Abstract
Hepatitis C is a major global health burden and Egypt has the highest prevalence of hepatitis C virus (HCV) worldwide. The current study was designed to evaluate the beneficial therapeutic effects of ethanolic extracts of Nigella sativa, Zingiber officinale and their mixture in Egyptian HCV patients. Sixty volunteer patients with proven HCV and fifteen age matched healthy subjects were included in this study. Exclusion criteria included patients on interferon alpha (IFN-α) therapy, infection with hepatitis B virus, drug-induced liver diseases, advanced cirrhosis, hepatocellular carcinoma (HCC) or other malignancies, blood picture abnormalities and major severe illness. Liver function enzymes, albumin, total bilirubin, prothrombin time and concentration, international normalized ratio, alpha fetoprotein and viral load were all assessed at baseline and at the end of the study. Ethanolic extracts of Nigella sativa and Zingiber officinale were prepared and formulated into gelatinous capsules, each containing 500 mg of Nigella sativa and/or Zingiber officinale. Clinical response and incidence of adverse drug reactions were assessed initially, periodically, and at the end of the study. Both extracts as well as their mixture significantly ameliorated the altered viral load, alpha fetoprotein, liver function parameters; with more potent effect for the combined therapy. In conclusion, administration of Nigella sativa and/or Zingiber officinale ethanolic extracts to HCV patients exhibited potential therapeutic benefits via decreasing viral load and alleviating the altered liver function, with more potent effect offered by the mixture.
Keywords: Hepatitis C, Nigella sativa, Zingiber officinale, viral load
Introduction
Hepatitis C virus (HCV) infection often is conducive to chronic hepatitis due to the low viral clearance rate, leading to liver cirrhosis (LC) and subsequent progression to hepatocellular carcinoma (HCC) (Alter, 2007[7]; Lavanchy, 2011[44]). It has been shown that there is an alarming increase in the incidence of HCC in HCV patients in Egypt (Anwar et al., 2008[8]). According to the World Health Organization (WHO) there are 130-170 million people infected with the hepatitis C virus, corresponding to 2-2.5 % of the world's total population (WHO, 2013[80]). Egypt has the highest prevalence of HCV worldwide (15 %) and the highest prevalence of HCV-4 (67 %) with a predominance of subtype 4a (55 %) (Nguyen and Keeffe, 2005[59]; Elkady et al., 2009[22]; Khattab et al., 2011[41], WHO, 2013[80]).
The standard therapy, which is based on a combination of pegylated interferon alpha (IFN-α) and ribavirin (McHutchison et al., 1998[52]), results in highly variable outcomes, is very expensive and has severe side effects that are difficult to endure for the patients (Di Bisceglie and Hoofnagle, 2002[17]; Calland et al., 2012[13]). Therefore, the establishment of a new treatment modality without serious adverse effects is desirable (Vermehren and Sarrazin, 2011[76]).
Medicinal plants have been found to play a major role in managing human diseases. Historically, numerous important modern drugs have been developed from molecules originally isolated from natural sources (Lee, 2000[46]; Balunas and Kinghorn, 2005[9]). The search for new bioactive molecules in plants in key therapeutic areas such as immunosuppression, infectious diseases, cancer and metabolic disorders is still an active part of pharmaceutical research (Newman and Cragg, 2012[58]). About 40 new drugs launched on the market between 2000 and 2010, originate from terrestrial plants, terrestrial microorganisms, marine organisms, and terrestrial vertebrates and invertebrates (Brahmachari, 2011[12]).
Ginger (Zingiber officinale) is a well-known and widely used herb, which contains several interesting bioactive constituents and possesses health-promoting properties (Ajayi et al., 2013[1]). From its origin in Southeast Asia and its spread to Europe, it has a long history of use as herbal medicine to treat a variety of ailments including vomiting, pain, indigestion, and cold induced syndromes (White, 2007[79]). More recently, it was reported that ginger also possessed anti-cancer, anticlotting, anti-inflammatory, and analgesic activities (Chrubasik et al., 2005[15]; Ali et al., 2008[4]).
Nigella sativa (N. sativa) is used as a food condiment in the Middle East, and its seeds/oil have been shown to possess anti-inflammatory, antiviral and antineoplastic activity in various in vitro and in vivo studies (Zaher et al., 2008[81]). There are many reports of its biological activities including: immunopotentiation, antitumor, anti-inflammatory, analgesic, antihypertensive, antidiabetic, respiratory stimulation, antibacterial, antifungal, anticestode and antinematode effects (Ali and Blunden, 2003[3]; Al-Naggar et al., 2003[5]).
To date, studies addressed the use of ethanolic extracts of Z. officinale, N. sativa and their mixture in HCV patients are scanty. Hence, we sought to evaluate the efficacy, safety, and tolerability of Z. officinale and N. sativa extracts, individually and in combination, as an alternative therapy in the management of HCV patients.
Materials and Methods
This was a prospective, single-armed, self-controlled pilot study, conducted at the Hepatology Unit, Gastroentrology Department, Beni-Suef University Hospital, Beni-Suef University, Egypt.
Patients
Sixty volunteer patients with proven chronic HCV who were selected from admitted patients at the Hepatology Unit, Beni-Suef University Hospital, with fifteen age-matched healthy controls were included in this study.
