Abstract
BACKGROUND
Fyn is a kinase that is upregulated in a subset of metastatic castration-resistant prostate cancer. Saracatinib potently inhibits Fyn activation. We have noted a relationship between Fyn expression and directional motility, a cellular process related to metastasis. As such we hypothesized that treatment with saracatinib would increase the time required to develop new metastatic lesions.
METHODS
Patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel were eligible for enrollment. This study was executed as a randomized discontinuation trial. During a lead-in phase of two 28-Day cycles, all patients received saracatinib. Afterward, patients with radiographically stable disease were randomized to either saracatinib or placebo. Patients continued treatment until evidence of new metastasis.
RESULTS
Thirty-one patients were treated. Only 26% of patients had stable disease after 8 weeks and thus proceeded to randomization. This required early termination of the study for futility. The 70% of patients who progressed after the lead-in phase exhibited expansion of existing lesions or decompensation due to clinical progression without new metastatic lesions. Fatigue was reported in more than 25% of patients (all grades) with only two patients experiencing grade 3 toxicity. Other grade 3 adverse events included dehydration, thrombocytopenia, and weakness.
CONCLUSIONS
This study was unable to determine if saracatinib had potential as metastasis inhibitor. Metastasis inhibition by saracatinib may still be viable in an earlier time in the disease history.
Keywords: prostate cancer, Fyn, azd0530, castration-resistant, saracatinib
INTRODUCTION
The Src-family kinases (SFKs) have been considered one the most important non-tyrosine kinase signaling groups in multiple cancers including prostate cancer. While much of the work in this field has focused on the prototypical member of the family, Src, there are a total of nine members including Fyn, Lyn, Blk, Fgr, Hck, Lck. Several of these, especially Fyn and Lyn, have been implicated in cancer progression.
Our laboratory has specifically identified a relationship between Fyn and directional motility in response to chemotactic factors such as hepatocyte growth factor (HGF) [1–3]. Our studies also have shown that knockdown of Fyn results in suppression of tumor cell growth and invasion. Other groups have also shown anticancer effects related to inhibition of Src [4–7] and Lyn [8,9]. Saracatinib (AZD0530) is a novel anilinoquinazoline that inhibits activation of most SFKs including Fyn, Lyn, and Src [10]. This preclinical data led us to predict that the pharmacologic inhibition of Fyn and other SFKs with saracatinib would impair successful migration of metastatic tumor cells to a secondary site for colonization. As such we hypothesized that treatment with saracatinib would increase the time required to develop new metastatic lesions.
Given the contemporary understanding of metastatic castration-resistant prostate cancer (mCRPC), we identified the population at greatest risk of new metastatic lesions as the population of patients with existing metastatic disease. Our previous studies suggested that these patients should also have higher expression levels of Fyn and SFKs as compared to those with non-metastatic or castration-sensitive disease [4].
Like other advanced malignant conditions, we anticipated a considerable degree of heterogeneity in the test population. As such, it was advantageous to apply a strategy to further refine the test population so as to optimize the testing of our hypothesis. We selected the use of the randomized discontinuation trial (RDT) design to meet this need [11,12].
Herein we report our clinical trial in the advanced disease population using new metastasis as a primary clinical endpoint.
MATERIALS AND METHODS
Patients
Eligible patients had histologically confirmed progressive metastatic prostate cancer despite castration and docetaxel-based chemotherapy. Progressive disease was defined as new clinical or radiographic metastasis or rising PSA of greater than 1.0 μg/L with at least two consecutive rises separated by at least 10 days. Prior chemotherapy, surgery, or radiotherapy needed to be administered at least 2 weeks prior to start of the trial. At the time of the study, abiraterone, enzulatamide, and radium-223 were not FDA-approved and available for general use. Other inclusion criteria included Eastern Cooperative Oncology Group (ECOG) score of ≤1 and adequate organ function.
Exclusion criteria included concurrent usage of non-FDA approved medications or other investigational agents, allergic reactions to compounds or chemicals similar to saracatinib, or usage of CYP3A4-active agents that may have potential drug interactions with saracatinib. Other exclusion criteria included a history of pneumonitis, cardiac dysrhythmias, prolonged QTc interval (>480 msec), or unresolved toxicity from previous treatments. Use of bisphosphonates was permitted. All patients provided informed consent in compliance with the declaration of Helsinki to participate on this University of Chicago Institutional Review Board approved protocol.
