Abstract
Mutations in NLGN4X have been identified in individuals with autism spectrum disorders and other neurodevelopmental disorders. A previous study reported that adult male mice lacking neuroligin4 (Nlgn4) displayed social approach deficits in the three-chambered test, altered aggressive behaviors and reduced ultrasonic vocalizations. To replicate and extend these findings, independent comprehensive analyses of autism-relevant behavioral phenotypes were conducted in later generations of the same line of Nlgn4 mutant mice at the National Institute of Mental Health in Bethesda, MD, USA and at the Institut Pasteur in Paris, France. Adult social approach was normal in all three genotypes of Nlgn4 mice tested at both sites. Reciprocal social interactions in juveniles were similarly normal across genotypes. No genotype differences were detected in ultrasonic vocalizations in pups separated from the nest or in adults during reciprocal social interactions. Anxiety-like behaviors, self-grooming, rotarod and open field exploration did not differ across genotypes, and measures of developmental milestones and general health were normal. Our findings indicate an absence of autism-relevant behavioral phenotypes in subsequent generations of Nlgn4 mice tested at two locations. Testing environment and methods differed from the original study in some aspects, although the presence of normal sociability was seen in all genotypes when methods taken from Jamain et al. (2008) were used. The divergent results obtained from this study indicate that phenotypes may not be replicable across breeding generations, and highlight the significant roles of environmental, generational and/or procedural factors on behavioral phenotypes.
Keywords: Adult social interaction, autism, juvenile social interaction, mouse models, neuroligin4, resident–intruder, three-chambered social approach task, ultrasonic vocalizations
Autism spectrum disorders (ASDs) are diagnosed on the basis of two main behavioral features: impairments in reciprocal social interactions, communication, restricted interests and/or stereotyped behaviors, with restricted interests (American Psychiatric Association and DC, 2011). The clinical heterogeneity of ASD ranges from profound to moderate impairments and mild personality traits. Cognitive and language deficits are not always present as observed in patients with Asperger syndrome. In 15–25% of the cases of ASD, a genetic mutation is detected using genomic arrays or sequencing (O’Roak et al. 2012a,b; Pinto et al. 2010; Sanders et al. 2011, 2012). Mutations can be structural genomic imbalances such as copy number variants, small insertions/deletions, or single nucleotide variants. Network analyses indicate a significant enrichment in genes coding for proteins involved in synapse formation or functions (Geschwind 2011; Gilman et al. 2011; Toro et al. 2010; Voineagu et al. 2011). These include synaptic scaffolding proteins (e.g. SHANK1-3) and cell adhesion molecules [e.g. neurexin (NRXN) and neuroligins (NLGN)].
The NLGNs are crucial factors for synaptic contact initiation, recruitment of presynaptic and postsynaptic proteins, synapse maturation/stabilization or elimination and synaptic plasticity (Bourgeron 2009; Sudhof 2008). Five NLGNs are present in the human genome (NLGN1, NLGN2, NLGN3, NLGN4X and NLGN4Y). Of these, the X-linked genes, namely NLGN3 and NLGN4X, were the first to be associated with ASD (Jamain et al. 2003). Remarkably, the phenotype of patients carrying mutations in these genes is largely variable, even between patients from the same family and carrying identical mutations. The first study reported a frame-shift mutation in NLGN4X gene in two brothers, one with typical autism and the second with Asperger syndrome (Jamain et al. 2003). In the same study, a non-synonymous point mutation (R451C) of the NLGN3 gene was reported in two brothers diagnosed respectively with typical autism and Asperger syndrome. Mutations within NLGN4X were subsequently associated with other neurodevelopmental disorders such as intellectual developmental delay (Laumonnier et al. 2004), typical autism (Yan et al. 2005) and Gilles de la Tourette syndrome (Lawson-Yuen et al. 2008; Macarov et al. 2007), a neurological disorder characterized by motor and vocal tics and behavioral anomalies (Robertson 2012). To date, only a single case of a NLGN4X deletion in a male with normal intelligence and apparently no autistic-like features has been reported (Macarov et al. 2007).