Inclusion criteria included all patients diagnosed with HCV and negative for HBV. Exclusion criteria included patients on IFN-α therapy; infection with HBV or hepatitis immunodeficiency virus; drug-induced liver diseases; advanced cirrhosis; HCC or other malignancies; blood picture abnormalities such as anemia (hemoglobin concentration of 10 g/dl or less), leucopenia (white blood cells 1.500/µl or less) and thrombocytopenia (platelets count 80.000/µl or less); major severe illness such as renal failure, congestive heart failure, respiratory failure or autoimmune disease; or non-compliance to treatment. Informed consent was obtained from all patients, and the institutional ethical committee approved the study protocol, which conformed with the ethical guidelines of the 1975 Declaration of Helsinki.
Extract preparation
N. sativa seeds and Z. officinale rhizomes were purchased from the local herbalist in Egypt. The seeds and rhizomes were authenticated by a specialist of Plant Taxonomy in Botany Department, Faculty of Science, Beni-Suef University, Egypt. Both were cleaned, dried, mechanically powdered, extracted with 96 % ethanol and evaporated with rotary evaporator to render the extract alcohol free. The extracts were made into soft gelatin capsules, each containing 500 mg of N. sativa and/or Z. officinale extracts. The above procedures were undertaken in the Arab Company for Pharmaceuticals & Medicinal Plants (Mepaco-Medifood), Enshas EL Raml, Sharkeiya Factory, Sharkeiya, Egypt.
Treatment protocol
Included patients and controls were classified into five groups each comprising fifteen patients as follows; Group 1 served as healthy subjects; Group 2 (HCV) served as HCV control; Group 3 (HCV+N) received a capsule containing 500 mg N. sativa extract twice daily; Group 4 (HCV+G) received a capsule containing 500 mg Z. officinale extract twice daily and Group 5 (HCV+NG) received a capsule containing 500 mg of each extract twice daily. Patients were followed up weekly throughout the study period for assessing treatment adherence, tolerability and incidence of adverse reactions.
Clinical and laboratory assessment
All eligible patients and controls were subjected to the following at enrollment and after 1 month therapy: (1) Full clinical assessment with an emphasis on hepato- and/or splenomegaly, jaundice, palmar erythema, flapping tremors, spider naevi, lower-limb edema, and ascites; (2) Laboratory investigation of liver functions including aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were assayed according to the method of Schumann and Klauke (2003[67]) using reagent kit purchased from Noble Diagnostic (Egypt); lactate dehydrogenase (LDH) was determined according to the method of Teitz (1986[73]) using reagent kit purchased from Human (Germany); alkaline phosphatase (ALP) was assayed according to the International Federation of Clinical Chemistry (IFCC, 1980[32]) using BioSystem (Spain) commercial kit, gamma glutamyl transferase (γGT) assay was performed according to Persijn and van der Slik (1976[60]) using kits from Reactivos GPL (Spain); albumin was assayed according to the method of Webster (1974[78]) using kits of Diamond Diagnostic (Egypt); total bilirubin, was determined using reagent kit purchased from Diamond Diagnostic (Egypt), according to the method of Kaplan (1984[40]) and prothrombin time (PT), prothrombin concentration (PC) and international normalized ratio (INR) were determined according to the method of Wanger and Dati (1998[77]) using kits supplied by Siemens Healthcare Diagnostic (USA) and (3) Serum α-fetoprotein (AFP) was assayed according to Forest and Pugeat (1987[24]) using kits purchased from VIDAS bio Merieux (France) and quantitative real time polymerase chain reaction (qRT-PCR) for HCV using Roche Amplicor HCV monitor version 2.0 (Roche Diagnostics, Branchburg, NJ), with lower detection limit < 50 IU/ml.
Statistical analysis
The data were statistically analyzed using SPSS v.16. Results were expressed as Mean ± Standard error (SE) and all statistical comparisons were made by means of one-way ANOVA test followed by Duncan's multiple range test post hoc analysis. A P value less than 0.05 was considered significant.
Results
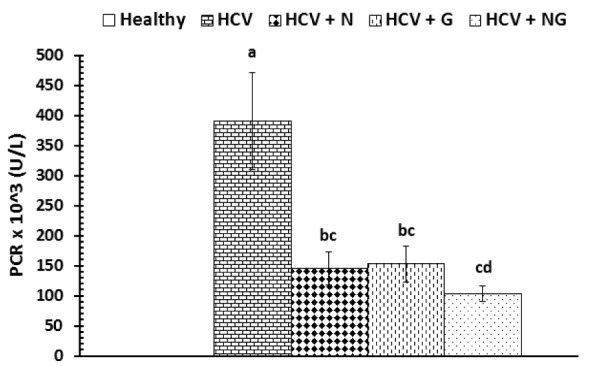
The effects of N. sativa, Z. officinale and their mixture on viral load of control and treated patients were depicted in Figure 1(Fig. 1). After one month treatment with N. sativa and/or Z. officinale ethanolic extracts, patients exhibited a significant (P<0.001) decrease in viral load as compared to HCV control group. The recorded data were 145.13 ± 26.93, 152.13 ± 29.73 and 102.93 ± 12.89 for HCV+N, HCV+G and HCV+NG groups, respectively. Treatment with the mixture of both extracts seemed to be the most effective in reducing viral load and there were five cases who showed a non-detectable viremia.
Figure 1. Viral load in control and treated patients. Data are expressed as mean ± SE, means which share the same superscript symbol(s) are not significantly different, P<0.001.
