Abstract
Introduction:
Data on effect of clozapine on metabolic syndrome in adolescent patients with psychosis are limited. This study aimed to evaluate the prevalence and incidence of metabolic syndrome in children and adolescents with psychotic disorders prior to clozapine and while receiving clozapine. Secondary aims were to study the effectiveness and side effect profile of clozapine.
Materials and Methods:
Thirteen child and adolescent patients were evaluated at baseline, 3 months, and a follow-up beyond 6 months. Assessments were made for metabolic profile, effectiveness by positive and negative syndrome scale (PANSS), and side effects.
Results:
Prior to starting of clozapine, the prevalence of metabolic syndrome was 23%. After 3 months on clozapine, 38.5% (5/13) patients fulfilled criteria of metabolic syndrome and further on follow-up beyond 6 months (with last observation carried forward) 46.2% (6/13) had developed metabolic syndrome. There was a significant reduction in PANSS scores at 3 months and follow-up more so in those who developed metabolic syndrome at 3 months. Among the other side effects, hypersalivation was the most common side effect (100%) followed by sedation (69%).
Conclusion:
Half the prevalence of metabolic syndrome in adolescents on clozapine can be attributed to other factors prior to starting of clozapine, and another half can be attributed to clozapine. Clozapine is effective in an adolescent population.
Keywords: Adolescent, clozapine, metabolic syndrome, schizophrenia
INTRODUCTION
Clozapine, an effective antipsychotic for management of psychosis particularly treatment-resistant schizophrenia in adults[1] is often indicated in early onset schizophrenia after the failure of two antipsychotic trials.[2,3,4] Long-term studies of 12 weeks to 9 years have shown significant clinical improvement with clozapine in children and adolescents.[5,6,7,8]
In recent times, there is an increase in the awareness of metabolic side effects associated with various antipsychotics. Taking this into consideration various associations have come up with guidelines with regard to monitoring of metabolic side effects while a patient is on antipsychotics.[9,10] Clozapine is considered to have the highest risk for the development of metabolic syndrome. Studies in adult population have reported the prevalence rate of metabolic syndrome to vary from 11% to 64% in patients receiving clozapine.[11] However, some of the studies have shown that all the metabolic syndromes in patients receiving clozapine must not be attributed to clozapine, as many patients, in fact, have metabolic syndrome prior to starting of clozapine.[12,13]
Despite the availability of several studies in adults, no study in children and adolescent has evaluated the prevalence of metabolic syndrome while receiving clozapine. However, several studies in children and adolescents report significant weight gain,[5,14] hypertriglyceridemia (defined at triglycerides >125 mg/dl), and prediabetes (fasting blood sugar >100) status in children and adolescent treated with clozapine.[15] Another study which reported US Food and Drug Administration data over 8 years, showed that 11 adolescents on clozapine (dose range 100-1000 mg) developed hyperglycemia, all within 6 months of clozapine initiation.[16] It has suggested that initial signs such as weight gain manifest in the first 3 months on clozapine, but lipid abnormalities may develop at a later stage. Hence longer duration studies are required in children and adolescents.[17]
Besides the metabolic side effects, the side effect profile of clozapine remains understudied in children and adolescent population. A recent review of literature on efficacy and tolerability of clozapine in children and adolescents reported high incidence rates of hypersalivation (80-90%), sedation (56-90%), constipation (13-50%), enuresis (15-61%), akathisia (15-31%), seizures (3%), neutropenia (6-15%), tachycardia (35%), hypotension (12.5%), and hypertension (6%).[18]
It is suggested that because of the scare of side effects of clozapine, it is generally underused, especially in children and adolescents.[18,19] As there is limited literature on metabolic disturbances with clozapine in children and adolescents and there are no studies on the prevalence of metabolic syndrome, there is a need to study this association of clozapine and metabolic syndrome further. Accordingly, the aim of this paper is to evaluate the data of adolescent patients initiated on clozapine and followed up prospectively and to assess their metabolic profile at baseline (preclozapine), 3 months after clozapine therapy, and further at follow-up beyond 6 months duration. Our secondary aim was to study the effectiveness and other adverse events associated with the use of clozapine in adolescents.
MATERIALS AND METHODS
This study was done in a Multi-Specialty Tertiary Care Hospital in North India after obtaining approval from the Institute Ethics Committee. This study included patients of all age groups started on clozapine during the study period. However, in this paper we present the data pertaining to adolescents aged ≤19 years only.
This study followed a naturalistic prospective design. No interference was done with respect to the decision to start clozapine. However, those patients who were considered for clozapine by their treating psychiatrists were monitored for side effects and effectiveness. No treatment decisions were altered as part of the study. All the patients considered for clozapine were informed about the study, and informed consent was obtained from the patients and/or the parents.