NLGNs are highly conserved evolutionarily, except for mouse Nlgn4 that rapidly evolved from other mammalian NLGNs, suggesting that its function in the brain is under less stringent control than that of other NLGNs (Bolliger et al. 2008). In mice, the Nlgn4 gene is located on the pseudoautosomal region 1 located on the top of the X and Y chromosome and exhibits sequence variations among mouse strains (Bolliger et al. 2008). Despite its divergence, mouse Nlgn4 binds NRXNs and is transported into dendritic spines, suggesting that the core properties of NLGNs are preserved. Nlgn4 is preferentially targeted at inhibitory synapses in the retina and in several areas of the central nervous system, including thalamus, colliculi, brainstem and spinal cord, and forms complexes with the inhibitory postsynaptic proteins gephyrin and collybistin (Hoon et al. 2011). Following the initial association between NLGN mutations and ASD, mice lacking Nlgn4 were generated (Jamain et al. 2008). On the behavioral level, the Nlgn4 null mutant mice (Nlgn4−/−) displayed reduced interest for social interactions in the three-chambered social approach test, decreased contact time in free interactions between males of the same genotype and increased latency to the first attack with no correlation between attack and escape behaviors in the resident–intruder test. Interestingly, Nlgn4−/− males emitted fewer ultrasonic vocalizations in the presence of an estrus female, with longer latencies to the first vocalization, in comparison with wild-type males. In addition, Nlgn4−/− males displayed reduced volumes of the whole brain, cerebellum, and brainstem, as compared to wild-type mice (Jamain et al. 2008).
Considering the large diversity of phenotypes in patients carrying similar genetic mutations, we decided to replicate and extend the findings reported by Jamain et al. (2008) in new cohorts of the same line of Nlgn4−/− mice. The study was conducted at two different sites [National institute of Mental Health (NIMH), Bethesda, MD, USA and Institut Pasteur, Paris, France], using later generations of Nlgn4−/− mice generated in the original laboratory and generously contributed by Prof. Nils Brose (MPI Experimental Medicine, Göttingen, Germany). A third site in Göttingen, Germany is currently pursuing parallel analyses of a revitalized earlier generation of the same Nlgn4 line (El-Kordi et al., in press).
Materials and methods
Subjects
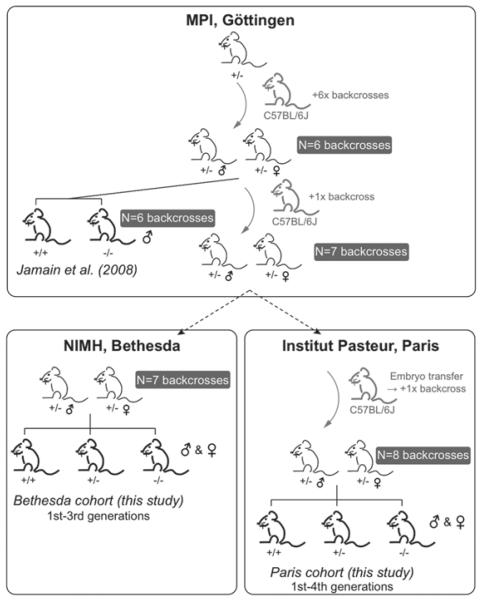
Mice with a null mutation in Nlgn4 were generated at the Max Planck Institute, as previously described (Jamain et al. 2008). The original Nlgn4 line was generated using a 129P2/OlaHsd embryonic stem-cell (ES-cell) clone (XST093) carrying a gene trap insertion 340 bp downstream of the first exon of Nlgn4 (BayGenomics, San Francisco, CA, USA). One chimeric mouse was obtained after blastocyst injection of XST093 ES cells at BayGenomics and bred with C57BL/6J females. Mutant animals were obtained after germ line transmission of the ES cells. Subjects used in the Jamain study were backcrossed onto the C7BL/6J (B6) background for six generations. After an additional backcross, mice were exported from the Max Planck Institute to the NIMH (Bethesda, MD, USA) and the Institut Pasteur (Paris, France) where two cohorts of mice were independently generated and tested in two laboratories. The Bethesda cohort was established by mating heterozygous males and heterozygous females directly imported from the Max Planck Institute, with no additional back-crossing. At the Paris site, mice from the Max Planck Institute were backcrossed onto B6 for an additional time before heterozygous (het) heterozygous (het) breeding began. Hence the Bethesda cohort had a total of seven backcrosses and the Paris cohort had eight back-crosses. At both the Bethesda and the Paris sites, later generations were generated by inbreeding non-sibling heterozygous males with heterozygous females. Wild-type, heterozygous and homozygous littermate offspring from these het × het mating pairs were used for all behavioral experiments (Fig. 1). Genotype was determined by polymerase chain reaction (PCR) analysis, with Nlgn4 specific primer sequence [Bethesda cohort: forward (Nlgn4+/+ and Nlgn4−/−): CTGCCTGTACCTCAACCTCTACGTG; reverse (Nlgn4+/+): TAGGGAAAGCG GAATTGAGTGTAAC; reverse (Nlgn4−/−): TACACTCCAACCTCCG CAAACTCCT. Paris cohort: forward (Nlgn4+/+ & Nlgn4−/−): GTAC CTCAACCTCTACGTGC; reverse (Nlgn4+/+): CACAGGGACGCGAC CTCGC; reverse (Nlgn4−/−): ACACTCCAACCTCCGCAAACTCCT]. Mice of the Bethesda cohort were weaned at 3 weeks of age, and group housed by sex in cages of 2–4 littermates per cage. Standard rodent chow and tap water were available ad libitum. In addition to standard bedding, a Nestlet square and a cardboard tube were provided in each cage. The colony room was maintained on a 12:12 light/dark