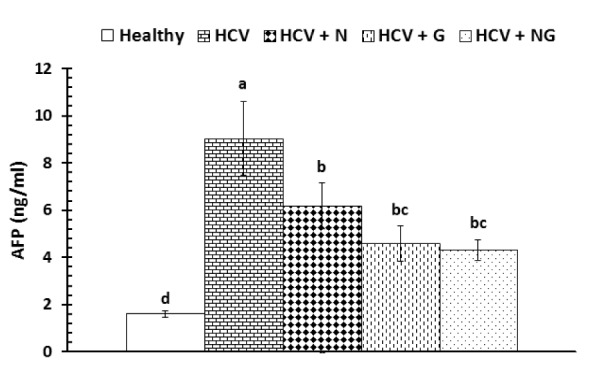
The serum concentrations of AFP were significantly (P<0.001) elevated in HCV control patients as compared to the corresponding healthy subjects (Figure 2(Fig. 2)). On the other hand, all treatments were very effective in alleviating the elevated AFP levels. However, while all used treatments have more or less similar effects, the used mixture seemed to be more effective in reducing serum AFP levels.
Figure 2. Serum AFP in healthy, HCV control and treated patients. Data are expressed as mean ± SE, means which share the same superscript symbol(s) are not significantly different, P<0.001.
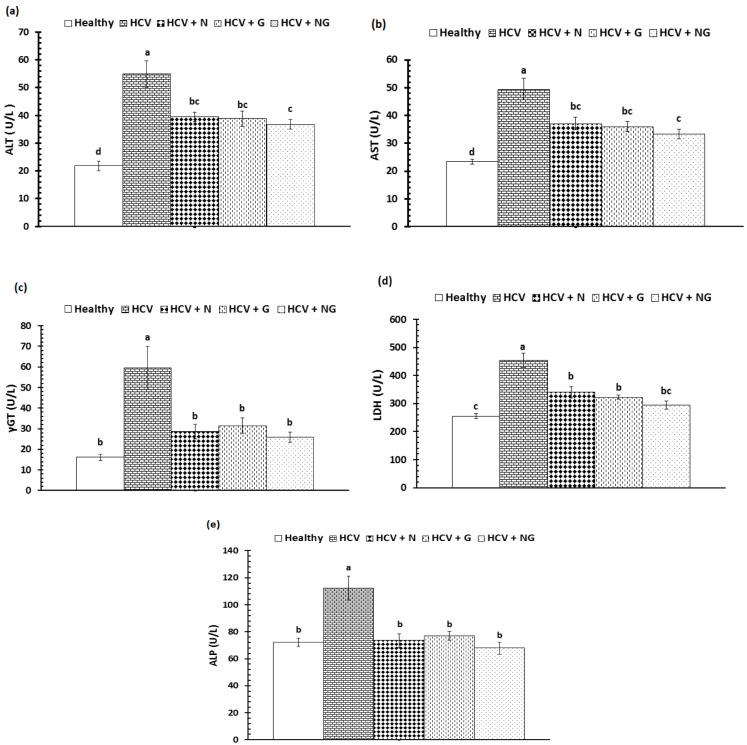
The activities of serum liver marker enzymes, AST, ALT, γGT, LDH and ALP, were represented in Figure 3a-e(Fig. 3). The HCV control group exhibited significantly (P<0.001) elevated serum activities of all assayed enzymes when compared to the corresponding healthy subjects. Administration of N. sativa, Z. officinale and their mixture significantly (P<0.001) ameliorated the elevated liver marker enzymes. Although there were non-significant differences between the administered treatments on liver marker enzymes, mixture of both extracts offered more potent effect on declining the elevated enzyme activities.
Figure 3. Serum liver enzymes of healthy, HCV control and HCV treated patients; (a) ALT, (b) AST, (c) γGT, (d) LDH and (e) ALP. Data are expressed as mean ± SE, means which share the same superscript symbol(s) are not significantly different, P<0.001.
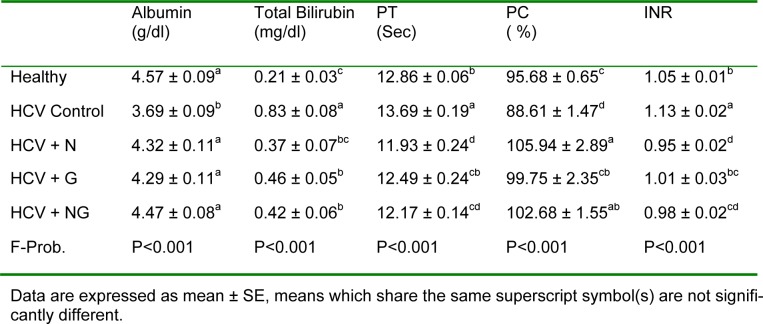
Serum albumin of the HCV control group exhibited a significant (P<0.001) decrease in comparison to healthy control group. On the other hand, serum total bilirubin showed an opposite pattern, where it was significantly (P<0.001) elevated in HCV control patients as compared to their corresponding healthy subjects (Table 1(Tab. 1)). Similarly, PT and INR were significantly (P<0.01) elevated in HCV control group, while PC was significantly (P<0.05) declined. All previously mentioned parameters were potentially improved following treatment with N. sativa and/or Z. officinale.
Table 1. Albumin, total bilirubin, PT, PC and INR in healthy, HCV and HCV treated patients.