In our setting clozapine is usually considered for children and adolescents who have treatment-resistant schizophrenia. Clozapine is usually started in the inpatient setting after detailed evaluation. Patients who are started on clozapine usually remain in the inpatient setting for the duration of 1-3 months. All the patients started on clozapine undergo baseline assessment in the form of recording of anthropometry and psychopathology on positive and negative symptom scale (PANSS). Patients and also undergo investigations in the form of assessment of heamogram, liver function test, renal function test, serum electrolytes, electrocardiogram, fasting blood sugar levels, lipid profile, electroencephalogram, and other investigations as per the requirement.
For this study children and adolescents who were started on clozapine in inpatient or outpatient setting between the periods of March 2010 and July 2013 were assessed at the baseline and followed up prospectively. The metabolic parameters were repeated at 3 months period and whenever possible after this period. Other side effects encountered during the continuation of clozapine were documented.
Metabolic syndrome was diagnosed as per the modified National Cholesterol Education Program-Adult Treatment Panel-III criteria.[20] Statistical Package for the Social Science Version 14 (SPSS for Windows, Version 14.0. Chicago, SPSS Inc.) was used for analysis. Frequencies with percentages were calculated for the categorical variables. Mean and standard deviation (SD) were computed for the continuous variables. Comparisons were done by using Pearson's Chi-square test (with and without Yate's correction), Fischer exact test, paired t-test, and repeat measure ANOVA test. Last observation carried forward (LOCF) analysis was used to study the prevalence of metabolic syndrome in case the patients dropped out of follow-up after 3 months.
RESULTS
A total of 13 adolescents were started on clozapine during the study and females slightly outnumbered males (53.7% vs. 46.3%). The mean age of the study sample was 17 (SD = 1.22) years with an age range of 16-19 years. Majority of the patients were Hindu by religion (69.23%) and were studying (61.5%). Majority of them were from nuclear families (84.61%), of urban background (61.53%) and had a family income of more than 6000 rupees per month (69.23%).
The mean age of onset of psychiatric illness was 15.38 (SD = 1.71) years, and the mean duration of illness was 1.61 (SD = 1.19) years. Majority (n = 10; 76.9%) were diagnosed to have schizophrenia, two patients were diagnosed to have psychosis NOS (15.4%), and one patient was diagnosed to have schizoaffective disorder (7.7%). In term of subtypes of schizophrenia, most common subtype was that of undifferentiated schizophrenia (n = 7; 53.8%), followed by paranoid schizophrenia (n = 2; 15.4%), and catatonic schizophrenia (n = 1; 7.7%). Three patients (23%) had a history of comorbid mental retardation and one among them had a diagnosis of attention deficit hyperactive disorder as well. In terms of severe adverse effects with antipsychotic, 1 (7.7%) patient had past history of neuroleptic malignant syndrome.
Irrespective of the primary diagnosis, all patients had received two or more adequate antipsychotic trials, each of at least 8-12 weeks duration with the therapeutic dose of specified antipsychotic medication. Family history of hypertension was present in 15.4% (n = 2) of cases. Few patients had family history of diabetes mellitus (n = 1; 7.7%) and cerebrovascular accident (n = 1; 7.7%).