cycle, with lights on at 0700 h, at approximately 20°C and 55% humidity. Adult mice from the first, second and third generations bred from the original pairs received at NIMH were tested between 2 and 4 months of age. All experiments were conducted between 0900 h and 1700 h. Equipments were cleaned with 70% ethanol and water between subjects. Mice of the Paris cohort were weaned at 4 weeks of age, and thereafter housed by sex with 2–4 animals per cage. Pups tested for ultrasonic vocalizations and developmental milestones were from the first and second generations bred from the original pairs received at Institut Pasteur. Adult mice used for all other tests were from the third and fourth generations bred from the original pairs received at Institut Pasteur and were tested between 3 and 4 months of age. Adult males were isolated 3 weeks before the start of experiments; females were not isolated. Both sexes were tested in most behavioral experiments, with the exception of the male–female social interaction test, a test designed only for males, and of the open field exploration at the Paris site. Besides sawdust bedding, no additional enrichments were provided in the housing cages. The colony room was maintained at 23 ± 1 C on a 12:12 h light/dark cycle, with lights on at 0800 h. All experiments were conducted between 0930 h and 1800 h. Equipment was cleaned with soap and water and dried with paper towels between subjects. When bedding was used, the cage was cleaned with soap and water, dried, and new fresh bedding was used. Mice were individually identified (Bethesda cohort: paw tattoos and Paris cohort: paw tattoos in pups, ear punches in adults). Data were collected and analyzed by experimenters blind to the genotype of the animals. All procedures were conducted in strict compliance with National Institute of Health regulations and approved by the National Institute of Mental Health Animal Care and Use Committee, and by the ethical committee of Ile-de-France (CEEA Ile-de-France Comité 1), in the Bethesda and Paris cohorts, respectively.
Figure 1. An illustration of breeding strategies used in the Jamain et al. (2008) study and the present study.
In the Jamain study, Nlgn4 mutations were generated with 129P2/OlaHsd ES cells. The mutation was back-crossed onto the C57BL/6J (B6) strain for six generations before behavioral experiments started. The mice were backcrossed onto B6 for one additional generation before being exported to Bethesda and Paris. Mice arrived at the Bethesda site did not undergo further backcrossing. Mice arrived at the Paris site were backcrossed onto B6 for an additional time. The standard heterozygous × heterozygous breeding scheme was used in the Jamain study, as well as at both sites in this study.
Developmental milestones and pup ultrasonic vocalizations
The Bethesda cohort was tested for assays of developmental milestones every other day from postnatal days 2, 4, 6, 8, 10, 12 and 14, as previously described (Chadman et al. 2008; Scattoni et al. 2008). Physical developmental milestones were body weight, body and tail lengths, fur development, eye opening, pinna detachment and incisor eruption. Behavioral developmental milestones were righting reflex, negative geotaxis, cliff aversion, forepaw grasping reflex, auditory startle, level screen, screen climbing and bar holding. The cut-off latency for the righting reflex was 30 seconds. The Paris cohort was tested for separation-induced ultrasonic vocalizations and developmental milestones on the same days (postnatal days 2, 4, 6, 8, 10 and 12) as described below. Each pup was taken from the nest and placed in a small container with a soft plastic bottom, inside a sound-attenuating chamber maintained at 23 ± 1°C. Isolation calls were recorded for 5 min with a Condenser ultrasound microphone Polaroid, the interface UltraSoundGate 416–200, and the software avisoft saslab pro v.4.40 recorder v3.2 from Avisoft Bioacoustics (Berlin, Germany; 16 bit format; sampling frequency: 300 kHz). At least 30 min after the vocalization test, each pup was weighed and tested for behavioral developmental milestones, including body weight, righting reflex (from P2 to P10) and negative geotaxis (from P2 to P12). The cut-off latency for the righting reflex is 120 seconds. Olfactory functions in pups were evaluated on P7, in the home cage odor preference test. The pup was placed in a cage (20 × 30 × 6 cm) divided into three zones. One side was covered with bedding from the nest cage and the other side with fresh bedding. The separation between these two zones was made of a neutral zone (width: 2.5 cm). The pup was placed in the middle of the neutral zone. Cumulative time with the nose in each zone was scored for 60 seconds. Each pup was tested twice in this test on the same day and mean time was considered for statistical analysis.