Discussion
Chronic hepatitis C is a major global health burden with an estimated 160 million infected individuals worldwide. This long-term disease evolves slowly, often leading to chronicity and potentially to liver failure (Calland et al., 2012[13]). The lack of vaccine for HCV infections, the high cost of the drugs specially in low-income countries with a high prevalence of HCV, ineffective therapy and the rapid emergence of new drug-resistant viruses have urged a growing need for developing new, more effective chemotherapeutic agents with less side effects for successful HCV treatment (Sheir et al., 2013[68]). In the current study, administration of N. sativa, Z. officinale and their mixture resulted in a significant decrease in viral load and AFP, with a more potent effect offered by the mixture. We hypothesized that efficacy of the mixture might be attributed to the synergistic effect of the included active compounds.
Barakat et al. (2013[10]) revealed that N. sativa administration resulted in a significant decrease in viral load, with 16.67 % of patients becoming seronegative, and 50 % showing a significant decrease in the quantitative viral count. Among these, 66.7 % had cirrhosis and 33.3 % had chronic liver disease, implying antiviral activity. Also, the anti-inflammatory, antiviral and antineoplastic activities of N. sativa have been previously documented in various in vitro and in vivo studies (Zaher et al., 2008[81]). To our best knowledge no studies have explored the therapeutic effects of Z. officinale extract in HCV patients. Hence, we assumed that the observed beneficial therapeutic effects of the used extract might be attributed to its rich content of the antioxidant and anti-inflammatory compounds.
It is relevant here to mention the involvement of oxidative stress in the pathogenesis of hepatic dysfunction in human which has been investigated for many years (Spirli et al., 2001[72]; Alpini et al., 2002[6]; Cesaratto et al., 2004[14]; Jabłonowska et al., 2005[36]). HCV infection often leads to liver fibrosis and cirrhosis, various metabolic alterations including steatosis, insulin and interferon resistance, and development of hepatocellular carcinoma or non-Hodgkin lymphoma. Multiple molecular mechanisms that trigger the emergence and development of each of these pathogenic processes have been identified so far. One of these involves marked induction of reactive oxygen species (ROS) in infected cells leading to oxidative stress (Ivanov et al., 2013[35]). In addition, HCV-related fibrosis, cirrhosis and liver failure have been found to be the result of an adaptive immune response to HCV-infected cells (Nelson, 2001[57]), which is mediated by induction of endoplasmic reticulum and oxidative stress, and downregulation of the antiapoptotic proteins, nuclear factor-κB and Bcl-xl, in infected hepatocytes (Joyce and Tyrrell, 2010[38]).
Thymoquinone, the main constituent of N. sativa seeds, protects organs against oxidative damage induced by a variety of free radical generating pathologies (Tekeoglu et al., 2007[74]; Kanter, 2008[39]; Radad et al., 2009[62]). In addition, many studies have addressed the hepatoprotective effects of thymoquinone (Nagi and Mansour, 2000[56]; Meral et al., 2001[53]; El-Dakhakhny et al., 2002[21]; Mahmoud et al., 2002[49]). Furthermore, a study by Salem and Hossain (2000[65]) recorded a striking reduction of murine cytomegalovirus titer in both spleen and liver of mice treated with N. sativa compared with control mice.
Regarding antioxidant content, Z. officinale is the third amongst 1000 food items analysed by Halvorsen et al. (2006[29]). [6]-Dehydroshogaol, [6]-shogaol and 1-dehydro-[6]-gingerdione were shown to be potent inhibitors of nitric oxide (NO) synthesis in activated macrophages as reported by Li et al. (2011[47]). The antioxidant activity of [6]-gingerol against linoleic acid autoxidation and phospholipids peroxidation has been demonstrated earlier (Ippoushi et al., 2003[33]). In addition, it has been found to protect against cellular damages induced by peroxynitrite (Ippoushi et al., 2003[33]). It protected DNA from lipopolysaccharide-induced oxidation damage in rats (Ippoushi et al., 2007[34]). In the study conducted by Dugasani et al. (2010[19]), [6]-shogaol has exhibited the most potent antioxidant properties, compared to [6]-gingerol, [8]-gingerol and [10]-gingerol. The recorded antioxidant efficacy of [6]-shogaol can be attributed to the presence of α,β-unsaturated ketone moiety (Dugasani et al., 2010[19]). Moreover, the contained phenolic compounds of ginger, such as gingerol and shogoal, possess antioxidant (Jeyakumar et al., 1999[37]), anti-cancer (Shukla and Singh, 2007[71]), anti-inflammatory (Hudson et al., 2006[31]; Habib et al., 2008[28]), anti-angiogenesis (Huang et al., 2000[30]) and anti-artherosclerotic properties (Coppola and Novo, 2007[16]). Furthermore, the phagocytic activity of blood leukocytes was increased in rainbow trout fed with plant extracts containing food, especially 1 % ginger (Dügenci et al., 2003[20]). Zhou et al. (2006[82]) demonstrated that the volatile oil of ginger influences both cell-mediated immune response and non-specific proliferation of T lymphocyte, and may exert beneficial effects in a number of clinical conditions, such as chronic inflammation and autoimmune diseases. Hence, the observed antiviral potential of N. sativa and/or Z. officinale was mediated through their antioxidant, immunomodulatory and anti-inflammatory efficacies.