Baseline metabolic profile
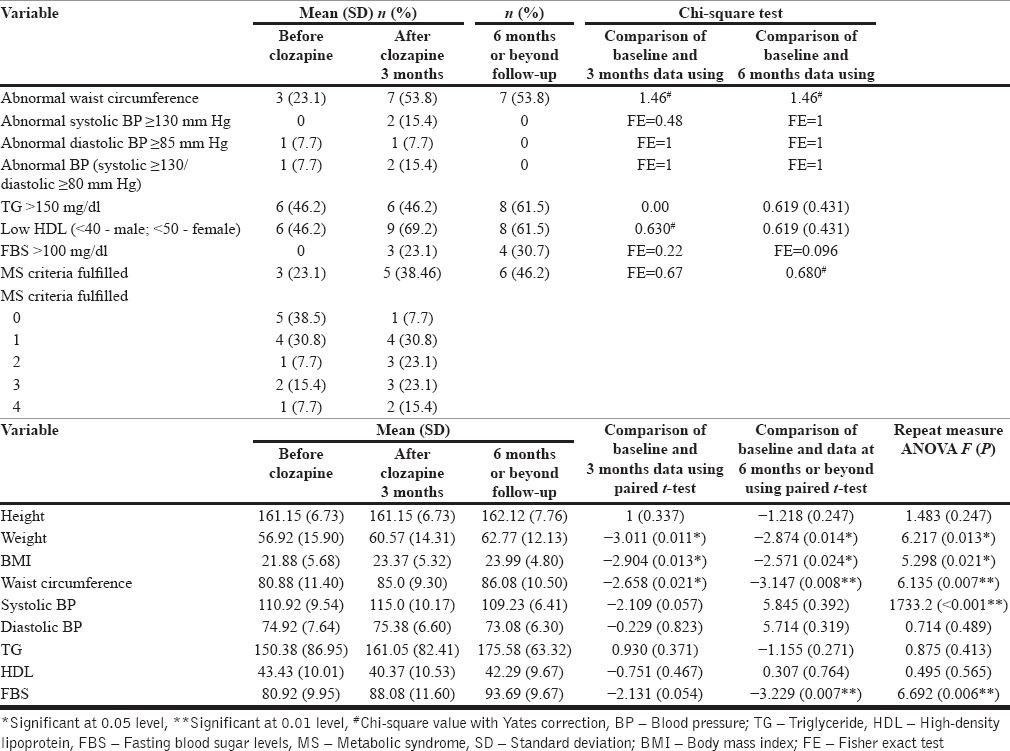
As shown in Table 1, 3 (23%) patients fulfilled the diagnosis of metabolic syndrome at the baseline, with 46.2% (n = 6) having abnormal triglyceride and high-density lipoprotein (HDL) levels. None of the patients had abnormal fasting blood sugar level at the baseline.
Table 1.
Metabolic profile of adolescents on clozapine at baseline, after 3 months, follow-up beyond 6 months (n = 13)
The mean dose of clozapine used in the patients was 256.7 (SD = 99.3) mg/day with a range of 75-400 mg, with the majority (n = 8; 61.5%) of the patients receiving a dose between 200 and 350 mg/day at 3 months assessment.
Follow-up data at 3 months after starting clozapine were available for all the patients (n = 13), and follow-up data at or beyond 6 months were available for nine patients (69.2%) with longest follow-up data available for 30 months and mean duration of follow-up of 15.3 (SD = 7.4) months.
As is evident from Table 1, by 3 months, 9 (69.2%) patients had abnormal HDL levels, and 7 (53.8%) had abnormal waist circumference. When compared to the baseline data, at 3 months, there was an increase in the number of patients fulfilling the various criteria of metabolic syndrome, but none of the difference was statistically significant. In terms of absolute value, there was significant increase in the body weight, body mass index, and waist circumference. The trend for an increase in systolic blood pressure and fasting blood glucose levels were also seen. However, the significant values for these were 0.057 and 0.054, respectively.
The prevalence of metabolic syndrome increased to 38.5% (n = 5) at 3 months follow-up, but the difference between baseline and 3 months assessment was not significant. When we looked at the data further, it was evident that none of the patients who had metabolic syndrome at the baseline had a reversal of the same and 2 (15.4%) new cases of metabolic syndrome were noticed while the patients were on clozapine during the first 3 months. Accordingly the incidence of metabolic syndrome with clozapine at 3 months was 15.4%.
Out of the 13 patients, 4 patients dropped out of treatment after 6 months. Unfortunately, the metabolic profile of these patients was not available after the 3 month assessment. The metabolic profile of remaining nine patients was repeated after a mean duration of 15.3 months with a range of 6-30 months. Of the four patients who dropped out of treatment 3 were positive for metabolic syndrome at 3 months assessment.
As shown in Table 2, there was a secular trend of an increase in the weight, body mass index, waist circumference, fasting blood glucose, and triglyceride levels from baseline to follow-up duration. However, there was a reduction in the blood pressure levels after the 3 months follow-up. Similarly, there was an increase in the levels of HDL after the 3 months follow-up. In terms of statistical difference, there was a significant increase in the waist circumference, and fasting blood glucose level over the period and there was a significant reduction in the systolic blood pressure.
Table 2.
Metabolic profile at 3 months and follow-up beyond 6 months (n = 9)

Out of the nine patients, available at all the three assessments, only 11.1 % (n = 1) patients met the criteria of metabolic syndrome at the baseline which increased to 22.2% (n = 2) at 3 months follow-up and 33.3% (n = 3) at 6 months or beyond follow-up. It was further seen that those patients who fulfilled one or more criteria of metabolic syndrome at baseline went on to develop metabolic syndrome later on.
Further analysis of data revealed that out of the two patients who fulfilled metabolic syndrome at 3 months follow-up, 1 had a reversal of metabolic syndrome and actually there were 2 new cases of metabolic syndrome during the follow-up period of 6 months and beyond.