Treatment and Design
The primary purpose of this study was to test the scientific hypothesis that treatment with saracatinib would delay the onset of new metastases in men with mCRPC. As a matter of good clinical practice, however, radiographic progression of existing lesions needed to be included. As such, the primary clinical endpoint of the study was radiographic progression-free survival. This study was executed as a RDT (Fig. 1). All patients were required to undergo a lead-in phase of saracatinib alone at 175 mg daily for two 28-Day cycles prior to randomization. Only patients who were stable by imaging after the lead-in were randomized to saracatinib or placebo. In this manner, patients whose cancers were clearly not sensitive to saracatinib were excluded from further study, as were any who were clearly responding to the treatment. We allowed for crossover at post-randomization progression to enhance protocol accrual.
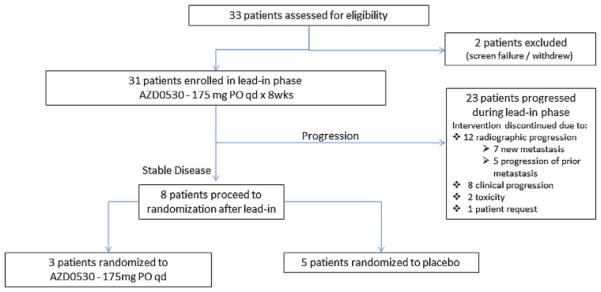
Fig. 1.
Trial design.
Randomized patients continued treatment until evidence of new metastasis, clinical decompensation, growth of existing lesions by RECIST criteria, or unmanageable drug-related toxicity. Progression by PSA was excluded since prior studies indicated saracatinib had little effect on PSA [16].
Clinical assessments were performed every 4 weeks and radiographic assessments were required every 8 weeks. Images were evaluated using RECIST 1.1 criteria. Bone scans were evaluated using a modification of PCWG2 criteria that required at least two new lesions to be present to declare disease progression. Dose reductions to 125 mg or 100 mg daily were allowed for excess toxicity that recovered to a grade one or better. Patients randomized to placebo had the opportunity to crossover to saracatinib if they were fit to continue treatment. Toxicity on trial was captured using the Common Toxicity Criteria for Adverse events (CTCAE) v 4.0.
Statistical Considerations
This study was designed with a full accrual of 125 patients. This sample size assumed a randomization rate of 70% (assuming a 30% loss to rapid progression during the lead-in period and no overt responders). With 88 randomized patients, the study was able to detect a hazard ratio of 1.75 in time to new metastases with a one-sided α of 0.1 and 80% power based on a log rank test.
Two early termination rules were applied based on the results observed in the lead-in phase. If after the first 40 patients less than 50% (19 or fewer) of the subjects had been randomized, the study would be reviewed for termination. Also, if after the first 40 patients, more than 90% (36 or greater) of the subjects had shown an absence of disease progression, saracatinib would be considered sufficiently active and appropriate for evaluation in a larger trial and the current trial would be terminated (the lower limit of the 95% confidence interval for proportion progression-free after two cycles would be at least 81%).
RESULTS
Patient Accrual and Demographics
A total of 33 patients from eight sites were consented from February 2011 to June 2012. Two patients did not proceed to treatment. Thirty-one entered the lead-in phase of this RDT (Fig. 2). After accrual of 31 patients, it was clear that early termination for futility would be required, and the study was closed.
Fig. 2.
Trial profile.
Patient characteristics are summarized in Table I. The patient population enrolled had a median age of 71 years (range 48–87). Approximately 26% of the patients were >75 years old. Baseline PSA in the study population was high with the average at 360 μg/L (range 2–1,480 μg/L). One-third of the study population had known visceral metastasis prior to enrollment. More than 80% of the study group had received five or more therapeutic treatment maneuvers prior to study enrollment. As noted earlier, all patients had prior docetaxel therapy.
TABLE 1.