The role of liver enzymes in the assessment of chronic hepatitis C remains important due to the fact that the majority of clinical indexes estimating the degree of liver fibrosis are based on liver transaminases (Giannini and Testa, 2003[26]; Forns et al., 2004[25]; Lackner et al., 2005[43]). These enzymes are considered the most sensitive markers of liver injury as they are found in the cytoplasm of liver cells, thus damage of these cells lead to their rapid leakage into the blood circulation (Ramaiah, 2007[64]). The present investigation showed a significant increase in serum ALT, AST, LDH, γGT and ALP activities and total bilirubin levels of untreated HCV patient as compared to healthy subjects. The significantly elevated liver enzymes could be attributed to viral-induced hepatocellular damage. On the other hand, administration of N. sativa, Z. officinale and their mixture greatly improved the altered serum liver enzymes and this may be mediated through their hepatoprotective effects.
In consistent with our results, a recent study by Barakat et al. (2013[10]) showed that N. sativa seed oil potentially alleviated serum liver marker enzymes in Egyptian HCV patients. They also revealed that N. sativa oil administration was tolerable, safe, decreased viral load, and improved oxidative stress and clinical condition. Mallikarjuna et al. (2008[50]) demonstrated that oral administration of 200 mg/kg ginger ethanolic extract along with country-made liquor significantly alleviated the elevated serum AST, ALT and ALP activities as well as tissue lipid peroxide levels in experimental animals. Moreover, Lebda et al. (2012[45]) reported that rabbits fed with basal diet supplemented with 2 % ginger powder and ginger extract; hot and cold for 30 days showed significant decrease of serum AST, ALT, ALP and γGT activities as compared with control group. Furthermore, many studies had addressed the potent hepatoprotective effect of ginger extract in induced hepatotoxicity (Mallikarjuna et al., 2008[50]; El-Sharaky et al., 2009[23]; Motawi et al., 2011[54]).
Serum albumin is the most abundant plasma protein (Don and Kaysen, 2004[18]) and is essential for maintaining oncotic pressure of the vascular system (Quinlan et al., 2005[61]). Serum albumin and prothrombin time can be considered useful tools, alone or combined in clinical scores, for evaluating liver function (Botta et al., 2003[11]). Chronic HCV patients may suffer a decrease in serum albumin level (Mason et al., 1999[51]), and improvement in hypoalbuminemia has been shown to improve prognosis (Nagao and Sata, 2010[55]) and quality of life (Kotoh et al., 2005[42]). In addition, a recent study by Shin and Moon (2010[70]) reported that in chronic liver diseases, the serum albumin level is reduced due to protein synthesis disruption in the liver. In the current study, administration of ginger and N. sativa as well as their mixture significantly increased serum albumin levels, indicating an improvement in clinical condition. Prior studies have shown similar effects of N. sativa administration to animals (Al-Gaby, 1998[2]; Tollba and Hassan, 2003[75]; Shewita and Taha, 2011[69]) and to HCV patients (Barakat et al., 2013[10]). Similarly, Motawi et al. (2011[54]) revealed the potency of Z. officinale in alleviating serum total protein and bilirubin levels in carbon tetrachloride-induced liver fibrosis in rats.
Therefore, the potent therapeutic effects offered by the mixture of N. sativa and Z. officinale in this study may be attributed to the synergism between their active constituents. Guimarães et al. (2011[27]) reported that the most potent antioxidant activity was found in combinations of different herbs, suggesting synergistic effects. So the synergistic effect of using mixture of herbs are designed to work together to produce a gentle, balanced and greater effect to correct underlying imbalances than temporary relief (Liu and Du, 2012[48]; Sarg et al., 2012[66]; Rajput and Mandal, 2012[63]).
In conclusion, our findings suggest that administration of both N. sativa and/or Z. officinale ethanolic extracts to HCV patients was safe, tolerable, decreased viral load, and improved clinical condition. In addition, the current study recommends the use of mixtures of herbal therapy to increase their synergetic effects against HCV and decrease their side effects.