Overall it was seen that in total 7 (53.84%) patients fulfilled metabolic syndrome while on clozapine. Of this 4 (30.76%) were new cases of metabolic syndrome while on clozapine. Of this, one patient had a reversal of metabolic syndrome while on continued use of clozapine.
Overall we compared all the three assessments by using the absolute number of patients available at a particular assessment and also by using the LOCF method for all the 13 patients. As is evident from the Tables 1 and 2, there was no significant increase in the presence or absence of each component of metabolic syndrome at the follow-up assessments, nor was there a significant increase in the prevalence of metabolic syndrome. However, there was a secular trend of increase in body weight, body mass index, waist circumference, and fasting blood sugar during follow-up. There was a significant variance in the systolic blood pressure too; however, there was no secular trend for this change. When the data of nine patients for all the three assessments was analyzed, a significant reduction in number of patients with abnormal HDL was seen between the 3 months assessment and assessment at 6 months or beyond.
Effectiveness of clozapine
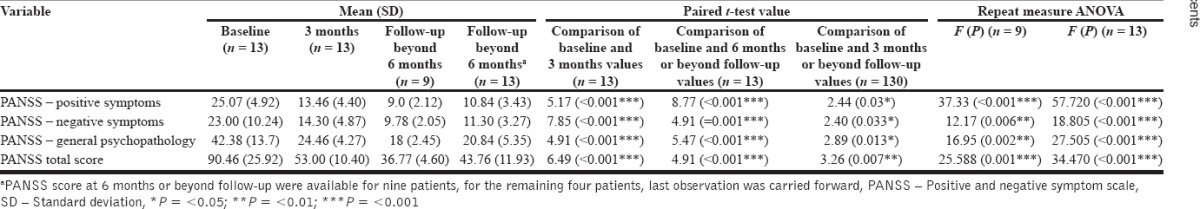
As is evident from Table 3, there was a significant reduction in the PANSS positive, negative, and general psychopathology scores at 3 months and further follow-up. When further comparisons were made by using paired t-test it was seen that compared to baseline, there was a significant reduction in all subscales and total PANSS score at 3 months and at further follow-up.
Table 3.
Effectiveness of clozapine at 3 months and further follow-up
Other adverse events with clozapine
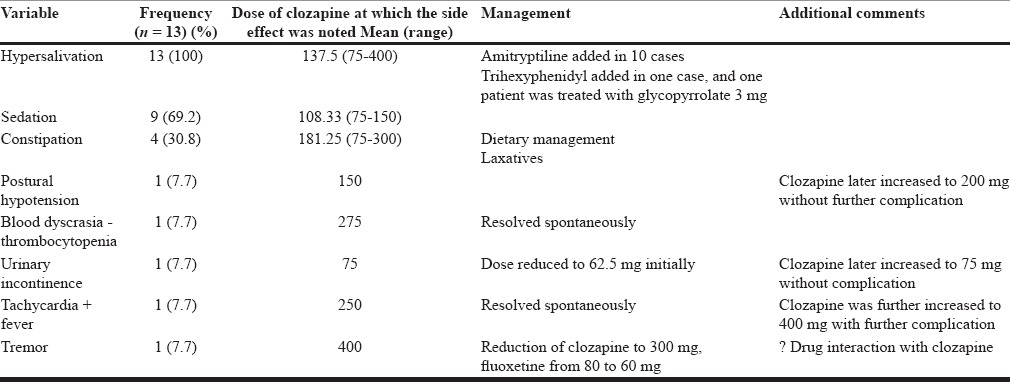
As is evident from Table 4, the incidence of hypersalivation was 100%, and that of sedation was 69%. One patient each experienced transient thrombocytopenia (platelet count <100,000), postural hypotension, urinary incontinence, fever with tachycardia, and tremor. None of these side effects required discontinuation of clozapine. As is evident from the Table 4, most of the side effects started at the dose of 75 mg/day or at a higher dose.
Table 4.
Side effects with clozapine (n = 13)
Relationship of metabolic syndrome with demographic and clinical variables
There was no difference in the age, gender, locality, age at onset, duration of illness between those who did not have metabolic syndrome and those who had metabolic syndrome at 3 months, at follow-up at a later date.
Relationship of metabolic syndrome and dose of clozapine
When the mean dose of clozapine received by those who had metabolic syndrome (295.0 [SD = 99.05] mg/day) at 3 months and those did not have metabolic syndrome (232.8 [SD = 97.95] mg/day) was compared, no significant difference was found (t = 1.1; P = 0.29). There was no difference in the dose of clozapine between those found to have metabolic syndrome and not found to have metabolic syndrome at 6 months and beyond (191.67 [SD = 141.15] vs. 285.42 [SD = 83.07] mg/day; t = −1.28; P = 0.24). Similarly, there was no difference in the dose of clozapine in those who ever had and who never had metabolic syndrome while receiving clozapine.