Patient Characteristics
| Patient characteristics (n = 31) | ||
|---|---|---|
| Age | Value | Range and percentage |
| Median | 71 | (48–87) |
| ≥75 years | 8 | (26%) |
| Ethnic origin | Number of patients |
Percentage |
| White | 23 | (74%) |
| Black | 7 | (23%) |
| Asian | 1 | (3%) |
| Baseline disease |
Number of patients |
Percentage |
| Visceral | 10 | (32%) |
| None | 8 | (26%) |
| Unknown | 13 | (42%) |
| Baseline PSA | Value (μLg/L) | Range |
| Mean | 360 | |
| Median | 230 | (2–1,480) |
| Prior therapy | Value | Range and percentage |
| Median | 6 prior therapies | (3–13) |
| ≥5 | 25 patients | (81%) |
Efficacy Analysis
Out of the 31 patients who received saracatinib during the lead in, only eight patients (26%) were considered to have stable disease after 8 weeks of lead-in therapy and were thus eligible for randomization. Given the low rate of patients randomized, the study was closed. Details of the entire treatment population are shown in Table II.
TABLE II.
Patients Enrolled in Trial
| Patients enrolled in trial (n = 31) | ||
|---|---|---|
| Median time to progression | Weeks 9 |
Range (2–19) |
| Average rise in PSA | PSA (μg/L) 349 |
Range (0–1,984) |
| Reason for termination of therapy |
Number of patients |
Percentage |
| New metastatic lesionsa | 10 | (32%) |
| Target lesion growth | 5 | (16%) |
| Clinical progression | 11 | (35%) |
| Toxicity | 3 | (10%) |
| Patient choice | 2 | (6%) |
Six patients with new bony metastasis, two patient with new visceral metastasis, two patients with new visceral and bony metastasis (Percentages may not add to 100 due to rounding).
Of the 23 patients who did not proceed to randomization, 12 (52%) had radiographic progression, but only seven patients (30%) had evidence of new metastasis (Table III). In comparison to patients who were not randomized, three (38%)% of the randomized patients ultimately developed radiographic progression, all with new metastatic lesions.
TABLE III.
Comparison of Randomized and Non-randomized Patients
| Patients not randomized (n = 23) |
Patients randomized (n = 8) |
|
|---|---|---|
| Median age (years) | 72 (range 48–87) | 69 (range 50–73) |
| ≥75 years | 8 (35%) | 0 (0%) |
| Mean baseline serum | 370 (range 2–1,480) | 330 (range |
| PSA (μg/L) | 8–1,375) | |
| Baseline visceral disease |
7 (30%) | 3 (38%) |
| ≥5 prior therapies | 18 (78%) | 7 (88%) |
| Mean rise in PSA (μg/L) |
400 (range 0–1984) | 201 (range 1–627) |
| Median time to progression (weeks) |
— | 16 (range 9–19) |
| Radiographic progression |
12 (52%) | 3 (38%) |
| New Metastasis | 7 (30%) | 3 (38%) |
| Target lesion growth | 5 (22%) | 0 (0%) |
| Clinical progression | 8 (35%) | 3 (38%) |
| Toxicity | 2 (9%) | 1 (13%) |
| Patient choice | 1 (4%) | 1 (13%) |
Percentages may not add to 100 due to rounding.
Of the eight patients who proceeded to the randomization phase of the study, three were randomized to saracatinib and five were randomized to placebo. None of the patients on placebo chose to cross to saracatinib as the protocol allowed at the time of progression. Those who were randomized to saracatinib had a median duration of stable disease (including lead-in period) of 18 weeks (range 17–19) and those who received placebo had a median duration of 12 weeks (range 9–17+) (one patient censored) (P = 0.12) No significant declines in serum PSA concentration were noted. All patients randomized to saracatinib remained on therapy until objective radiographic progression: two patients developed new bone lesions, and one patient developed a new soft tissue lesion. The patients randomized to placebo all discontinued treatment prior to objective radiographic progression. Three decompensated clinically from growth of existing lesions, one experienced unacceptable toxicity (see below), and one patient withdrew after randomization due to anxiety about the randomization without report of clinically significant toxicity or clinical/radiographic progression.