Supplementary Material
References
- 1.Ajayi OB, Akomolafe SF, Akinyemi FT. Food value of two varieties of Ginger (Zingiber officinale) commonly consumed in Nigeria. ISRN Nutr. 2013;2013:359727. doi: 10.5402/2013/359727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Gaby AM. Amino acid composition and biological effects of supplementing broad bean and corn proteins with Nigella sativa (black cumin) cake protein. Nahrung. 1998;42:290–294. doi: 10.1002/(sici)1521-3803(199810)42:05<290::aid-food290>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17:299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 4.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46:409–20. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 5.Al-Naggar TB, Gómez-Serranillos MP, Carretero ME, Villar AM. Neuropharmacological activity of Nigella sativa L. extracts. J Ethnopharmacol. 2003;88:63–68. doi: 10.1016/s0378-8741(03)00157-0. [DOI] [PubMed] [Google Scholar]
- 6.Alpini G, McGill J, Larusso N. The pathobiology of biliary epithelia. Hepatology. 2002;35:1256–1268. doi: 10.1053/jhep.2002.33541. [DOI] [PubMed] [Google Scholar]
- 7.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anwar WA, Khaled HM, Amra HA, El-Nezami H, Loffredo CA. Changing pattern of hepatocellular carcinoma (HCC) and its risk factors in Egypt: possibilities for prevention. Mutat Res. 2008;659:176–184. doi: 10.1016/j.mrrev.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78:431–41. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Barakat EMF, El Wakeel LM, Hagag RS. Effects of Nigella sativa on outcome of hepatitis C in Egypt. World J Gastroenterol. 2013;19:2529–2536. doi: 10.3748/wjg.v19.i16.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botta F, Giannini E, Romagnoli P, Fasoli A, Malfatti F, Chiarbonello B, et al. MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: a European study. Gut. 2003;52:134–139. doi: 10.1136/gut.52.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmachari G. Natural products in drug discovery: impacts and opportunities - an assessment. In: Brahmachari G, editor. Bioactive natural products. Singapore: World Scientific Publishing Co.; 2011. pp. 1–199. [Google Scholar]
- 13.Calland N, Dubuisson J, Rouillé Y, Séron K. Hepatitis C virus and natural compounds: a new antiviral approach? Viruses. 2012;4:2197–2217. doi: 10.3390/v4102197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cesaratto L, Vascotto C, Calligaris S, Tell G. The importance of redox state in liver damage. Ann Hepatol. 2004;3:86–92. [PubMed] [Google Scholar]
- 15.Chrubasik S, Pittler MH, Roufogalis BD. Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine. 2005;12:684–701. doi: 10.1016/j.phymed.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Coppola G, Novo S. Statins and peripheral arterial disease: effects on claudication, disease progression, and prevention of cardiovascular events. Arch Med Res. 2007;38:479–488. doi: 10.1016/j.arcmed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Di Bisceglie AM, Hoofnagle JH. Optimal therapy of hepatitis C. Hepatology. 2002;36:S121–7. doi: 10.1053/jhep.2002.36228. [DOI] [PubMed] [Google Scholar]
- 18.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17:432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 19.Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol. 2010;127:515–520. doi: 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Dügenci SK, Arda N, Candan A. Some medicinal plants as immunostimulant for fish. J Ethnopharmacol. 2003;88:99–106. doi: 10.1016/s0378-8741(03)00182-x. [DOI] [PubMed] [Google Scholar]
- 21.El-Dakhakhny M, Mady N, Lembert N, Ammon HP. The hypoglycemic effect of Nigella sativa oil is mediated by extrapancreatic actions. Planta Med. 2002;68:465–466. doi: 10.1055/s-2002-32084. [DOI] [PubMed] [Google Scholar]
- 22.Elkady A, Tanaka Y, Kurbanov F, Sugauchi F, Sugiyama M, Khan A, et al. Genetic variability of hepatitis C virus in South Egypt and its possible clinical implication. J Med Virol. 2009;81:1015–1023. doi: 10.1002/jmv.21492. [DOI] [PubMed] [Google Scholar]
- 23.El-Sharaky AS, Newairy AA, Newairy AA, Kamel MA, Eweda SM. Protective effect of ginger extract against bromobenzene-induced hepatotoxicity in male rats. Food Chem Toxicol. 2009;47:1584–1590. doi: 10.1016/j.fct.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Forest MG, Pugeat M, editors. Binding proteins of steroid hormones. Colloque INSERM. Montrouge: Libbey; 1987. pp. 53–86. [Google Scholar]
- 25.Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2004;39:862–863. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 26.Giannini E, Testa R. Noninvasive diagnosis of fibrosis: The truth is rarely pure and never simple. Hepatology. 2003;38:1312–1313. doi: 10.1053/jhep.2003.50500. [DOI] [PubMed] [Google Scholar]
- 27.Guimarães R, Barros L, Carvalho AM, Ferreira IC. Infusions and decoctions of mixed herbs used in Folk Medicine synergism in antioxidant potential. Phytother Res. 2011;25:1209–1214. doi: 10.1002/ptr.3366. [DOI] [PubMed] [Google Scholar]
- 28.Habib SH, Makpol S, Abdul Hamid NA, Das S, Ngah WZ, Yusof YA. Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics (Sao Paulo) 2008;63:807–813. doi: 10.1590/S1807-59322008000600017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halvorsen BL, Carlsen MH, Phillips KM, Bøhn SK, Holte K, Jacobs DR, Jr, et al. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am J Clin Nutr. 2006;84:95–135. doi: 10.1093/ajcn/84.1.95. [DOI] [PubMed] [Google Scholar]