Juvenile reciprocal social interactions
The Bethesda cohort was tested for juvenile reciprocal social interactions between age days 21 and 24. The test was conducted in the Noldus PhenoTyper Observer 3000 chamber (25 × 25 × 35 cm, Noldus Information Technology, Leesburg, VA, USA) as previously described (Yang et al. 2009, 2012). The floor of the arena was covered with a 0.5-cm layer of clean bedding. Each subject mouse was singly housed in a clean cage for 1 h before the test. After this brief isolation period, the subject mouse and an age- and sex-matched B6 partner mouse were simultaneously placed in the arena and their interactions were videotaped for 10 min. Social interactions were scored by a highly trained observer, using the noldus observer v.5.0 software. Parameters of social behaviors included nose-to-nose sniff (sniffing the nose and snout region of the partner), front approach (moving toward the partner from a distance of approximately half a body length, in a head-on manner), follow (walking straight behind the partner, keeping pace with the one ahead), nose-to-anogenital sniff (sniffing the anogenital region of the partner) and push-crawl (pushing the head underneath the partner’s body, squeezing between the wall/floor and the partner, and crawling over or under the partner’s body are similar behaviors which were combined into a single parameter). Besides social behaviors, non-social arena exploration (walking around the arena, rearing, or sniffing the wall) and bouts of self-grooming were scored as measures of exploratory activity and repetitive behavior, respectively. All behaviors were analyzed for frequency of occurrence, i.e. number of bouts.
Automated three-chambered social approach task
Identical automated three-chambered equipment and accompanying software (NIMH Research Services Branch, Bethesda, MD, USA) was used in Bethesda and in Paris (Silverman et al. 2011, 2012; Yang et al. 2012). Time spent sniffing the object and time spent sniffing the novel mouse during the 10-min sociability session, as well as time spent sniffing the two target mice during the 10-min preference for social novelty test session, were later scored from video recordings of the sessions, by observers using stopwatches.
Two batches of animals in the Bethesda cohort were tested. One cohort was tested using the method routinely used in our Laboratory of Behavioral Neuroscience (LBN) (Silverman et al. 2010, Yang et al. 2011; Silverman et al. 2012; Yang et al. 2012). This method began by placing the subject mouse in the center chamber for a 10-min habituation to the center only. The doors to the side chambers were then lifted, and the subject was allowed to explore all three empty chambers for another 10 min. Lack of innate side preference was confirmed during the second 10-min habituation. The subject was then briefly confined to the center chamber while the clean novel object (an inverted stainless steel wire pencil cup, Galaxy, Kitchen Plus, http://www.kitchen-plus.com, Columbus, OH, USA) was placed in one of the side chambers. A novel 129S1/SvImJ mouse previously habituated to the enclosure was placed in an identical wire cup located in the other side chamber. A disposable plastic drinking cup containing a lead weight was placed on the top of each inverted wire pencil cup to prevent the subject from climbing on top. The side containing the novel object and the novel mouse alternated between the left and right chambers across the subjects. After both stimuli were positioned, the two side doors were simultaneously lifted and the subject was allowed access to all three chambers for 10 min. The light intensity in the side chambers was approximately 15 lx. The apparatus was cleaned with 70% ethanol and water between subjects. The light level in the side chambers was approximately 15 lx. 129S1/SvImJ was used as the target novel mouse because this strain is generally inactive, passive and does not exhibit aggressive behaviors toward subject mice (Yang et al. 2011). Using a minimally active partner is a strategy that allows all approaches to be initiated by the subject mouse only.
