Abstract
Traumatic brain injury (TBI) is among the significant causes of morbidity and mortality in the present world. Around 1.6 million persons sustain TBI, whereas 200,000 die annually in India, thus highlighting the rising need for appropriate cognitive rehabilitation strategies. This literature review assesses the current knowledge of various cognitive rehabilitation training strategies. The entire spectrum of TBI severity; mild to severe, is associated with cognitive deficits of varying degree. Cognitive insufficiency is more prevalent and longer lasting in TBI persons than in the general population. A multidisciplinary approach with neuropsychiatric evaluation is warranted. Attention process training and tasks for attention deficits, compensatory strategies and errorless learning training for memory deficits, pragmatic language skills and social behavior guidance for cognitive-communication disorder, meta-cognitive strategy, and problem-solving training for executive disorder are the mainstay of therapy for cognitive deficits in persons with TBI. Cognitive impairments following TBI are common and vary widely. Different cognitive rehabilitation techniques and combinations in addition to pharmacotherapy are helpful in addressing various cognitive deficits.
Keywords: Cognitive impairment, cognitive rehabilitation, traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) is a steadily rising public health concern and one of the significant causes of morbidity and mortality in India.[1] Around 10 million people sustain TBI worldwide annually.[2] The recent global status report on road safety by the World Health Organization, 2013 has clearly highlighted the existing and growing enormity of this problem across the world and has emphasized on the outmost need for well-designed and evaluated programs in prevention, management, and rehabilitation. As India continues to progress to greater urbanization with rapid development in terms of motorization, incidence of TBIs will increase significantly. Data from an epidemiological study undertaken in Bengaluru have shown incidence, mortality, and case fatality rates of 150/100,000, 20/100,000 and 10%, respectively.[3] An estimated 2 million people sustain brain injuries with nearly a million requiring rehabilitation services at the national level.[3]
Persons, who survive TBI, end up with chronic disability.[1] It significantly impacts on an individual's life, in terms of cognitive, behavioral, psychosocial and physical factors, and vocational issues.[4] Among them, cognitive disabilities are often the most disabling and distressing for the affected persons, family members, and the society. Cognitive deficits can significantly impair activities of daily living (ADL), employment, social relationships, recreation, and active participation in the community.
TBI is classified as mild, moderate, and severe depending on the level of consciousness, particularly duration of coma and posttraumatic amnesia (PTA).[4,5] In moderate to severe TBI, cognition appears to be markedly impaired around 1-month postinjury[6] or shortly after resolution of PTA.[7,8] Cognitive impairments persisting even after 3 months were found to be associated with higher frequency disability.[9] In moderate to severe TBI, cognitive recovery does not return to baseline even after 2 years of injury. In contrast, the cognitive recovery tends to be rapid in patients with mild TBI, returning almost to “normal baseline functioning” within 3 months.[10,11]
Literature and studies have reported that effective cognitive rehabilitation interventions initiated post-TBI enhance the recovery process and minimize the functional disability. Hence, it is necessary to have a proper guideline for the cognitive rehabilitation of traumatic brain injured persons with multiple cognitive impairments. This article has been adapted from various literatures and outlines briefly the commonly encountered cognitive deficits following TBI. It also provides a summary of effective rehabilitation strategies for the cognitively impaired persons.
OBJECTIVE
Objectives of this study were to discuss the various cognitive training strategies for persons with TBI with cognitive deficits and to aid in establishing the appropriate technique for cognitive rehabilitation in persons TBI sequelae.
METHODOLOGY
An online literature search using the terms attention, memory, language, cognition, communication, executive, problem solving, reasoning, remediation, training, rehabilitation, and in the Medline and EMBASE databases was conducted. A total of 99 studies on cognitive rehabilitation were assessed and evaluated.
Common cognitive impairments
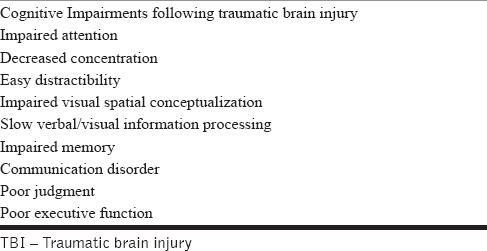
TBI can cause a plethora of cognitive impairments. Table 1 shows the common cognitive impairments following TBI. Arciniegas et al.[12] have reported that posttraumatic attention, memory deficits, and disturbances in executive functioning are the most commonly encountered neurocognitive deficits. Attention and memory deficits may exacerbate or cause additional disturbances in executive function, interpersonal communication skill, and other complex cognitive functions.
Table 1.
Common cognitive impairments following TBI
Cognitive assessment
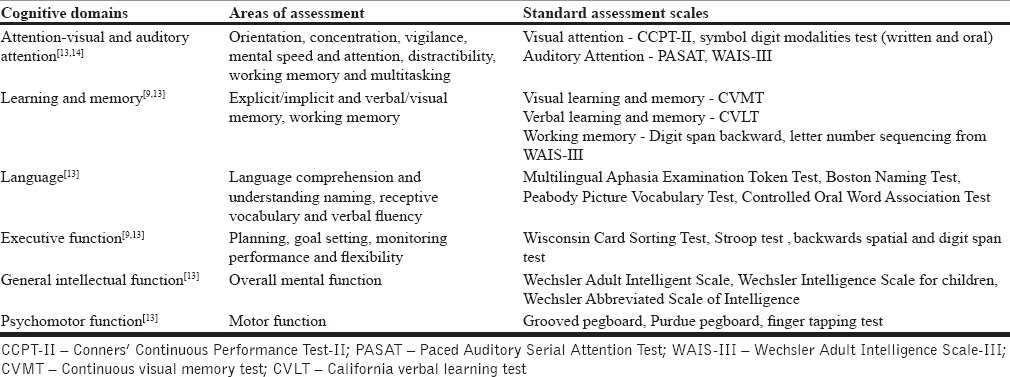
A detailed neuropsychiatric assessment, to assess the residual cognitive abilities and inabilities of the TBI person, is necessary before starting of cognitive rehabilitation. In addition, repeat neuropsychological assessments, at a regular interval, are necessary to evaluate the effectiveness of ongoing treatment. Table 2 shows the commonly used standardized assessment scales for neuropsychological assessments for cognitive function.
Table 2.