- 30.Huang S, DeGuzman A, Bucana CD, Fidler IJ. Nuclear factor-kappa B activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice. Clin Cancer Res. 2000;6:2573–2581. [PubMed] [Google Scholar]
- 31.Hudson EA, Fox LH, Luckett JCA, Manson MM. Ex vivo cancer chemoprevention research possibilities. Environ Toxicol Pharmacol. 2006;21:204–214. doi: 10.1016/j.etap.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 32.IFCC Methods for the measurement of catalytic concentration of enzymes. J Clin Chem Clin Biochem. 1980;18:521–534. [PubMed] [Google Scholar]
- 33.Ippoushi K, Azuma K, Ito H, Horie H, Higashio H. [6]-Gingerol inhibits nitric oxide synthesis in activated J774.1 mouse macrophages and prevents peroxynitrite-induced oxidation and nitration reactions. Life Sci. 2003;73:3427–3437. doi: 10.1016/j.lfs.2003.06.022. [DOI] [PubMed] [Google Scholar]
- 34.Ippoushi K, Takeuchi A, Ito H, Horie H, Azuma K. Antioxidative effects of daikon sprout (Raphanus sativus L.) and ginger (Zingiber officinale Roscoe) in rats. Food Chem. 2007;102:237–242. [Google Scholar]
- 35.Ivanov AV, Bartosch B, Smirnova OA, Isaguliants MG, Kochetkov SN. HCV and oxidative stress in the liver. Viruses. 2013;5:439–469. doi: 10.3390/v5020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jabłonowska E, Tchórzewski H, Lewkowicz P, Kuydowicz J. Reactive oxygen intermediates and serum antioxidative system in patients with chronic C hepatitis treated with IFN-alpha and thymus factor X. Arch Immunol Ther Exp (Warszaw) 2005;53:529–33. [PubMed] [Google Scholar]
- 37.Jeyakumar SM, Nalini N, Menon VP. Antioxidant activity of ginger (Zingiber officinale) in rats fed a high fat diet. Med Sci Res. 1999;27:341–344. [Google Scholar]
- 38.Joyce MA, Tyrrell DL. The cell biology of hepatitis C virus. Microbes Infect. 2010;12:263–271. doi: 10.1016/j.micinf.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Kanter M. Effects of Nigella sativa and its major constituent, thymoquinone on sciatic nerves in experimental diabetic neuropathy. Neurochem Res. 2008;33:87–96. doi: 10.1007/s11064-007-9419-5. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan LA. Glucose. In: Kaplan LA, Pesce AJ, editors. Clinical chemistry: theory, analysis, and correlation. St. Louis, MO: Mosby; 1984. pp. 1032–1036. [Google Scholar]
- 41.Khattab MA, Ferenci P, Hadziyannis SJ, Colombo M, Manns MP, Almasio PL, et al. Management of hepatitis C virus genotype 4: recommendations of an international expert panel. J Hepatol. 2011;54:1250–1262. doi: 10.1016/j.jhep.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Kotoh K, Nakamuta M, Fukushima M, Matsuzaki C, Enjoji M, Sakai H, et al. High relative fat-free mass is important for maintaining serum albumin levels in patients with compensated liver cirrhosis. World J Gastroenterol. 2005;11:1356–1360. doi: 10.3748/wjg.v11.i9.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lackner C, Struber G, Liegl B, Leibl S, Ofner P, Bankuti C, et al. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis. Hepatology. 2005;41:1376–1382. doi: 10.1002/hep.20717. [DOI] [PubMed] [Google Scholar]
- 44.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–115. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 45.Lebda MA, Taha NM, Korshom MA, Mandour AEA, El-Morshedy AM. Biochemical effect of ginger on some blood and liver parameters in male New Zealand rabbits. Online J Anim Feed Res. 2012;2(2):197–202. [Google Scholar]
- 46.Lee KH. Research and future trends in the pharmaceutical development of medicinal herbs from Chinese medicine. Public Health Nutr. 2000;3:515–22. doi: 10.1017/s1368980000000604. [DOI] [PubMed] [Google Scholar]
- 47.Li F, Wang Y, Parkin KL, Nitteranon V, Liang J, Yang W, et al. Isolation of quinone reductase (QR) inducing agents from ginger rhizome and their in vitro anti-inflammatory activity. Food Res Int. 2011;44:1597–1603. [Google Scholar]
- 48.Liu A-L, Du G-H. Antiviral properties of phytochemicals. In: Patra AK, editor. Dietary phytochemicals and microbes. Dordrecht: Springer Sciene+Business Media; 2012. pp. 93–126. [Google Scholar]
- 49.Mahmoud MR, El-Abhar HS, Saleh S. The effect of Nigella sativa oil against the liver damage induced by Schistosoma mansoni infection in mice. J Ethnopharmacol. 2002;79:1–11. doi: 10.1016/s0378-8741(01)00310-5. [DOI] [PubMed] [Google Scholar]
- 50.Mallikarjuna K, Sahitya Chetan P, Sathyavelu Reddy K, Rajendra W. Ethanol toxicity: rehabilitation of hepatic antioxidant defense system with dietary ginger. Fitoterapia. 2008;79:174–178. doi: 10.1016/j.fitote.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–333. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]
- 52.McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485–92. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 53.Meral I, Yener Z, Kahraman T, Mert N. Effect of Nigella sativa on glucose concentration, lipid peroxidation, anti-oxidant defence system and liver damage in experimentally-induced diabetic rabbits. J Vet Med A Physiol Pathol Clin Med. 2001;48:593–599. doi: 10.1046/j.1439-0442.2001.00393.x. [DOI] [PubMed] [Google Scholar]