Relationship of metabolic syndrome with duration of clozapine use
When the relationship of metabolic syndrome was evaluated with total duration of clozapine use there was no significant difference between total duration of clozapine use between those who did not have metabolic syndrome (n = 6; mean = 18.16 [7.27] months) and those who had metabolic syndrome (n = 3; mean = 9.66 [4.04] months).
Relationship of metabolic syndrome with treatment response
No significant difference in the PANSS score was seen at baseline and at 3 months among those who had and who did not have metabolic syndrome at 3 months. However, it was seen that those patients who had lower scores in the subscale of general psychopathology (18.66 [1.52] vs. 25.5 [1.87]; t = 5.43; P = 0.001) and total PANSS (37.66 [5.50] vs. 56.33 [6.12]; t = 4.43; P = 0.003) at 3 months were more likely to have metabolic syndrome beyond 3 months.
DISCUSSION
To be the best our knowledge, this is the first study which has prospectively followed up adolescent patients receiving clozapine for the prevalence of metabolic syndrome in adolescents.
Findings of this study can be interpreted from various perspectives such as:
Do adolescent patients with psychotic disorders have a higher rate of metabolic syndrome?
Do the adolescents receiving clozapine have a higher rate of metabolic syndrome compared to those receiving other antipsychotic?
Can all the metabolic disturbances in adolescents receiving clozapine be attributed to clozapine itself?
We attempt to compare the findings of this study with existing literature on children and adolescents on metabolic syndrome and also in adults, and try to interpret the findings of this study.
In terms of prevalence of metabolic syndrome seen in this study at the baseline, the prevalence rate of 23% is significantly higher than that reported for the general population in our catchment area. Two studies have evaluated prevalence rate of metabolic syndrome in children and adolescents aged 10-18 years among the school-attending adolescents and those taken from general population, and these suggest that prevalence rate of metabolic syndrome in this age group is 2.7-4.2%,[21,22] with higher prevalence rate of 4.8% in those aged 16-18 years.[23] Accordingly, it can be concluded that adolescents with severe mental illness, who are on treatment with psychotropics have a higher prevalence rate of metabolic syndrome.
Few studies from various parts of the world have evaluated the prevalence of metabolic syndrome in children and adolescents receiving antipsychotics. Small sample studies (n = 8-26) in patients of schizophrenia have reported a lack of metabolic syndrome in children and adolescents at the baseline and emergence of metabolic syndrome in none or very few patients treated with risperidone.[24,25] When we compare the finding of this study with this, it appears that the incidence rate of metabolic syndrome with clozapine is probably higher than that seen with other antipsychotics. However, it must be understood that total prevalence of metabolic syndrome in adolescent patients receiving clozapine cannot be attributed totally to clozapine.
In terms of prevalence of metabolic syndrome, if we consider all the seven patients who had metabolic syndrome “ever” while receiving clozapine, the prevalence rate of metabolic syndrome in adolescents receiving clozapine in this study is 53.84%. In terms of incidence, i.e., new-onset metabolic syndrome, four patients “ever” had metabolic syndrome while receiving clozapine, with an incidence rate of 30.76%. These prevalence and incidence rate are comparable to those noted in studies done in the adult population.[11,12,13] These findings suggest that adolescents are as prone to develop metabolic syndrome as adults, and there is a need to monitor the metabolic profile and institute preventive measures to reduce the risk of development of metabolic syndrome.
Studies suggest that compared to adult onset schizophrenia, childhood, and adolescent onset schizophrenia has a relatively poor prognosis and delay in institution of treatment and starting of clozapine are associated with poor prognosis.[26] If one attempts to look at the increase in the rate of metabolic syndrome with clozapine, findings of this study, suggest a nonsignificant increase in the prevalence of metabolic syndrome in adolescents receiving clozapine. In term of absolute number, over the mean follow-up period of 15.3 months, compared to the baseline, twice the number of the patient had metabolic syndrome, but the difference was not significant. This finding suggests that clinicians should not withhold clozapine for long in patients who are not responding to clozapine, because of the scare of metabolic disturbances.