Toxicity
Toxicities occurring in two or more (>5%) of the patients and considered at least possibly related to study drug are summarized in Table IV. No grade four toxicities were encountered on this study as a result of saracatinib. No patients developed pneumonitis during the course of this study by either clinical or radiographic assessments. There were three patients who discontinued for toxicity. During the lead-in phase, one patient discontinued due to grade two fatigue and diarrhea and another discontinued due to grade one vomiting. A patient who was randomized to placebo after lead-in discontinued due to grade two transaminitis, nausea, and diarrhea.
TABLE IV.
Patient Toxicities Considered At Least Possibly Related To Study Drug
| Patient toxicities (n = 31) | ||||
|---|---|---|---|---|
| Adverse event | Number of patients with any grade toxicity |
% of patients |
Number of patients with grade 3 toxicity |
% of patients |
| Fatigue | 9 | 29 | 2 | 6% |
| Dehydration | 3 | 10 | 1 | 3% |
| Thrombocytopenia | 3 | 10 | 1 | 3% |
| Weakness | 1 | 3 | 1 | 3% |
| Anorexia | 8 | 26 | — | — |
| Nausea | 8 | 26 | — | — |
| Transaminitis | 6 | 19 | — | — |
| Vomiting | 7 | 23 | — | — |
| Diarrhea | 5 | 16 | — | — |
| Anemia | 4 | 13 | — | — |
| Constipation | 3 | 10 | — | — |
| Renal Dysfunction | 3 | 10 | — | — |
| Edema | 2 | 6 | — | — |
| Fever | 2 | 6 | — | — |
| Flu-like symptoms | 2 | 6 | — | — |
| Hematuria | 2 | 6 | — | — |
| Leukopenia | 2 | 6 | — | — |
| Myalgia | 2 | 6 | — | — |
DISCUSSION
Prostate cancer continues to be the most common cancer affecting American men and the second leading cause of cancer death [13]. The transition to CRPC represents an important clinical hallmark that indicates an increased risk of death. Since 2004, the number of treatment options for patients has increased [14-18]. Although these therapeutic breakthroughs are encouraging, there remains a need for additional strategies offering benefit with acceptable toxicity. Since metastatic progression precedes end organ failure, metastasis inhibition theoretically is a viable form of treatment.
While most studies of SFKs in general and in prostate cancer specifically have focused on classical Src kinase c-Src, our laboratory has demonstrated that Fyn is the most upregulated SFK in advanced prostatic malignancies. Fyn is known to regulate activity of focal adhesion kinase (FAK) and paxillin, key regulators of cell shape and motility. Studies from our laboratory have confirmed that these phenotypic alterations are related to the Fyn kinase utilizing a variety of cell lines, xenograft mouse models, and clinical samples [19]. These findings have led us to investigate the potential clinical benefit related to SFK inhibition in prostate cancer. More specifically, our laboratory data led us to hypothesize that the clinical benefit of SFK inhibition would manifest as inhibition of metastatic progression and hence prolonged time to new metastasis.
There is one prior study of saracatinib in CRPC by Lara et al. [20] whose primary endpoint was response based upon modified PSA response criteria (i.e., a 30% decrease from baseline). As was observed in our study, no PSA response was noted. However, various kinase inhibitors have been shown to increase PSA secretion from cells independent of an effect on growth [21–23] adding to concerns that PSA measurements may not optimally reflect clinical benefit.
Our trial was unfortunately unable to detect an effect on metastasis, principally due to the paucity of patients who were randomized. As such, this study was unable to fully test the central hypothesis that inhibition of Fyn and other SFKs by saracatinib would delay the development of new metastatic lesions. There are three possible explanations for our clinical observation. It is possible that the underlying hypothesis that saracatinib would improve outcomes was erroneous since a high percentage of patients progressed during the lead-in phase, although most progressions were not due to new lesions. It is also possible that the suppression of Fyn or other SFKs by saracatinib was not sufficient to alter Fyn/SFK-driven metastatic progression. Finally, the population chosen for study was not preselected by biomarker (e.g., Fyn) expression and may not have been optimal for metastasis inhibition. These were generally older, medically fragile, heavily pre-treated, late-stage patients with high tumor burdens (reflected by scans and PSA) when compared to other contemporary mCRPC studies (Table V). Many had developed visceral metastases which have been associated with poorer outcomes [24–26]. This population was apt to discontinue therapy due to overall clinical decompensation from disease progression even without the development of new metastatic lesions. While the growth of pre-existing lesions was not directly relevant to the molecular hypothesis, ethically it prevented investigators from continuing study treatment.