Commonly used standardized neuropsychological assessment scale
As a caveat, an improvement in the neuropsychological test does not necessarily mean that the patient has improved in functional ADL contemporaneously. Hence, assessment for functional outcome measurement tools (functional independence measure [FIM], Disability Rating Scale [DRS]) to live independently and to return to work, should be considered when attempting to plan appropriate cognitive rehabilitation programs for survivors of TBI. It is evident from literature[15,16,17] that the neuropsychological test results, as measures of cognitive ability, have been found to correlate significantly with functional outcome measures (e.g., FIM and DRS). Hanks et al.[17] reported that cognitive performances, measured on neuropsychological assessments, better predicted 1-year outcomes than functional measures. Similarly, Neese et al.[16] demonstrated that neuropsychological performances in intellectual, academic, executive, and visuoperceptual domains correlated significantly with DRS scores and hence, suggested that cognitive assessments could predict the level of function throughout the rehabilitation process.
Cognitive rehabilitation
The goal of cognitive rehabilitation following TBI is to enhance the persons' ability to process and interpret information and to improve the person's ability to perform mental functions. Silver et al.[18] reported that cognitive rehabilitation is best suited for well-motivated and functionally independent persons with mild to moderate cognitive impairments.
Cognitive rehabilitation cannot be seen as a “stand alone” therapy for persons with cognitive deficits. It has always shown more benefits when administered as the part of the multidisciplinary/interdisciplinary approach. Multidisciplinary team approach encompasses physician, neuropsychologists, speech-language pathologists, occupational therapists, physical therapist, and social workers.
Cognitive rehabilitation consists of diverse interventions; however, there is a consensus in literature that cognitive rehabilitation has to be tailored to individual needs.[5] Studies have divided cognitive rehabilitation therapy into two components: Restorative and compensatory approach.[5] The restorative approach aims at reinforcing, strengthening, or restoring the impaired skills. It includes the repeated exercise of standardized cognitive tests of increasing difficulty, targeting specific cognitive domains (e.g., selective attention, memory for new information). Compensatory approach teaches ways of bypassing or compensating for the impaired function.[5] Various authors[5,19] have reported the effective use of assistive technologies (AT), calendars, electronic memory devices, alarms, or reminders as compensatory techniques.
Pharmacotherapy based on two principles, catecholaminergic and cholinergic augmentation has been found to be a useful adjunct in cognitive rehabilitation.[18]
Attention
Tsaousides and Gordon[13] describes attention as a “complex mental activity that refers to how an individual receives and begins to process internal and external stimuli.” Attention deficits are more commonly encountered in persons with severe TBI, and may include difficulties in sustained attention/concentration, delayed reaction time, distractibility, decreased processing speed, and impaired dual or multitasking (e.g., walking and talking).[13,20]
Attention can be improved significantly with a specific skill training after acquired brain injury.[21] Attention process training (APT)[13] is a direct attention training program, intended to be restorative, has been designed to improve visual and auditory attention. APT targets five components of attention: Focused attention, sustained attention, selective attention, alternating attention, and divided attention. The training program consists of tasks with a hierarchical progression of increasing attention demands, graduating from simple to complex distracters.[13]
APT can significantly improve complex attention.[22] Sohlberg and Mateer[22] noted traumatic brain injured persons, who were undergoing APT, performed better in the Paced Auditory Serial Addition Test, Stroop Test, and the Trail Making Test. Tiersky et al.,[23] in his randomized control trial, has demonstrated that mild to moderate brain injured persons, who were receiving cognitive remediation and cognitive behavioral psychotherapy, performed better in divided auditory attention. Compared to the control group, the treatment group showed significantly improved emotional functioning, reduced psychological distress. Cognitive remediation program included direct attention training and compensatory strategy training with memory notebook and problem-solving strategies. Westerberg et al.[24] noted significant improvements in attention in brain injured persons, who were undergoing direct attention training, an automated, and computerized training program (computer software) for 5 weeks. A comprehensive review article by Cicerone et al.[25] has recommended direct attention training and metacognitive training for TBI persons with attention deficit. Metacognitive training targets the development of the compensatory strategy. However it is noteworthy, that there is not enough evidence to differentiate the effectiveness of specific attention training during the acute stage versus gains that occur from spontaneous recovery or general cognitive interventions.[26]
Pharmacotherapy
Studies[1,27] have shown that treatment with amantadine, if started within the first few days following TBI, improves the arousal and accelerates the rate of functional recovery and ultimately improves attention, visuospatial function (constructional praxis), executive function, and general cognitive function of persons with TBI. Similarly, few studies[28,29,30] have reported that methylphenidate may improve hypoarousal, attention and processing speed, and general cognitive function. Although methylphenidate was found to improve cognitive functions in several studies the results were conflicting, which until date does not have enough evidence to support its usage among moderate to severe brain injury patients.
Memory
Memory impairment is one of the most common cognitive impairments after TBI.[31] It is frequently the first function to be notably impaired and one of the last function to be regained in the recovery process.
Cognitive rehabilitation therapy interventions aim either to restore or compensate the memory deficits.[13] Restorative approaches for memory intervention include the word list, paragraph listening, visual imagery, and mnemonic strategies.[13] Cappa et al.[32] and Cicerone et al.[33] in their review, reported that memory remediation treatments like memory drills, computer-assisted cognitive rehabilitation are not much helpful for TBI persons for long-term memory. Though, computer-assisted strategies have been found to be useful to improve overall general cognitive functioning, attention, memory, and executive skills as a whole.
There is strong evidence supporting the use of external memory aids in compensating the memory impairments in TBI persons. Compensatory strategy training, including internalized strategy training (e.g., visual imagery) and external memory compensations (e.g., memory notebooks, AT tools), is found to be effective in mild memory impairments after TBI.[25] A memory notebook usually includes section of orientation (injury related information), memory log, calendar, to-do lists, transportation (maps, public transportation schedule, and taxi phone numbers), a feelings log, names, etc.[13] AT tools encompass portable electronic devices, personal computers, personal digital assistants, voice recorders, pagers, etc.[13]
Studies[25,34,35,36,37] have mentioned that errorless learning (EL) is another useful strategy for teaching specific information or procedures. EL technique facilitates compensatory strategies training targeting personally relevant memory problems, such as taking medications at meal time, or keeping keys in a consistent location. Dou et al.[34] have shown that TBI persons with memory impairment, who were undergoing a computerized assisted or therapist-assisted EL program, performed better scoring in neuropsychological tests after memory training compared to no treatment control group.
