 11-deoxycorticosteroid and ACTH response to metyrapone (plasma metyrapone test). J Clin Endocrinol Metab. 1969;29(1):6–11. doi: 10.1210/jcem-29-1-6. [DOI] [PubMed] [Google Scholar]
- 33.Kamenický P, Droumaguet C, Salenave S, et al. Mitotane, metyrapone, and ketoconazole combination therapy as an alternative to rescue adrenalectomy for severe ACTH-dependent Cushing's syndrome. J Clin Endocrinol Metab. 2011;96(9):2796–2804. doi: 10.1210/jc.2011-0536. [DOI] [PubMed] [Google Scholar]
- 34.Verhelst JA, Trainer PJ, Howlett TA, et al. Short and long-term responses to metyrapone in the medical management of 91 patients with Cushing's syndrome. Clin Endocrinol (Oxf) 1991;35(2):169–178. doi: 10.1111/j.1365-2265.1991.tb03517.x. [DOI] [PubMed] [Google Scholar]
- 35.Peer A, Gottfried M, Sinibaldi V, et al. Comparison of abiraterone acetate versus ketoconazole in patients with metastatic castration resistant prostate cancer refractory to docetaxel. Prostate. 2014;74(4):433–440. doi: 10.1002/pros.22765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vasaitis TS, Bruno RD, Njar VCO. CYP17 inhibitors for prostrate cancer therapy. J Steroid Mol Biol. 2011;125(1–2):23–31. doi: 10.1016/j.jsbmb.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]