TABLE V.
Characteristics of Saracatinib RDT Patients Compared to Previously Studied Patients with mCRPC
| Study | Median age (years) |
≥75 years (average %) |
Baseline PSA (μg/L) |
Visceral disease (average %) |
|---|---|---|---|---|
| Saracatinib RDT |
71 | 26 | 360 | 32 |
| TAX 327a | 68 | 20 | 115 | 23 |
| Cabazitaxelb | 68 | 19 | 136 | 25 |
| Radium - 223c |
71 | 29 | 160 | 0 |
N Engl J Med 2004; 351:1,502–12.
Lancet 2010; 376:1,147–54.
N Engl J Med 2013; 369:213–23.
Averages were taken for the studies with two treatment arms.
Another noteworthy aspect of our approach was the use of RDT design. Most assays for measuring Fyn or SFK activation in clinical specimens have not been well validated or standardized. It is unclear what level of target expression and/or activation is required to predict sensitivity to saracatinib. This trial design allowed the drug under investigation to select the population as opposed to an assay that has not been validated. A large number of patients in this study were unable to complete the lead-in phase of treatment due to reasons unrelated to the formation of new metastasis. While this led to premature closure of the study, the RDT design allowed us to stop accrual in an efficient manner, while still maximizing patient exposure in a way that may not have been accomplished using a traditional randomized phase 2 trial design with 1:1 or even 2:1 allocation.
Our clinical experience during this study points to the need for careful patient selection (in this case, patient stratification based on the status of Fyn kinase in patients) and study design, especially when testing novel biological hypotheses in a highly heterogeneous population. In this advanced and highly heterogeneous population, the use of new metastatic lesions as a primary endpoint is sub-optimal. But given the biology of saracatinib, our hypothesis was that if the drug was effective we would expect some shift in this endpoint. However, there is no way to prove that new radiographic metastatic lesions are due to cell migration (thus a potential target of SFK inhibition) or simply represent differential growth of pre-existing micro metastases. We would speculate that as the other fields mature, such as that of circulating tumor cells (CTCs), an alternative endpoint may be used. In this case, if SFK inhibition resulted in impaired motility, it may be the case that this would result in a notable decline in CTC counts, which may correlate with reduced metastatic potential. Moreover, our lab and others have been studying the relationship between solid tumors and CTCs hoping to use them as tissue surrogates. These studies are early but show promise and may 1day allow for minimally invasive molecular classification of patients [27,28].
We continue to propose that metastasis inhibition in advanced CRPC remains a novel trial endpoint that is viable and clinically relevant. Given this particular experience, the methodology of testing study hypotheses must further consider patient factors and drug toxicity in future studies. Given the emerging trends in the field and an expanding knowledge of biology and biomarkers of disease progression and metastasis, an earlier disease population (post-op and or non-metastatic CRPC), and efficient study design may be identified in the future.
ACKNOWLEDGMENTS
This work was supported by NCI/CTEP phase 2 consortium contract number N01-CM-2011-00071C as well as the Department of Defense Prostate Cancer Research Program Idea Award contract number W81XWH-11-1-0422, a Prostate Cancer Foundation Young Investigator Award, St. Anthony Prostate Cancer Research Fund, the CD McKinnon Memorial Neuroendocrine Prostate Cancer Fund, and the Berns Family Prostate Cancer Fund.
Footnotes
Conflicts of interests: The authors report no financial or conflict of interest relevant to the subject of this article.
Grant sponsor: NCI/CTEP Phase 2 Consortium; Grant number: N01-CM-2011-00071C; Grant sponsor: Department of Defense Prostate Cancer Research Program Idea Award; Grant number: W81XWH-11-1-0422; Grant sponsor: Prostate Cancer Foundation Young Investigator Award; Grant sponsor: St. Anthony Prostate Cancer Research Fund; Grant sponsor: CD McKinnon Memorial Neuroendocrine Prostate Cancer Fund; Grant sponsor: Berns Family Prostate Cancer Fund.