Computer assisted training is useful for improving general cognitive functioning. It has been found to have several benefits such as allowing flexibility in retraining procedures, programs that can be customized for individuals, and finally it reduces the direct time a therapist needs to be with a patient. Several studies have shown computer-assisted strategies to improve attention, memory, and executive skills.[38,39,40]
Pharmacotherapy: Zhang et al.[41] reported that donepezil, a centrally-selective acetylcholinesterase inhibitor, may improve attention and memory impairments during the subacute postinjury period. Another drug, rivastigmine, which is an acetylcholinesterase and butyrylcholinesterase inhibitor, (3-6 mg/day) is safe and well tolerated and may improve attention and working memory.[42] Neurobehavioral Guidelines Working Group[43] has recommended the use of donepezil (5-10 mg daily) and rivastigmine (3-6 mg daily) to enhance attention and memory for persons with moderate to severe TBI during subacute and chronic periods of recovery.[18] Citicholine (cytidine diphosphate choline) though termed as a neuroprotective agent, has not been found to have a significant effect on functional and cognitive recovery. However, Levin[44] in his study reported that citicholine may reduce postconcussive symptoms and improve cognition memory during the early period after mild to moderate TBI. In contrast, Zafonte et al.[45] did not find in any significant improvement in cognition and functional status even after 90 days trial of citicholine in traumatic brain injured persons. Similarly, Tj et al.[46] also did not find any difference in the quality of life in brain injured persons after trial of citicholine.
Visuospatial perception
Visuospatial perception changes such as unilateral neglect, impairments of body scheme, and constructional skills are common in severe TBI persons.[47] Agnosia and apraxia are not uncommon. When such deficits combine with cognitive impairments, they have a significant impact in rehabilitation participation and ADL along with posing as a safety concern.
Using visuospatial cues to direct attention to the areas of residual vision, in vision restoration therapy (VRT), some improvement in vision in persons with visual field defect has been documented. It has the potential to enhance neural plasticity and ultimately increase conscious visual perception.[48] Similarly, Mueller et al.[49,50] showed that VRT improvesvisual functions in persons with central nervous system disorders. Pizzamiglio et al.[51] used spatial scanning with optokinetic stimulation in patients with the hemineglect disorder, but it failed to show any additional benefit in their performance. A study by Cicerone et al.[25] has found visual scanning training, isolated microcomputer exercises, and electronic technologies to be useful. Likewise, prism adaptation has also been found to be useful in gaze abnormalities.[52] Nonconfrontive, behavioral therapy approaches have been reportedly beneficial in anosognosia. Anosognosia (impaired self-awareness or denial) is a very common and serious consequence of brain injury. Brain-injured persons with anosognosia face difficulties in the adoption of compensatory strategies, which ultimately comes in the way of rehabilitation.[53] Virtual reality game[54,55,56,57] has been found to improve self-awareness and some attention factors. However, pharmacotherapy has not found to be any role for visual perceptual impairments.
Language and communication
Communication is very complex and involves processing of both verbal and nonverbal information. Language and communication disorder in the TBI can be categorized into four main groups: Apraxia, aphasia, dysarthria, and cognitive communication disorder. Apraxia is the inability to carry out a motor act despite intact motor and sensory pathways.[20] An apraxia in brain injured persons has been found of three types: Ideomotor, ideational, and constructional apraxia.[20] The type of speech and language impairment is dependent on the extent and location of the brain injury. Broca's aphasia (26.49%) is the most common type, followed by anomic aphasia (19.6%), and transcortical motor aphasia (15.6%).[58] Dysarthria along with swallowing deficits has been reported, affecting respiration, phonation, resonance, articulation, and prosody.[59] Cognitive communication disorder or inappropriate communication following TBI may impair social interacting and reintegrating which can ultimately lead to frustrating or embarrassing experiences.[20,60] Persons with TBI can suffer from delayed word recall to reduced emotion while communicating with others. They find difficulty specially in word finding[61] and language processing.[62] Brain-injured persons show impairments in self-focused conversation[20] and in interpreting linguistic humor.[63]
Language functions are significantly associated with the functional and cognitive status of the brain injured persons.[64] Speech and language therapy, including constraint-induced aphasia therapy (CIAT),[65,66,67] computer-assisted therapy,[68,69,70,71] melodic intonation therapy,[72,73,74] and neurostimulation techniques like transcranial direct current stimulation (tDCS),[75] have been found to improve dysarthria and aphasia in acquired brain injured persons. The principle behind CIAT is massed practice, with language tasks of increasing difficulty and using constraint of compensatory (nonverbal) communication strategies.[65]
The Lee Silverman voice treatment (LSVT) has been found to improve loudness, sustained phonation and connected speech, word and sentence intelligibility in persons with dysarthria following brain injury.[76] The focus of the LSVT treatment is on respiratory, laryngeal muscles, and articulatory function to improve the speech clarity by graded exercises. Studies[25,77,78] have reported that pragmatic language skills, social behaviors, and cognitive training along with psychotherapy for emotional adjustment, can significantly improve the social communication skill of the traumatic brain injured persons. Similar results were reported by McDonald et al.,[78] on acquired brain injury persons, which predominantly involved persons with TBI. Group-based interventions[25] and specialized computer and internet training material[20,79] were found to be additional useful methods of rehabilitating social communication skills after TBI. Bornhofen and McDonald[80] suggested that EL and self-instruction training both can improve in emotional perception abilities of TBI persons and indirectly can improve communication with the general population.
For those with apraxia, studied by Smania et al.[81] showed that gesture production exercises helped in addition to improvement in their ADL functioning. The gesture production exercises were made up “transitive, intransitive-symbolic, and intransitive-nonsymbolic gestures.”[81,82] In those undergoing gesture production exercises, achieved significant improvement in neuropsychological tests including ideomotor and ideational apraxia tests.[82]
Executive function
Executive function can be defined as the mental capacity to “engage successfully in independent, purposive, self-serving behavior.”[83] Executive function allows the person to plan or set goals, initiate behavior, solve problems, anticipate consequences, monitor performance, and respond flexibly and adaptively. Impairments in executive functions may include an inability to perform these cognitive processes and impede daily activities.