In terms of the type of metabolic disturbances, findings of this study suggest that overall lipid abnormalities (i.e., high triglyceride levels and low HDL levels) followed by an increase in the waist circumference are the most common abnormalities in adolescents receiving clozapine. This profile is slightly different than that seen in adult patients receiving clozapine in our setting.[11,13]
Many previous studies have evaluated the components of metabolic syndrome in children and adolescents receiving clozapine or other antipsychotics. These studies suggest that patients receiving clozapine on an average gain 2.5-3.6 kg during the initial 6-8 weeks and gain about 9.5 kg weight by 1 year.[5,14,26,27] In our study, mean increase in weight was 3.65 (SD = 4.37) kg at 3 months and 6.33 (SD = 8.28) kg at the mean follow-up duration of 15.33 months. Accordingly findings of this study support the observation of previous studies. Further findings of this study suggest a secular trend of weight gain with the passage of time in patients receiving clozapine. This suggests that measures must be taken right from the beginning of clozapine to manage the body weight, and these must be continued throughout the period of clozapine use.
Previous studies suggest that about two-third (63.6%) of children and adolescents with early onset schizophrenia develop hypertension.[5] Findings of this study suggest that very few adolescent patients develop hypertension while receiving clozapine and when it develops, it is possibly temporary. Previous studies have reported an incidence rate of hypertriglyceridemia to be in the range of 8.3-22.2%.[5,15] Withstanding the differences in the cut-offs used for defining hypertriglyceridemia in this and earlier studies, findings of this study suggest that there is no increase in the rate of hyperglyceridemia with clozapine in short-term (3 months) and in the long-term the incidence rate is about 15%. These differences could be due to small sample size of this study as well as the previous studies. In addition, the difference could be due to different cut-offs used and possibly due to ethnic and dietary differences, which have been reported to influence the various components of metabolic syndrome.[28]
Another finding which is important to note is that most of the patients who developed metabolic syndrome while receiving clozapine had one or more metabolic abnormality to start with. This finding is similar to that reported in the adult population.[13] Accordingly, it is important to identify this high-risk population at the beginning itself, and measures must be taken to reduce the risk of development of metabolic syndrome since the beginning of clozapine therapy.
In this study, there was no relationship between the sociodemographic variables, clinical variables, dose of clozapine, and duration of clozapine use with the development of metabolic syndrome. Certain studies in adult population also suggest a lack of such relationship with most of these variables.[11]
The clinical effectiveness of clozapine was clearly demonstrated in our study with significant improvement in all the subscales of PANSS. Previous open-label and randomized controlled trials have also proven the efficacy and effectiveness of clozapine in childhood-onset schizophrenia and in adolescents with schizophrenia.[4,5,6,8,15,18] This finding suggests that clozapine is a useful choice in adolescent patients with treatment-resistant psychotic disorders, and must be considered as and when indicated. The risk and benefit of clozapine must be weighed in before reaching a decision, and clinicians should not be scared of using clozapine in this age group. Another important observation in this study is a continued improvement with clozapine in the long run. Previous studies in adults, children, and adolescents also suggest that improvement with clozapine is not only seen during the initial period, but it also continues over the initial 6 months or more.[5,6,8] Accordingly, clozapine trial should not be suspended at 3 months in patients who initially showed some response to clozapine.
However, an interesting finding which was noted in this study was a higher risk of developing metabolic syndrome in long-term follow-up in patients who have a higher reduction in PANSS at 3 months. This finding provides credence to the hypothesis proposed by some of the researchers, who suggest that risk of metabolic syndrome is related to treatment response.[29] Accordingly there is a need to further evaluate this relationship.
In this study besides metabolic side effect, significantly high proportion of patients had hypersalivation (100%) and sedation (69.2%). Previous studies have also reported high prevalence rate for these side effects, and our findings are comparable.[18] Similarly, the rates of constipation are also comparable to the reported literature.[19] However, the rate of enuresis, tachycardia, postural fall, and urinary incontinence seen in this study are slightly less than that reported in literature.[18] These differences could be due to doses used. In contrast to some of the studies from the West, in general, lower doses were used in our patients. In this study, one patient developed transient thrombocytopenia, which is similar to some of the observations in the adult population.[30]
Conclusions this study adds to the growing literature describing metabolic disturbances with clozapine and is the first of its kind in studying metabolic syndrome as an entity in child and adolescent population. The strengths of our study are its prospective design which enabled optimal monitoring of metabolic disturbances, side effects, and effectiveness of clozapine. Most previous studies especially related to metabolic disturbances and side effects were retrospective[16] or reported individual metabolic abnormalities only.[5,14,15] To conclude, this study suggests 23.1%, 38.46%, and 46.2% of adolescents receiving clozapine have metabolic syndrome at baseline, 3 months, and at long-term follow-up of 15.33 months, respectively. However, these differences are statistically not significant. There is a significant increase in the weight, body mass index, waist circumference, and fasting blood glucose level with continued use of clozapine. Clozapine is effective in the management of treatment-resistant schizophrenia in adolescents and a higher level of response at 3 months is associated with the development of metabolic syndrome later on. Among other side effects, hypersalivation and sedation are the most common side effects of clozapine in adolescents.