REFERENCES
- 1.Jensen AR, David SY, Liao C, Dai J, Keller ET, Al-Ahmadie H, Dakin-Hache K, Usatyuk P, Sievert MF, Paner GP, Yala S, Cervantes GM, Natarajan V, Salgia R, Posadas EM. Fyn is downstream of the HGF/MET signaling axis and affects cellular shape and tropism in PC3 cells. Clin Cancer Res. 2011;17(10):3112–3122. doi: 10.1158/1078-0432.CCR-10-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saito YD, Jensen AR, Salgia R, Posadas EM. Fyn: A novel molecular target in cancer. Cancer. 2010;116(7):1629–1637. doi: 10.1002/cncr.24879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Posadas EM, Al-Ahmadie H, Robinson VL, Jagadeeswaran R, Otto K, Kasza KE, Tretiakov M, Siddiqui J, Pienta KJ, Stadler WM, Rinker-Schaeffer C, Salgia R. FYN is overexpressed in human prostate cancer. BJU Int. 2009;103(2):171–177. doi: 10.1111/j.1464-410X.2008.08009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drake JM, Graham NA, Lee JK, Stoyanova T, Faltermeier CM, Sud S, Titz B, Huang J, Pienta KJ, Graeber TG, Witte ON. Metastatic castration-resistant prostate cancer reveals intrapatient similarity and interpatient heterogeneity of therapeutic kinase targets. Proc Natl Acad Sci USA. 2013;110(49):E4762–E4769. doi: 10.1073/pnas.1319948110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake JM, Graham NA, Stoyanova T, Sedghi A, Goldstein AS, Cai H, Smith DA, Zhang H, Komisopoulou E, Huang J, Graeber TG, Witte ON. Oncogene-specific activation of tyrosine kinase networks during prostate cancer progression. Proc Natl Acad Sci USA. 2012;109(5):1643–1648. doi: 10.1073/pnas.1120985109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai H, Smith DA, Memarzadeh S, Lowell CA, Cooper JA, Witte ON. Differential transformation capacity of Src family kinases during the initiation of prostate cancer. Proc Natl Acad Sci USA. 2011;108(16):6579–6584. doi: 10.1073/pnas.1103904108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai H, Babic I, Wei X, Huang J, Witte ON. Invasive prostate carcinoma driven by c-Src and androgen receptor synergy. Cancer Res. 2011;71(3):862–872. doi: 10.1158/0008-5472.CAN-10-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldenberg-Furmanov M, Stein I, Pikarsky E, Rubin H, Kasem S, Wygoda M, Weinstein I, Reuveni H, Ben-Sasson SA. Lyn is a target gene for prostate cancer: Sequence-based inhibition induces regression of human tumor xenografts. Cancer Res. 2004;64(3):1058–1066. doi: 10.1158/0008-5472.can-03-2420. [DOI] [PubMed] [Google Scholar]
- 9.Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, Gelovani JG, Kim SJ, Wang Z, Gallick GE. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008;68(9):3323–3333. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- 10.Green TP, Fennell M, Whittaker R, Curwen J, Jacobs V, Allen J, Logie A, Hargreaves J, Hickinson DM, WilkinBon RW, Elvin P, Boyer B, Carragher N, Ple PA, Bermingham A, Holdgate GA, Ward WH, Hermequin LF, Davies BR, Costello GF. Preclinical anticancer activity of the potent, oral Src inhibitor AZD0530. Mol Oncol. 2009;3(3):248–261. doi: 10.1016/j.molonc.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, Gore M, Desai AA, Patnaik A, Xiong HQ, Rowinsky E, Abbruzzese JL, Xia C, Simantov R, Schwartz B, O'Dwyer PJ. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(16):2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 12.Stadler WM, Rosner G, Small E, Hollis D, Rini B, Zaentz SD, Mahoney J, Ratain MJ. Surcessful implementation of the randomized discontinuation trial design: An application to the study of the putative antiangiogenic agent carboxyaminoimidazole in renal cell carcinoma-CALGB 69901. J Clin Oncol. 2005;23(16):3726–3732. doi: 10.1200/JCO.2005.44.150. [DOI] [PubMed] [Google Scholar]