A number of studies[84,85] have reported metacognitive strategy training (directed at improving self-monitoring and self-regulation) are more effective compared to conventional rehabilitation in improving posttraumatic executive dysfunction. Metacognitive strategy training helps to assess individual's performance and reduces or prevents errors by structured and repetitive cueing, or by encouraging repeated assessment and self-monitoring. Complex tasks can be broken into smaller steps and directly teaching individuals using step-by-step procedures.[86] Cicerone et al.[25] have also mentioned that metacognitive strategy training facilitates the treatment of attention, memory, language deficits, and social skills.
Besides the metacognitive training, problem-solving training (PST)[87] and goal management training[88] have shown favorable outcome in posttraumatic executive function. Hewitt et al.[89] have reported that autobiographical memory queuing can improve the performance on planning tasks and can be an effective component of PST. Charters et al.[90] have recommended the use of electronic reminder systems to help daily functioning for acquired brain injured persons.
Pharmacotherapy
Dopaminergic agents bromocriptine and amantadine have been found to improve executive function of brain injured persons. McDowell et al.[91] have reported that bromocriptine can improve “cognitive initiation” (i.e., diminished motivation or apathy) in the late postinjury period. Persons who were taking a low dose of bromocriptine (2.5 mg/daily) performed better on tests of executive functioning.[91] Kraus and Maki[1] in their case series recorded positive response in executive functioning with amantadine (maximum dose was given 400 mg/day). Amantadine is also an N-methyl D-aspartate glutamate receptor antagonist, protect neural cells against excitotoxicity.[92]
Cognitive behavior therapy and family therapy
TBI can also cause a tremendous impact on emotional, behavioral stability, and self-confidence of the injured persons. Primary caregivers of persons with TBI undergo a lot of emotional stress and burden. Albert et al.[93] showed that low-cost social work liaison intervention offered benefit in reducing the caregivers burden and improving satisfaction. A study done by Sinnakarupan et al.,[94] showed that an educational program, given to caregivers and family members of a TBI, helps in reducing their distress and coping abilities.
Behavioral changes, very common after traumatic brain injuries, usually include anger, depression, agitation, and verbal or physical aggression. Emotional stability is primarily necessary, otherwise the person with TBI is unable to attend to participate and benefit from the cognitive rehabilitation processes. Psychotherapy (individual, as well as group psychotherapy) stresses on emotional, and behavioral therapy, which ultimately facilitate the training of cognition-specific interventions. Studies have shown some benefit of coping skills training and anger management in reducing aggression.[95] In a study conducted by Baker et al.,[96] music therapy has shown some promise in improving mood and anger. Pharmacotherapy also aids in the management of behavioral issues, although discussion of this in details is beyond the scope of this article.
Noninvasive brain stimulation
Demirtas-Tatlidede et al.[97] have reported that various forms of noninvasive brain stimulation (NBS) techniques including transcranial magnetic stimulation and tDCS hold promise of diagnostic and therapeutic utility to enable functional restoration in TBI. In their review, they conclude that NBS may have positive changes in mood, visuospatial functions, language and working memory, and/or executive functions. Villamar et al.[98] reported evidence from animal and human studies, revealing potential benefit of NBS in enhancing plastic changes to facilitate learning and recovery of function. However, they caution that this evidence is mainly theoretical and recommend further studies for establishing definitive role of NBS in TBI.
Comprehensive holistic rehabilitation program
Cicerone et al.[99] found TBI persons who were undergoing comprehensive holistic neuropsychological rehabilitation achieved greater improvements in community functioning compared to those who received conventional rehabilitation. Comprehensive holistic rehabilitation programme (CHRP), a combination of therapeutic services, includes individual and group therapies, psychotherapy, psychoeducation, and family therapy. The holistic neuropsychological intervention stress on metacognitive and emotional regulation technique for cognitive deficits, emotional difficulties. CHRPs facilitate skill transfer and generalization, behavioral and affective regulation, and community integration.[99]
CONCLUSION
Cognitive deficits following TBI are common and lead to significant disability and morbidity. Our review study highlights the effectiveness of rehabilitation in reducing the impact of brain injury related cognitive impairments. Novel techniques have emerged and are further evolving.
On the other hand, it must be kept in mind that rehabilitation not only encompasses cognitive limitations but also physical and psychosocial issues. Although evidence regarding the efficacy of pharmacotherapy in persons with cognitive and behavioral deficits due to TBI is scarce, they may be used to support recovery.