The second batch was tested using the method described by Jamain et al. (2008). The Jamain method differs from the LBN method in that: (1) the subject was habituated to the center chamber for a single 5-min session, (2) B6 mice were used as novel mice and (3) fresh bedding was scattered on the floor of the apparatus. The Paris cohort was tested using a third method. The method differs from the LBN method in that: (1) there was no habituation to the center chamber only, (2) during the 10-min habituation session, an empty wire cup was placed in each side chamber, such that the subject was habituated to all three chambers and the wire cups. The wire cup was therefore not a novel object during the subsequent social test session, (3) B6 mice were used as novel mice, (4) brighter illumination (150 lx) was used. Methodological differences are summarized in Table S2. The Paris cohort was also tested for the preference for social novelty (Schmeisser et al. 2012). After the social approach session described above, the subject was again confined to the center chamber while a second unfamiliar B6 mouse of the same sex was placed under the previously empty cup. The subject mouse was then allowed to freely explore all three chambers for 10 min.
Same-sex resident–intruder test and male–female social interactions
Adult mice of the Paris cohort were tested in the resident–intruder paradigm as previously described (Bourgeron et al. 2005; Schmeisser et al. 2012). Prior to the interaction test, the subject mouse was placed in a clean Plexiglas cage (50 × 25 × 30 cm) with fresh bedding, for a 30 min habituation session. After the habituation period, an unfamiliar B6 mouse of the same sex was introduced into the cage. The two animals were allowed to freely interact for 4 min. Ultrasonic vocalizations were recorded using the same equipment and settings as described above. The test was conducted under low light illumination (100 lx).
Adult male mice of the Paris cohort were tested for male–female social interactions, as previously described (Jamain et al. 2008; Schmeisser et al. 2012). Male subjects used in the experiment were not sexually naïve, they were co-housed with a female for 3 days and then isolated again for 2 days before the test. The subject male was placed in a clean empty cage (Plexiglas, 50 × 25 × 30 cm) for a 10-min habituation session. An unfamiliar B6 female in estrus (detected by vaginal smears) was then introduced into the cage. Social interactions were videotaped for subsequent scoring of the latency for the first contact and time spent in contact. Ultrasonic vocalizations were recorded for 3 min with the same equipment and settings described above. The test was conducted under low light illumination (100 lx).
Repetitive self-grooming
The Bethesda cohort was scored for spontaneous grooming behaviors when placed individually in a clean, empty mouse cage without bedding, using methods previously described (Yang et al. 2007, 2009). Each mouse was given a 10-min habituation period in the empty cage and then rated for 10 min for cumulative time spent grooming all body regions. The test session was videotaped and scored later by two trained observers, blind to genotype. Inter-rater reliability was >95%.
Elevated plus-maze and light ↔ dark exploration tests of anxiety-like behaviors
The Bethesda cohort was tested for anxiety-like behaviors in the elevated plus-maze test and the light ↔ dark exploration test, using previously described methods (Chadman et al. 2008; Crawley & Goodwin 1980; Silverman et al. 2011; Yang et al. 2009). The elevated plus-maze consisted of two open arms (30 × 5 cm) and two closed arms (30 × 5 × 15 cm) extending from a central area (5 × 5 cm). Room illumination was approximately 30 lx. The test began by placing the subject mouse in the center, facing a closed arm. The mouse was allowed to freely explore the maze for 5 min. Time spent in the open arms and closed arms, and number of entries into the open arms and closed arms, were scored using observer software (Noldus Information Technology). The light ↔ dark exploration test was conducted in an automated chamber (NIMH Research Services Branch). The test began by placing the mouse in the light compartment facing away from the partition. The animal was allowed to freely explore the apparatus for 10 min. Time spent in each compartment and number of transitions between the light (350 lx) and dark (3 lx) compartments were automatically recorded.
Open field activity
The Bethesda cohort was tested for general exploratory locomotion in a square open field arena (40 × 40 cm, VersaMax Animal Activity Monitoring System, Accuscan, Columbus, OH, USA) for a 10-min session, under a dim illumination of 30 lx. Total distance traveled in the arena, vertical activity, horizontal activity and time spent in the center were automatically measured by software linked to the photocell detectors. The Paris cohort was tested in a circular open field arena (1 m in diameter) for 30 min, under a low illumination of 100 lx. The test session was video-recorded and analyzed for time spent in the central zone vs. time spent at the periphery, and total distance traveled (custom software, Labview National Instruments, Austin, TX, USA).