- 13.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 14.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Jr., Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Flechon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI, Investigators C-A- Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Flechon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS, Investigators A Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 16.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Investigators IS Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 17.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, Roessner M, Gupta S, Sartor AO, Investigators T Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment A randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 18.Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fossa SD, Chodacki A, Wiechno P, Logue J, Seke M, Widmark A, Johannessen DC, Hoskin P, Bottomley D, James ND, Solberg A, Syndikus I, Kliment J, Wedel S, Boehmer S, Dall'Oglio M, Franzen L, Coleman R, Vogelzang NJ, O'Bryan-Tear CG, Staudacher K, Garcia-Vargas J, Shan M, Bruland OS, Sartor O, Investigators A Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 19.Jensen AR, Saito YD, Liao C, Dai J, Keller ET, Al-Ahmadie HA, Dakin Hache K, Usatyuk P, Sievert MF, Paner G, Yala S, Cervantes GM, Natarajan V, Salgia R, Posadas EM. Fyn is downstream of the HGF/MET signaling axis and affects cellular shape and tropism in PC3 cells. Clin Cancer Res. 2011;17(10):3112–3122. doi: 10.1158/1078-0432.CCR-10-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lara PN, Longmate J, Evans CP, Quinn DI, Twardowski P, Chatta G, Posadas E, Stadler W, Gandara DR. A phase II trial of the Src-kinase inhibitor AZD0530 in patients with advanced castration-resistant prostate cancer: A California Cancer Consortium Study. Anticancer Drugs. 2009;20(3):179–184. doi: 10.1097/CAD.0b013e328325a867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aragon-Ching JB, Dahut WL. VEGF inhibitors and prostate cancer therapy. Current Mol Pharmacol. 2009;2(2):161–168. doi: 10.2174/1874467210902020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahut WL, Scripture C, Posadas E, Jain L, Gulley JL, Arlen PM, Wright JJ, Yu Y, Cao L, Steinberg SM, Aragon-Ching JB, Venitz J, Jones E, Chen CC, Figg WD. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clin Cancer Res. 2008;14(1):209–214. doi: 10.1158/1078-0432.CCR-07-1355. [DOI] [PubMed] [Google Scholar]
- 23.Dixon SC, Kruger EA, Bauer KS, Figg WD. Thalidomide upregulates prostate-specific antigen secretion from LNCaP cells. Cancer Chemother Pharmacol. 1999;43(Suppl):S78–S84. doi: 10.1007/s002800051103. [DOI] [PubMed] [Google Scholar]
- 24.Ost P, Decaestecker K, Lambert B, Fonteyne V, Delrue L, Lumen N, Ameye F, De Meerleer G. Prognostic factors influencing prostate cancer-specific survival in non-castrate patients with metastatic prostate cancer. Prostate. 2014;74(3):297–305. doi: 10.1002/pros.22750. [DOI] [PubMed] [Google Scholar]
- 25.Pond GR, Sonpavde G, de Wit R, Eisenberger MA, Tannock IF, Armstrong AJ. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur Urol. 2014;65(1):3–6. doi: 10.1016/j.eururo.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Li B, Zhang P, Yao Y, Chang J. Clinical characteristics and prognostic factors of prostate cancer with liver metastases. Tumour Biol. 2014;35(1):595–601. doi: 10.1007/s13277-013-1083-6. [DOI] [PubMed] [Google Scholar]
- 27.Lu YT, Zhao L, Shen Q, Garcia MA, Wu D, Hou S, Song M, Xu X, Ouyang WH, Ouyang WW, Lichterman J, Luo Z, Xuan X, Huang J, Chung LW, Rettig M, Tseng HR, Shao C, Posadas EM. NanoVelcro Chip for CTC enumeration in prostate cancer patients. Methods. 2013;64(2):144–152. doi: 10.1016/j.ymeth.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao L, Lu YT, Li F, Wu K, Hou S, Yu J, Shen Q, Wu D, Song M, Ouyang WH, Luo Z, Lee T, Fang X, Shao C, Xu X, Garcia MA, Chung LW, Rettig M, Tseng HR, Posadas EM. High-purity prostate circulating tumor cell isolation by a polymer nano-fiber-embedded microchip for whole exome sequencing. Adv Mater. 2013;25(21):2897–2902. doi: 10.1002/adma.201205237. [DOI] [PMC free article] [PubMed] [Google Scholar]