The complexity and the heterogeneous nature of brain injuries make it difficult to standardize treatment and rehabilitation. The development of guidelines is hindered by inadequate sample sizes and lack of appropriate comparison groups for clinical rehabilitation research. Further studies are required to arrive at the evidence based treatment protocol for cognitively impaired persons due to TBI. In a developing country like India, focus should be directed toward rehabilitation interventions that are not only effective and easily applicable but are also low cost. Increasing public awareness and attention will lead to further research and better advocacy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kraus MF, Maki PM. Effect of amantadine hydrochloride on symptoms of frontal lobe dysfunction in brain injury: Case studies and review. J Neuropsychiatry Clin Neurosci. 1997;9:222–30. doi: 10.1176/jnp.9.2.222. [DOI] [PubMed] [Google Scholar]
- 2.Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation. 2007;22:341–53. [PubMed] [Google Scholar]
- 3.Gururaj G. Epidemiology of traumatic brain injuries: Indian scenario. Neurol Res. 2002;24:24–8. doi: 10.1179/016164102101199503. [DOI] [PubMed] [Google Scholar]
- 4.De Lisa JA, Gans BM, Walsh NE. Physical Medicine and Rehabilitation: Principles and Practice. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 5.Koehler R, Wilhelm EE, Shoulson I. Cognitive Rehabilitation Therapy for Traumatic Brain Injury: Evaluating the Evidence. Washington, DC: National Academies Press; 2012. [Google Scholar]
- 6.Dikmen S, McLean A, Jr, Temkin NR, Wyler AR. Neuropsychologic outcome at one-month postinjury. Arch Phys Med Rehabil. 1986;67:507–13. [PubMed] [Google Scholar]
- 7.Boake C, Millis SR, High WM, Jr, Delmonico RL, Kreutzer JS, Rosenthal M, et al. Using early neuropsychologic testing to predict long-term productivity outcome from traumatic brain injury. Arch Phys Med Rehabil. 2001;82:761–8. doi: 10.1053/apmr.2001.23753. [DOI] [PubMed] [Google Scholar]
- 8.Kreutzer JS, Gordon WA, Rosenthal M, Marwitz J. Neuropsychological characteristics of patients with brain injury: Preliminary findings from a multicenter investigation. J Head Trauma Rehabil. 1993;8:47–59. [Google Scholar]
- 9.Skandsen T, Finnanger TG, Andersson S, Lydersen S, Brunner JF, Vik A. Cognitive impairment 3 months after moderate and severe traumatic brain injury: A prospective follow-up study. Arch Phys Med Rehabil. 2010;91:1904–13. doi: 10.1016/j.apmr.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Schretlen DJ, Shapiro AM. A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int Rev Psychiatry. 2003;15:341–9. doi: 10.1080/09540260310001606728. [DOI] [PubMed] [Google Scholar]
- 11.Vaishnavi S, Rao V, Fann JR. Neuropsychiatric problems after traumatic brain injury: Unraveling the silent epidemic. Psychosomatics. 2009;50:198–205. doi: 10.1176/appi.psy.50.3.198. [DOI] [PubMed] [Google Scholar]
- 12.Arciniegas DB, Held K, Wagner P. Cognitive Impairment Following Traumatic Brain Injury. Curr Treat Options Neurol. 2002;4:43–57. doi: 10.1007/s11940-002-0004-6. [DOI] [PubMed] [Google Scholar]
- 13.Tsaousides T, Gordon WA. Cognitive rehabilitation following traumatic brain injury: Assessment to treatment. Mt Sinai J Med. 2009;76:173–81. doi: 10.1002/msj.20099. [DOI] [PubMed] [Google Scholar]
- 14.Conners C. Conners' Continuous Performance Test (CPT II) Computer Programs for Windows Technical Guide and Software Manual. North Tonawanda: Multi-Health Systems; 2000. [Google Scholar]
- 15.Cullen NK, Weisz K. Cognitive correlates with functional outcomes after anoxic brain injury: A case-controlled comparison with traumatic brain injury. Brain Inj. 2011;25:35–43. doi: 10.3109/02699052.2010.531691. [DOI] [PubMed] [Google Scholar]
- 16.Neese LE, Caroselli JS, Klaas P, High WM, Jr, Becker LJ, Scheibel RS. Neuropsychological assessment and the Disability Rating Scale (DRS): A concurrent validity study. Brain Inj. 2000;14:719–24. doi: 10.1080/026990500413740. [DOI] [PubMed] [Google Scholar]
- 17.Hanks RA, Millis SR, Ricker JH, Giacino JT, Nakese-Richardson R, Frol AB, et al. The predictive validity of a brief inpatient neuropsychologic battery for persons with traumatic brain injury. Arch Phys Med Rehabil. 2008;89:950–7. doi: 10.1016/j.apmr.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Silver JM, McAllister TW, Arciniegas DB. Depression and cognitive complaints following mild traumatic brain injury. Am J Psychiatry. 2009;166:653–61. doi: 10.1176/appi.ajp.2009.08111676. [DOI] [PubMed] [Google Scholar]
- 19.Silver JM, Yudofsky SC, Hales RE. Neuropsychiatry of Traumatic Brain Injury. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 20.Halbauer JD, Ashford JW, Zeitzer JM, Adamson MM, Lew HL, Yesavage JA. Neuropsychiatric diagnosis and management of chronic sequelae of war-related mild to moderate traumatic brain injury. J Rehabil Res Dev. 2009;46:757–96. doi: 10.1682/jrrd.2008.08.0119. [DOI] [PubMed] [Google Scholar]
- 21.Park NW, Ingles JL. Effectiveness of attention rehabilitation after an acquired brain injury: A meta-analysis. Neuropsychology. 2001;15:199–210. doi: 10.1037//0894-4105.15.2.199. [DOI] [PubMed] [Google Scholar]
- 22.Sohlberg MM, Mateer CA. Effectiveness of an attention-training program. J Clin Exp Neuropsychol. 1987;9:117–30. doi: 10.1080/01688638708405352. [DOI] [PubMed] [Google Scholar]
- 23.Tiersky LA, Anselmi V, Johnston MV, Kurtyka J, Roosen E, Schwartz T, et al. A trial of neuropsychologic rehabilitation in mild-spectrum traumatic brain injury. Arch Phys Med Rehabil. 2005;86:1565–74. doi: 10.1016/j.apmr.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Westerberg H, Jacobaeus H, Hirvikoski T, Clevberger P, Ostensson ML, Bartfai A, et al. Computerized working memory training after stroke — A pilot study. Brain Inj. 2007;21:21–9. doi: 10.1080/02699050601148726. [DOI] [PubMed] [Google Scholar]