Considering the risk of various metabolic disturbances and metabolic syndrome with clozapine, it is important to monitor the patients regularly. Further, there is a need to encourage healthy habits such as diet changes (lower fat/cholesterol, smaller portions, increased fiber, increased water) and increased aerobic exercise (30-60 min/day), which helps prevent weight gain, constipation, blood pressure changes, and glucose abnormalities.[31] Parents and guardians must be advised to increase adherence with these habits in adolescents.
This study was limited by the small sample size. Despite the best efforts, 4 out of the 13 patients dropped out of treatment. There was no control group. No attempt was made to collect the information about the dietary pattern and lifestyle modifications followed by the patients. Further studies with larger sample size and with similar prospective design are needed in future considering the increased use of clozapine for the management of treatment-resistant schizophrenia in children and adolescent population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Citrome L. A systematic review of meta-analyses of the efficacy of oral atypical antipsychotics for the treatment of adult patients with schizophrenia. Expert Opin Pharmacother. 2012;13:1545–73. doi: 10.1517/14656566.2011.626769. [DOI] [PubMed] [Google Scholar]
- 2.Vitiello B, Correll C, van Zwieten-Boot B, Zuddas A, Parellada M, Arango C. Antipsychotics in children and adolescents: Increasing use, evidence for efficacy and safety concerns. Eur Neuropsychopharmacol. 2009;19:629–35. doi: 10.1016/j.euroneuro.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Sarkar S, Grover S. Antipsychotics in children and adolescents with schizophrenia: A systematic review and meta-analysis. Indian J Pharmacol. 2013;45:439–46. doi: 10.4103/0253-7613.117720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kranzler HN, Cohen SD. Psychopharmacologic treatment of psychosis in children and adolescents: Efficacy and management. Child Adolesc Psychiatr Clin N Am. 2013;22:727–44. doi: 10.1016/j.chc.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Shaw P, Sporn A, Gogtay N, Overman GP, Greenstein D, Gochman P, et al. Childhood-onset schizophrenia: A double-blind, randomized clozapine-olanzapine comparison. Arch Gen Psychiatry. 2006;63:721–30. doi: 10.1001/archpsyc.63.7.721. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y, Kim BN, Cho SC, Kim JW, Shin MS. Long-term sustained benefits of clozapine treatment in refractory early onset schizophrenia: A retrospective study in Korean children and adolescents. Hum Psychopharmacol. 2008;23:715–22. doi: 10.1002/hup.982. [DOI] [PubMed] [Google Scholar]
- 7.Sikich L, Frazier JA, McClellan J, Findling RL, Vitiello B, Ritz L, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: Findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420–31. doi: 10.1176/appi.ajp.2008.08050756. [DOI] [PubMed] [Google Scholar]
- 8.Sporn AL, Vermani A, Greenstein DK, Bobb AJ, Spencer EP, Clasen LS, et al. Clozapine treatment of childhood-onset schizophrenia: Evaluation of effectiveness, adverse effects, and long-term outcome. J Am Acad Child Adolesc Psychiatry. 2007;46:1349–56. doi: 10.1097/chi.0b013e31812eed10. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27:596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- 10.De Nayer A, De Hert M, Scheen A, Van Gaal L, Peuskens J, et al. On Behalf of the Consensus Group. Belgian consensus on metabolic problems associated with atypical antipsychotics. Int J Psychiatry Clin Pract. 2005;9:130–7. doi: 10.1080/13651500510018310. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra N, Grover S, Chakrabarti S, Kulhara P. Metabolic Syndrome in Schizophrenia: A review. Indian J Psychological Medicine. 2013;35:227–40. doi: 10.4103/0253-7176.119471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Josiassen RC, Filmyer DM, Curtis JL, Shaughnessy RA, Joseph A, Parson RL. An archival, follow-forward exploration of the metabolic syndrome in randomly selected, clozapine-treated patients. Clin Schizophr Relat Psychoses. 2009;3:87–96. [Google Scholar]
- 13.Nebhinani N, Grover S, Chakrabarti S, Kate N, Avasthi A. A longitudinal study of change in prevalence of metabolic syndrome and metabolic disturbances 3 months after Clozapine therapy. J Ment Health Hum Behav. 2013;18:9–17. [Google Scholar]