General health, neurological reflexes, pain sensitivity and motor coordination in adults
Measures of general health and neurological reflexes were evaluated in the Bethesda cohort as previously described (Silverman et al. 2011; Yang et al. 2009). General health was assessed by fur condition, whisker condition, body weight, body temperature, body and limb tone and three 15-min observations of home cage behaviors at different phases of the circadian cycle. Neurological reflexes were assessed by forepaw reaching, righting reflex, trunk curl, whisker twitch, pinna response, eyeblink response and auditory startle. Behavioral reactivity was evaluated as responsiveness to petting, intensity of dowel biting and level of audible vocalizations when handled. Empty cage behaviors were scored by placing the mouse into a clean, empty cage and noting wild running, stereotypies and exploratory behaviors such as rearing and jumping. Response to thermal stimulation of the feet and tail was measured as previously described (Chadman et al. 2008; Silverman et al. 2011). The hot plate test was conducted by placing the mouse on an arena surface kept at a constant temperature of 55 C (IITC Life Science Inc., Woodland Hills, CA, USA). Latency to first response, such as licking or shaking paws, was recorded. To prevent tissue damage, a cut-off latency of 30 seconds was applied. Motor coordination was assessed using a mouse accelerating rotarod (Ugo Basile, Collegeville, PA, USA). Mice were tested for three consecutive trials on the rotating drum that accelerated from 4 to 40 r.p.m over 5 min. The inter-trial interval was 1 min. Latency to fall was scored for each trial.
Analyses of audio recordings
We first confirmed the accuracy of automatic detection of pup isolation calls (pulse train detection analyzes by avisoft saslab pro, Avisoft, Germany; hold time: 7 milliseconds) in a subset of files in which ultrasonic vocalizations (USVs) were also manually detected by a highly trained experimenter. Pup isolation calls were then detected automatically. Vocalizations recorded in adult animals were detected manually using spectrograms generated by the software avisoft saslab pro (Avisoft Bioacoustics, Germany; FFT-length: 1024 points; 75% overlap; time resolution: 0.853 milliseconds; frequency resolution: 293 Hz; Hamming window). Ultrasonic vocalizations were quantified by measuring call rate in both pups and adults, and latency to the first call in adults.
To analyze call categories, pup vocalizations on P8, as well as adult vocalizations were manually labeled using avisoft saslab pro (FFT-length: 1024 points; 75% overlap; time resolution: 0.853 milliseconds; frequency resolution: 293 Hz; Hamming window). Each call was classified into 1 of 11 call categories. The categorization criteria were adapted from Scattoni et al. (2008), and are based on frequency modulations and call duration:
Short: duration shorter than 5 milliseconds; frequency range ≤ 6.25 kHz.
Flat: duration longer than 5 milliseconds and frequency range ≤ 6.25 kHz.
Upward: increase in frequency; frequency range >6.25 kHz with only one direction of frequency modulation.
Downward: decrease in frequency; frequency range >6.25 kHz with only one direction of frequency modulation.
Modulated: frequency modulations in more than one direction; frequency range >6.25 kHz.
Complex: addition of one or more frequency component (not necessarily harmonic).
One frequency jump: inclusion of one jump in frequency without time gap between the two frequency components.
Multiple frequency jumps: inclusion of more than one jump in frequency without time gaps between the two consecutive frequency components.
Mixed: inclusion of a noisy (‘unstructured’) part within a pure tone call.
Unstructured: no pure tone component identifiable; ‘noisy’ calls.
Others: include all the calls which did not fit in any of the preceding categories (e.g. calls combining features of several of the previous call types).
Statistical analyses
For most experiments, data of males and females were analyzed separately. Sexes were combined for statistical analyses of developmental milestones and pup vocalizations.
Bethesda cohort
For the three-chambered social approach test, Repeated Measures analysis of variances (anovas) were used to compare time spent in the two side chambers, with the factor of chamber side (novel mouse side vs. novel object side). Time spent sniffing the novel mouse vs. the novel object was similarly analyzed. Time spent in the center chamber is included on the graphs for illustrative purposes, but not included in the statistical analysis. The ANOVAs with repeated measurements (between-subject factors: genotype and within-subject factor: age) were used to analyze developmental milestones. All other behavioral tests conducted in Bethesda were analyzed using one-way anova, with Scheffe test for post hoc comparisons. Data of the Bethesda cohort were analyzed with statview v.5.0 software (SAS Institute Inc, Cary, NC, USA).