- 25.Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, et al. Evidence-based cognitive rehabilitation: Updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil. 2011;92:519–30. doi: 10.1016/j.apmr.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Cicerone KD, Dahlberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S, et al. Evidence-based cognitive rehabilitation: Updated review of the literature from 1998 through 2002. Arch Phys Med Rehabil. 2005;86:1681–92. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 27.Giacino JT, Whyte J, Bagiella E, Kalmar K, Childs N, Khademi A, et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med. 2012;366:819–26. doi: 10.1056/NEJMoa1102609. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Whyte J, Patel S, Europa E, Wang J, Coslett HB, et al. Methylphenidate modulates sustained attention and cortical activation in survivors of traumatic brain injury: A perfusion fMRI study. Psychopharmacology (Berl) 2012;222:47–57. doi: 10.1007/s00213-011-2622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willmott C, Ponsford J. Efficacy of methylphenidate in the rehabilitation of attention following traumatic brain injury: A randomised, crossover, double blind, placebo controlled inpatient trial. J Neurol Neurosurg Psychiatry. 2009;80:552–7. doi: 10.1136/jnnp.2008.159632. [DOI] [PubMed] [Google Scholar]
- 30.Kim YH, Ko MH, Na SY, Park SH, Kim KW. Effects of single-dose methylphenidate on cognitive performance in patients with traumatic brain injury: A double-blind placebo-controlled study. Clin Rehabil. 2006;20:24–30. doi: 10.1191/0269215506cr927oa. [DOI] [PubMed] [Google Scholar]
- 31.Rees L, Marshall S, Hartridge C, Mackie D, Weiser M. Erabi Group. Cognitive interventions post acquired brain injury. Brain Inj. 2007;21:161–200. doi: 10.1080/02699050701201813. [DOI] [PubMed] [Google Scholar]
- 32.Cappa SF, Benke T, Clarke S, Rossi B, Stemmer B, van Heugten CM. Task Force on Cognitive Rehabilitation; European Federation of Neurological Societies. EFNS guidelines on cognitive rehabilitation: Report of an EFNS task force. Eur J Neurol. 2005;12:665–80. doi: 10.1111/j.1468-1331.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- 33.Cicerone KD, Dahlberg C, Kalmar K, Langenbahn DM, Malec JF, Bergquist TF, et al. Evidence-based cognitive rehabilitation: Recommendations for clinical practice. Arch Phys Med Rehabil. 2000;81:1596–615. doi: 10.1053/apmr.2000.19240. [DOI] [PubMed] [Google Scholar]
- 34.Dou ZL, Man DW, Ou HN, Zheng JL, Tam SF. Computerized errorless learning-based memory rehabilitation for Chinese patients with brain injury: A preliminary quasi-experimental clinical design study. Brain Inj. 2006;20:219–25. doi: 10.1080/02699050500488215. [DOI] [PubMed] [Google Scholar]
- 35.Ehlhardt LA, Sohlberg MM, Glang A, Albin R. TEACH-M: A pilot study evaluating an instructional sequence for persons with impaired memory and executive functions. Brain Inj. 2005;19:569–83. doi: 10.1080/002699050400013550. [DOI] [PubMed] [Google Scholar]
- 36.Clare L, Jones RS. Errorless learning in the rehabilitation of memory impairment: A critical review. Neuropsychol Rev. 2008;18:1–23. doi: 10.1007/s11065-008-9051-4. [DOI] [PubMed] [Google Scholar]
- 37.Landis J, Hanten G, Levin HS, Li X, Ewing-Cobbs L, Duron J, et al. Evaluation of the errorless learning technique in children with traumatic brain injury. Arch Phys Med Rehabil. 2006;87:799–805. doi: 10.1016/j.apmr.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Bergquist T, Gehl C, Mandrekar J, Lepore S, Hanna S, Osten A, et al. The effect of internet-based cognitive rehabilitation in persons with memory impairments after severe traumatic brain injury. Brain Inj. 2009;23:790–9. doi: 10.1080/02699050903196688. [DOI] [PubMed] [Google Scholar]
- 39.Chen SH, Thomas JD, Glueckauf RL, Bracy OL. The effectiveness of computer-assisted cognitive rehabilitation for persons with traumatic brain injury. Brain Inj. 1997;11:197–209. doi: 10.1080/026990597123647. [DOI] [PubMed] [Google Scholar]
- 40.Tam SF, Man WK. Evaluating computer-assisted memory retraining programmes for people with post-head injury amnesia. Brain Inj. 2004;18:461–70. doi: 10.1080/02699050310001646099. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Plotkin RC, Wang G, Sandel ME, Lee S. Cholinergic augmentation with donepezil enhances recovery in short-term memory and sustained attention after traumatic brain injury. Arch Phys Med Rehabil. 2004;85:1050–5. doi: 10.1016/j.apmr.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Silver JM, Koumaras B, Chen M, Mirski D, Potkin SG, Reyes P, et al. Effects of rivastigmine on cognitive function in patients with traumatic brain injury. Neurology. 2006;67:748–55. doi: 10.1212/01.wnl.0000234062.98062.e9. [DOI] [PubMed] [Google Scholar]
- 43.Neurobehavioral Guidelines Working Group. Warden DL, Gordon B, McAllister TW, Silver JM, Barth JT, et al. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J Neurotrauma. 2006;23:1468–501. doi: 10.1089/neu.2006.23.1468. [DOI] [PubMed] [Google Scholar]
- 44.Levin HS. Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci. 1991;103(Suppl):S39–42. doi: 10.1016/0022-510x(91)90007-t. [DOI] [PubMed] [Google Scholar]
- 45.Zafonte RD, Bagiella E, Ansel BM, Novack TA, Friedewald WT, Hesdorffer DC, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT) JAMA. 2012;308:1993–2000. doi: 10.1001/jama.2012.13256. [DOI] [PubMed] [Google Scholar]
- 46.Aniruddha TJ, Pillai S, Devi BI, Sampath S, Chandramouli BA. Role of citicoline in the management of mild head injury. Indian J Neurotrauma. 2009;6:49–52. [Google Scholar]
- 47.McKenna K, Cooke DM, Fleming J, Jefferson A, Ogden S. The incidence of visual perceptual impairment in patients with severe traumatic brain injury. Brain Inj. 2006;20:507–18. doi: 10.1080/02699050600664368. [DOI] [PubMed] [Google Scholar]
- 48.Poggel DA, Kasten E, Sabel BA. Attentional cueing improves vision restoration therapy in patients with visual field defects. Neurology. 2004;63:2069–76. doi: 10.1212/01.wnl.0000145773.26378.e5. [DOI] [PubMed] [Google Scholar]