- 54.Motawi TK, Hamed MA, Shabana MH, Hashem RM, Aboul Naser AF. Zingiber officinale acts as a nutraceutical agent against liver fibrosis. Nutr Metab (London) 2011;8:40. doi: 10.1186/1743-7075-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagao Y, Sata M. Serum albumin and mortality risk in a hyperendemic area of HCV infection in Japan. Virol J. 2010;7:375. doi: 10.1186/1743-422X-7-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagi MN, Mansour MA. Protective effect of thymoquinone against doxorubicininduced cardiotoxicity in rats: a possible mechanism of protection. Pharmacol Res. 2000;41:283–9. doi: 10.1006/phrs.1999.0585. [DOI] [PubMed] [Google Scholar]
- 57.Nelson DR. The immunopathogenesis of hepatitis C virus infection. Clin Liver Dis. 2001;5:931–953. doi: 10.1016/s1089-3261(05)70202-6. [DOI] [PubMed] [Google Scholar]
- 58.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from. 1981 to. 2010. J Nat Prod. 2012;75:311–35. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen MH, Keeffe EB. Prevalence and treatment of hepatitis C virus genotypes 4, 5, and 6. Clin Gastroenterol Hepatol. 2005;3:S97–S101. doi: 10.1016/s1542-3565(05)00711-1. [DOI] [PubMed] [Google Scholar]
- 60.Persijn JP, van der Slik W. A new method for the determination of gamma-glutamyltransferase in serum. J Clin Chem Clin Biochem. 1976;14:421–427. doi: 10.1515/cclm.1976.14.1-12.421. [DOI] [PubMed] [Google Scholar]
- 61.Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41:1211–1219. doi: 10.1002/hep.20720. [DOI] [PubMed] [Google Scholar]
- 62.Radad K, Moldzio R, Taha M, Rausch WD. Thymoquinone protects dopaminergic neurons against MPP+ and rotenone. Phytother Res. 2009;23:696–700. doi: 10.1002/ptr.2708. [DOI] [PubMed] [Google Scholar]
- 63.Rajput S, Mandal M. Antitumor promoting potential of selected phytochemicals derived from spices: a review. Eur J Cancer Prev. 2012;21:205–215. doi: 10.1097/CEJ.0b013e32834a7f0c. [DOI] [PubMed] [Google Scholar]
- 64.Ramaiah S. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem Toxicol. 2007;45:1551–7. doi: 10.1016/j.fct.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 65.Salem ML, Hossain MS. In vivo acute depletion of CD8(+) T cells before murine cytomegalovirus infection upregulated innate antiviral activity of natural killer cells. Int J Immunopharmacol. 2000;22:707–718. doi: 10.1016/s0192-0561(00)00033-3. [DOI] [PubMed] [Google Scholar]
- 66.Sarg T, Abdel-Ghani A, Zayed R, El-Sayed M. Bioactive compounds from Phyllanthus atropurpureus. J Nat Prod. 2012;5:10–20. [Google Scholar]
- 67.Schumann G, Klauke R. New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: preliminary upper reference limits obtained in hospitalized subjects. Clin Chem Acta. 2003;327:69–79. doi: 10.1016/s0009-8981(02)00341-8. [DOI] [PubMed] [Google Scholar]
- 68.Sheir Z, Badra G, Salama O, Gomaa AI, Saber W. Effect of combination of some natural products and chloroquine on HCV infection in Egyptian patients: Pilot study. J Liver. 2013;2:116. [Google Scholar]
- 69.Shewita RS, Taha AE. Effect of dietary supplementation of different levels of black seed (Nigella Sativa L.) on growth performance, immunological, hematological and carcass parameters of broiler chicks. World Academy of Science, Engineering and Technology (WASET) 2011;53:788–794. [Google Scholar]
- 70.Shin M-O, Moon J-O. Effect of dietary supplementation of grape skin and seeds on liver fibrosis induced by dimethyl-nitrosamine in rats. Nutr Res Pract. 2010;4:369–74. doi: 10.4162/nrp.2010.4.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shukla Y, Singh M. Cancer preventive properties of ginger: A brief review. Food Chem Toxicol. 2007;45:683–690. doi: 10.1016/j.fct.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Spirli C, Nathanson M, Fiorotto R, Duner E, Denson L, Sanz J. Proinflammatory cytokines inhibit secretion in rat bile duct epithelium. Gastroenterology. 2001;121:156–169. doi: 10.1053/gast.2001.25516. [DOI] [PubMed] [Google Scholar]
- 73.Teitz N. Fundamentals of clinical chemistry. Philadelphia, PA: Saunders; 1986. [Google Scholar]
- 74.Tekeoglu I, Dogan A, Ediz L, Budancamanak M, Demirel A. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother Res. 2007;21:895–897. doi: 10.1002/ptr.2143. [DOI] [PubMed] [Google Scholar]
- 75.Tollba AAH, Hassan MSH. Using some natural additives to improve physiological and productive performance of broiler chicks under high temperature conditions 2-black cumin (Nigella Sativa) or garlic (Allium sativum) Egypt Poul Sci. 2003;23:327–340. [Google Scholar]
- 76.Vermehren J, Sarrazin C. New hepatitis C therapies in clinical development. Eur J Med Res. 2011;16:303–314. doi: 10.1186/2047-783X-16-7-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wanger C, Dati F. Prothrombin time (PT) test. In: Thomas L, editor. Cilinical laboratory diagnostics. Frankfurt: TH-books Verlagsges; 1998. pp. 599–601. [Google Scholar]
- 78.Webster D. A study of the interaction of bromcresol green with isolated serum globulin fractions. Clin Chim Acta. 1974;53:109–115. doi: 10.1016/0009-8981(74)90358-1. [DOI] [PubMed] [Google Scholar]
- 79.White B. Ginger: an overview. Am Family Phys. 2007;75:1689–91. [PubMed] [Google Scholar]
- 80.World Health Oranization. Hepatitis C. Updated July. Available from: http://www.who.int/mediacentre/factsheets/fs164/en/index.html.
- 81.Zaher KS, Ahmed WM, Zerizer SN. Observations on the biological effects of black cumin seed (Nigella sativa) and green tea (Camellia sinensis) Global Veterinaria. 2008;2:198–204. [Google Scholar]
- 82.Zhou H, Deng Y, Xie Q. The modulatory effects of the volatile oil of ginger on the cellular immune response in vitro and in vivo in mice. J Ethnopharmacol. 2006;105:301–305. doi: 10.1016/j.jep.2005.10.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.