Paris cohort
The ANOVAs with repeated measurements (between-subject factors: genotype, sex and within-subject factor: age) were used to estimate the effect of age, sex and genotype on the call rate for pup isolation calls. The χ2 tests were used to compare the distribution of the different call types and percentage of time spent in the nest bedding in the homing test. For the three-chambered social approach test, repeated measures anovas were used to compare time spent in the two side chambers, with the factor of chamber side (novel mouse side vs. empty cup side). Time spent sniffing the novel mouse vs. the novel object was similarly analyzed. For adult experiments with small sample size and non-normal distribution, Wilcoxon–Mann–Whitney U-tests were used to compare differences among genotypes. Data of the Paris cohort were analyzed with statistical software r (R Developmental Core Team 2009).
Results
Absence of significant genotype differences in developmental milestones and pup ultrasonic vocalizations
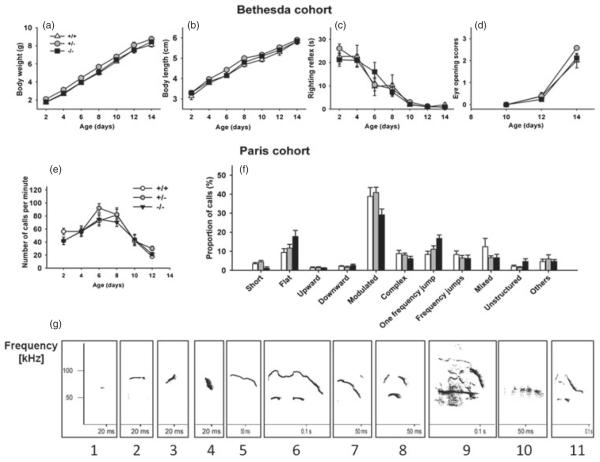
In the Bethesda cohort, no significant genotype differences were observed on measures of early developmental milestones, including body weight [F2,40 =0.33, nonsignificant (NS); Fig. 2a], body length (F2,40 =0.20, NS; Fig. 2b), righting reflex (F2,55 =0.01, NS; Fig. 2c) and eye opening (F2,40 =2.04, NS; Fig. 2d). Similar results were found in the Paris cohort (Figure S1). In the pup isolation paradigm, a significant effect of age was detected on the rate of calls (F1,58 =20.06, P <0.001). No significant differences were found between sexes (F1,58 =0.09, NS) nor across genotypes (F2,58 =0.99, NS; Fig. 2e). No significant genotype differences were found in the vocal repertoire. Separation-induced pup USVs consisted ofmore than 30% of modulated calls, 10% of flat calls and 10% of one-frequency-jump calls and small numbers of upward and downward calls (Fig. 2f,g).
Figure 2. No genotype differences were found in early developmental milestones (Bethesda cohort) and separation-induced pup ultrasonic vocalizations (Paris cohort) in Nlgn4 mice.
Analysis of markers of developmental milestones revealed no genotype differences in wild-type (+/+), heterozygous Nlgn4+/− (+/−) and null mutant Nlgn4−/− (−/−) pups between age day 2 and day 14, on measures of (a) body weight, (b) body length, (c) righting reflex and (d) eye opening. +/+, N =8; +/−, N =18; −/−, N =17. (e) Number of ultrasonic vocalizations emitted by pups separated from the nest did not differ significantly among genotypes. +/+, N =15–17; +/−, N =25–31; −/−, N =20. (f) Call repertoires were similar among genotypes on age day 8. +/+, N =11; +/−, N =18; −/−, N =14. (g) Spectrograms of the 11 call types analyzed. Numeric labels: 1=short; 2=flat; 3=upward; 4=downward; 5=modulated; 6=complex; 7=one frequency jump; 8=frequency jumps; 9=mixed; 10=unstructured; 11=others. Data are presented as mean±SEM in this figure and all other figures.
No evidence of impairments in juvenile reciprocal social interactions
No deficits were found in any measures of social behaviors, repetitive behaviors and exploratory activity during reciprocal social interactions in male Nlgn4−/− juveniles of the Bethesda cohort (Fig. 3). A significant genotype effect was found on nose-to-nose sniff (F2,29 =5.47, P <0.01; Fig. 3a). However, post hoc comparisons did not show significant differences between Nlgn4+/+ and Nlgn4−/− or between Nlgn4+/+ and Nlgn4+/−. No significant genotype effects were found on follow (F2,29 =0.46, NS; Fig. 3b), nose-to-anogenitial sniff (F2,29 =2.94. NS; Fig. 3c), push-crawl (F2,29 =0.47, NS; Fig. 3d), bouts of self-grooming (F2,29 =0.65, NS; Fig. 3e) and arena exploration (F2,29 =0.78, NS; Fig. 3f). Similar results were found in female juveniles of the Bethesda cohort (Figure S2).