- 49.Mueller I, Mast H, Sabel BA. Recovery of visual field defects: A large clinical observational study using vision restoration therapy. Restor Neurol Neurosci. 2007;25:563–72. [PubMed] [Google Scholar]
- 50.Mueller I, Poggel D, Kenkel S, Kasten E, Sabel B. Vision restoration therapy after brain damage: Subjective improvements of activities of daily life and their relationship to visual field enlargements. Vis Impair Res. 2003;5:157–78. [Google Scholar]
- 51.Pizzamiglio L, Fasotti L, Jehkonen M, Antonucci G, Magnotti L, Boelen D, et al. The use of optokinetic stimulation in rehabilitation of the hemineglect disorder. Cortex. 2004;40:441–50. doi: 10.1016/s0010-9452(08)70138-2. [DOI] [PubMed] [Google Scholar]
- 52.Marshall RS. Rehabilitation approaches to hemineglect. Neurologist. 2009;15:185–92. doi: 10.1097/NRL.0b013e3181942894. [DOI] [PubMed] [Google Scholar]
- 53.Katz N, Fleming J, Keren N, Lightbody S, Hartman-Maeir A. Unawareness and/or denial of disability: Implications for occupational therapy intervention. Can J Occup Ther. 2002;69:281–92. doi: 10.1177/000841740206900504. [DOI] [PubMed] [Google Scholar]
- 54.Lloréns R, Navarro MD, Alcañiz M, Noé E. Therapeutic effectiveness of a virtual reality game in self-awareness after acquired brain injury. Stud Health Technol Inform. 2012;181:297–301. [PubMed] [Google Scholar]
- 55.Bart O, Agam T, Weiss PL, Kizony R. Using video-capture virtual reality for children with acquired brain injury. Disabil Rehabil. 2011;33:1579–86. doi: 10.3109/09638288.2010.540291. [DOI] [PubMed] [Google Scholar]
- 56.Mumford N, Wilson PH. Virtual reality in acquired brain injury upper limb rehabilitation: Evidence-based evaluation of clinical research. Brain Inj. 2009;23:179–91. doi: 10.1080/02699050802695566. [DOI] [PubMed] [Google Scholar]
- 57.Fong KN, Chow KY, Chan BC, Lam KC, Lee JC, Li TH, et al. Usability of a virtual reality environment simulating an automated teller machine for assessing and training persons with acquired brain injury. J Neuroeng Rehabil. 2010;7:19. doi: 10.1186/1743-0003-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ozbudak Demir S, Görgülü G, Köseoglu F. Comparison of rehabilitation outcome in patients with aphasic and non-aphasic traumatic brain injury. J Rehabil Med. 2006;38:68–71. doi: 10.1080/16501970510041262. [DOI] [PubMed] [Google Scholar]
- 59.Morgan AT, Mageandran SD, Mei C. Incidence and clinical presentation of dysarthria and dysphagia in the acute setting following paediatric traumatic brain injury. Child Care Health Dev. 2010;36:44–53. doi: 10.1111/j.1365-2214.2009.00961.x. [DOI] [PubMed] [Google Scholar]
- 60.Kempf S, Lauer N, Corsten S, Voigt-Radloff S. Potential analysis of research on speech therapy-led communication training in aphasia following stroke. Z Evid Fortbild Qual Gesundhwes. 2014;108(Suppl 1):S45–52. doi: 10.1016/j.zefq.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 61.Larkins B. The application of the ICF in cognitive-communication disorders following traumatic brain injury. Semin Speech Lang. 2007;28:334–42. doi: 10.1055/s-2007-986530. [DOI] [PubMed] [Google Scholar]
- 62.Godfrey HP, Knight RG, Marsh NV, Moroney B, Bishara SN. Social interaction and speed of information processing following very severe head-injury. Psychol Med. 1989;19:175–82. doi: 10.1017/s0033291700011120. [DOI] [PubMed] [Google Scholar]
- 63.Docking K, Murdoch BE, Jordan FM. Interpretation and comprehension of linguistic humour by adolescents with head injury: A group analysis. Brain Inj. 2000;14:89–108. doi: 10.1080/026990500120952. [DOI] [PubMed] [Google Scholar]
- 64.Demir SO, Altinok N, Aydin G, Köseoglu F. Functional and cognitive progress in aphasic patients with traumatic brain injury during post-acute phase. Brain Inj. 2006;20:1383–90. doi: 10.1080/02699050601081844. [DOI] [PubMed] [Google Scholar]
- 65.Meinzer M, Djundja D, Barthel G, Elbert T, Rockstroh B. Long-term stability of improved language functions in chronic aphasia after constraint-induced aphasia therapy. Stroke. 2005;36:1462–6. doi: 10.1161/01.STR.0000169941.29831.2a. [DOI] [PubMed] [Google Scholar]
- 66.Pulvermüller F, Neininger B, Elbert T, Mohr B, Rockstroh B, Koebbel P, et al. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32:1621–6. doi: 10.1161/01.str.32.7.1621. [DOI] [PubMed] [Google Scholar]
- 67.Sickert A, Anders LC, Münte TF, Sailer M. Constraint-induced aphasia therapy following sub-acute stroke: A single-blind, randomised clinical trial of a modified therapy schedule. J Neurol Neurosurg Psychiatry. 2014;85:51–5. doi: 10.1136/jnnp-2012-304297. [DOI] [PubMed] [Google Scholar]
- 68.Laganaro M, Di Pietro M, Schnider A. Computerised treatment of anomia in acute aphasia: Treatment intensity and training size. Neuropsychol Rehabil. 2006;16:630–40. doi: 10.1080/09602010543000064. [DOI] [PubMed] [Google Scholar]
- 69.Nicholas M, Sinotte M, Helm-Estabrooks N. Using a computer to communicate: Effect of executive function impairments in people with severe aphasia. Aphasiology. 2005;19:1052–65. [Google Scholar]
- 70.Cherney LR, Halper AS, Holland AL, Cole R. Computerized script training for aphasia: Preliminary results. Am J Speech Lang Pathol. 2008;17:19–34. doi: 10.1044/1058-0360(2008/003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anastasia MR. Computerised training for impairments of word comprehension and retrieval in aphasia. Aphasiology. 2006;20:257–68. [Google Scholar]
- 72.Belin P, Van Eeckhout P, Zilbovicius M, Remy P, François C, Guillaume S, et al. Recovery from nonfluent aphasia after melodic intonation therapy: A PET study. Neurology. 1996;47:1504–11. doi: 10.1212/wnl.47.6.1504. [DOI] [PubMed] [Google Scholar]