- 14.Fleischhaker C, Heiser P, Hennighausen K, Herpertz-Dahlmann B, Holtkamp K, Mehler-Wex C, et al. Weight gain in children and adolescents during 45 weeks treatment with clozapine, olanzapine and risperidone. J Neural Transm (Vienna) 2008;115:1599–608. doi: 10.1007/s00702-008-0105-9. [DOI] [PubMed] [Google Scholar]
- 15.Kumra S, Kranzler H, Gerbino-Rosen G, Kester HM, DeThomas C, Cullen K, et al. Clozapine versus “high-dose” olanzapine in refractory early-onset schizophrenia: An open-label extension study. J Child Adolesc Psychopharmacol. 2008;18:307–16. doi: 10.1089/cap.2007.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koller E, Malozowski S, Doraiswamy PM. Atypical antipsychotic drugs and hyperglycemia in adolescents. JAMA. 2001;286:2547–8. doi: 10.1001/jama.286.20.2547. [DOI] [PubMed] [Google Scholar]
- 17.Pringsheim T, Lam D, Ching H, Patten S. Metabolic and neurological complications of second-generation antipsychotic use in children: A systematic review and meta-analysis of randomized controlled trials. Drug Saf. 2011;34:651–68. doi: 10.2165/11592020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Schneider C, Corrigall R, Hayes D, Kyriakopoulos M, Frangou S. Systematic review of the efficacy and tolerability of clozapine in the treatment of youth with early onset schizophrenia. Eur Psychiatry. 2014;29:1–10. doi: 10.1016/j.eurpsy.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Gogtay N, Rapoport J. Clozapine use in children and adolescents. Expert Opin Pharmacother. 2008;9:459–65. doi: 10.1517/14656566.9.3.459. [DOI] [PubMed] [Google Scholar]
- 20.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP). Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Singh R, Bhansali A, Sialy R, Aggarwal A. Prevalence of metabolic syndrome in adolescents from a North Indian population. Diabet Med. 2007;24:195–9. doi: 10.1111/j.1464-5491.2007.02066.x. [DOI] [PubMed] [Google Scholar]
- 22.Singh N, Parihar RK, Saini G, Mohan SK, Sharma N, Razaq M. Prevalence of metabolic syndrome in adolescents aged 10-18 years in Jammu, J and K. Indian J Endocrinol Metab. 2013;17:133–7. doi: 10.4103/2230-8210.107849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goeb JL, Marco S, Duhamel A, Kechid G, Bordet R, Thomas P, et al. Metabolic side effects of risperidone in early onset schizophrenia. Encephale. 2010;36:242–52. doi: 10.1016/j.encep.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Maayan LA, Vakhrusheva J. Risperidone associated weight, leptin, and anthropometric changes in children and adolescents with psychotic disorders in early treatment. Hum Psychopharmacol. 2010;25:133–8. doi: 10.1002/hup.1097. [DOI] [PubMed] [Google Scholar]
- 25.Eggers C, Bunk D. The long-term course of childhood-onset schizophrenia: A 42-year followup. Schizophr Bull. 1997;23:105–17. doi: 10.1093/schbul/23.1.105. [DOI] [PubMed] [Google Scholar]
- 26.Fraguas D, Correll CU, Merchán-Naranjo J, Rapado-Castro M, Parellada M, Moreno C, et al. Efficacy and safety of second-generation antipsychotics in children and adolescents with psychotic and bipolar spectrum disorders: Comprehensive review of prospective head-to-head and placebo-controlled comparisons. Eur Neuropsychopharmacol. 2011;21:621–45. doi: 10.1016/j.euroneuro.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Maayan L, Correll CU. Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol. 2011;21:517–35. doi: 10.1089/cap.2011.0015. [DOI] [PubMed] [Google Scholar]
- 28.Garduño-Diaz SD, Khokhar S. South Asian dietary patterns and their association with risk factors for the metabolic syndrome. J Hum Nutr Diet. 2013;26:145–55. doi: 10.1111/j.1365-277X.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- 29.Venkatasubramanian G, Rao NP, Arasappa R, Kalmady SV, Gangadhar BN. A longitudinal study of relation between side-effects and clinical improvement in schizophrenia: Is there a neuro-metabolic threshold for second generation antipsychotics? Clin Psychopharmacol Neurosci. 2013;11:24–7. doi: 10.9758/cpn.2013.11.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kate N, Grover S, Aggarwal M, Malhotra P, Sachdeva MS. Clozapine associated thrombocytopenia. J Pharmacol Pharmacother. 2013;4:149–51. doi: 10.4103/0976-500X.110913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barlow SE. Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120(Suppl 4):S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]