- 73.Zumbansen A, Peretz I, Hébert S. Melodic intonation therapy: Back to basics for future research. Front Neurol. 2014;5:7. doi: 10.3389/fneur.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schlaug G, Marchina S, Norton A. From singing to speaking: Why singing may lead to recovery of expressive language function in patients with Broca's Aphasia. Music Percept. 2008;25:315–323. doi: 10.1525/MP.2008.25.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Aguiar V, Paolazzi CL, Miceli G. tDCS in post-stroke aphasia: The role of stimulation parameters, behavioral treatment and patient characteristics. Cortex. 2015;63:296–316. doi: 10.1016/j.cortex.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 76.Wenke RJ, Theodoros D, Cornwell P. The short- and long-term effectiveness of the LSVT for dysarthria following TBI and stroke. Brain Inj. 2008;22:339–52. doi: 10.1080/02699050801960987. [DOI] [PubMed] [Google Scholar]
- 77.Dahlberg CA, Cusick CP, Hawley LA, Newman JK, Morey CE, Harrison-Felix CL, et al. Treatment efficacy of social communication skills training after traumatic brain injury: A randomized treatment and deferred treatment controlled trial. Arch Phys Med Rehabil. 2007;88:1561–73. doi: 10.1016/j.apmr.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 78.McDonald S, Tate R, Togher L, Bornhofen C, Long E, Gertler P, et al. Social skills treatment for people with severe, chronic acquired brain injuries: A multicenter trial. Arch Phys Med Rehabil. 2008;89:1648–59. doi: 10.1016/j.apmr.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 79.Egan J, Worrall L, Oxenham D. An Internet training intervention for people with traumatic brain injury: Barriers and outcomes. Brain Inj. 2005;19:555–68. doi: 10.1080/02699050400013659. [DOI] [PubMed] [Google Scholar]
- 80.Bornhofen C, McDonald S. Comparing strategies for treating emotion perception deficits in traumatic brain injury. J Head Trauma Rehabil. 2008;23:103–15. doi: 10.1097/01.HTR.0000314529.22777.43. [DOI] [PubMed] [Google Scholar]
- 81.Smania N, Aglioti SM, Girardi F, Tinazzi M, Fiaschi A, Cosentino A, et al. Rehabilitation of limb apraxia improves daily life activities in patients with stroke. Neurology. 2006;67:2050–2. doi: 10.1212/01.wnl.0000247279.63483.1f. [DOI] [PubMed] [Google Scholar]
- 82.Smania N, Girardi F, Domenicali C, Lora E, Aglioti S. The rehabilitation of limb apraxia: A study in left-brain-damaged patients. Arch Phys Med Rehabil. 2000;81:379–88. doi: 10.1053/mr.2000.6921. [DOI] [PubMed] [Google Scholar]
- 83.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- 84.Goverover Y, Johnston MV, Toglia J, Deluca J. Treatment to improve self-awareness in persons with acquired brain injury. Brain Inj. 2007;21:913–23. doi: 10.1080/02699050701553205. [DOI] [PubMed] [Google Scholar]
- 85.Cheng SK, Man DW. Management of impaired self-awareness in persons with traumatic brain injury. Brain Inj. 2006;20:621–8. doi: 10.1080/02699050600677196. [DOI] [PubMed] [Google Scholar]
- 86.Kennedy MR, Coelho C, Turkstra L, Ylvisaker M, Moore SM, Yorkston K, et al. Intervention for executive functions after traumatic brain injury: A systematic review, meta-analysis and clinical recommendations. Neuropsychol Rehabil. 2008;18:257–99. doi: 10.1080/09602010701748644. [DOI] [PubMed] [Google Scholar]
- 87.von Cramon D, Matthes-von Cramon G, Mai N. Problem-solving deficits in brain-injured patients: A therapeutic approach. Neuropsychol Rehabil. 1991;1:45–64. [Google Scholar]
- 88.Levine B, Robertson IH, Clare L, Carter G, Hong J, Wilson BA, et al. Rehabilitation of executive functioning: An experimental-clinical validation of goal management training. J Int Neuropsychol Soc. 2000;6:299–312. doi: 10.1017/s1355617700633052. [DOI] [PubMed] [Google Scholar]
- 89.Hewitt J, Evans JJ, Dritschel B. Theory driven rehabilitation of executive functioning: Improving planning skills in people with traumatic brain injury through the use of an autobiographical episodic memory cueing procedure. Neuropsychologia. 2006;44:1468–74. doi: 10.1016/j.neuropsychologia.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 90.Charters E, Gillett L, Simpson GK. Efficacy of electronic portable assistive devices for people with acquired brain injury: A systematic review. Neuropsychol Rehabil. 2015;25:82–121. doi: 10.1080/09602011.2014.942672. [DOI] [PubMed] [Google Scholar]
- 91.McDowell S, Whyte J, D'Esposito M. Differential effect of a dopaminergic agonist on prefrontal function in traumatic brain injury patients. Brain. 1998;121(Pt 6):1155–64. doi: 10.1093/brain/121.6.1155. [DOI] [PubMed] [Google Scholar]
- 92.Nickels JL, Schneider WN, Dombovy ML, Wong TM. Clinical use of amantadine in brain injury rehabilitation. Brain Inj. 1994;8:709–18. doi: 10.3109/02699059409151025. [DOI] [PubMed] [Google Scholar]
- 93.Albert SM, Im A, Brenner L, Smith M, Waxman R. Effect of a social work liaison program on family caregivers to people with brain injury. J Head Trauma Rehabil. 2002;17:175–89. doi: 10.1097/00001199-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 94.Sinnakaruppan I, Downey B, Morrison S. Head injury and family carers: A pilot study to investigate an innovative community-based educational programme for family carers and patients. Brain Inj. 2005;19:283–308. doi: 10.1080/02699050400003924. [DOI] [PubMed] [Google Scholar]
- 95.O'Leary CA. Reducing aggression in adults with brain injuries. Behav Interv. 2000;15:205–16. [Google Scholar]
- 96.Baker F, Wigram T, Gold C. The effects of a song-singing programme on the affective speaking intonation of people with traumatic brain injury. Brain Inj. 2005;19:519–28. doi: 10.1080/02699050400005150. [DOI] [PubMed] [Google Scholar]
- 97.Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Bernabeu M, Tormos JM, Pascual-Leone A. Noninvasive brain stimulation in traumatic brain injury. J Head Trauma Rehabil. 2012;27:274–92. doi: 10.1097/HTR.0b013e318217df55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Villamar MF, Santos Portilla A, Fregni F, Zafonte R. Noninvasive brain stimulation to modulate neuroplasticity in traumatic brain injury. Neuromodulation. 2012;15:326–38. doi: 10.1111/j.1525-1403.2012.00474.x. [DOI] [PubMed] [Google Scholar]
- 99.Cicerone KD, Mott T, Azulay J, Sharlow-Galella MA, Ellmo WJ, Paradise S, et al. A randomized controlled trial of holistic neuropsychologic rehabilitation after traumatic brain injury. Arch Phys Med Rehabil. 2008;89:2239–49. doi: 10.1016/j.apmr.2008.06.017. [DOI] [PubMed] [Google Scholar]