Figure 3. Juvenile reciprocal social interaction behaviors in 21-day old male Nlgn4 mice of the Bethesda cohort.
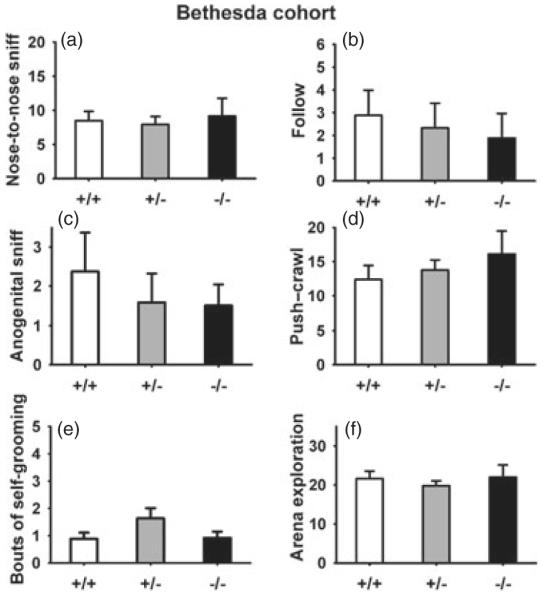
No significant genotype differences were found on measures of (a) nose-to-nose sniff, (b) follow, (c) nose-to-anogenital sniff and (d) push–crawl, or on bouts of (e) self-grooming and (f) arena exploration. +/+, N = 9; +/−, N = 13; −/−, N = 10.
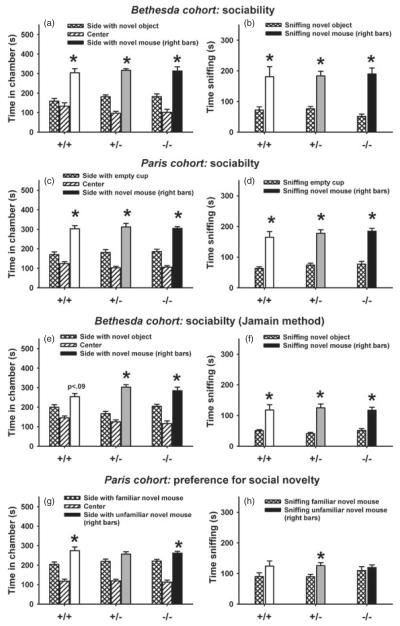
No evidence of impaired adult social approach in the automated three-chambered task
Figure 4 shows normal sociability in adult male Nlgn4 mice of the Bethesda cohort tested using the LBN method, of the Bethesda cohort tested using methods from Jamain et al. (2008), and of the Paris cohort tested using the Paris method. Males of all genotypes spent significantly more time in the chamber containing the novel mouse than in the chamber containing the novel object (Bethesda cohort; Fig. 4a,e) or the empty cup (Paris cohort; Fig. 4c), and spent more time sniffing the novel mouse than the novel object (Fig. 4b,f) or the empty cup (Fig. 4d), with the exception of wild-type males tested in Bethesda using the Jamain method (Fig. 4e). (a, b) Bethesda cohort males tested with LBN method. Chamber time: +/+, F1,6 =21.55, P <0.01; +/−, F1,11 =76.54, P <0.001; −/−, F1,12 =15.90, P <0.01. Sniff time: +/+, F1,6 =8.50, P <0.05; +/−, F1,11 =29.91, P <0.001; −/−, F1,12 =38.70, P <0.001. (c, d) Paris cohort males tested with Paris method. Chamber time: +/+, F1,10 =22.32, P <0.001; +/−, F1,14 =17.89, P <0.001; −/−, F1,13 =38.19, P <0.001. Sniff time: +/+, F1,0 =22.22, P <0.001; +/−, F1,14 =44.18, P <.001; −/−, F1,13 =46.77, P <0.001. (e, f) Bethesda cohort males tested with the Jamain method. Chamber time: +/+, F1,8 =3.65, P =0.09; +/−, F1,7 =34.38, P <0.001; −/−, F1,7 =10.35, P <0.05. Sniff time: +/+, F1,8 =17.01, P <0.01; +/−, F1,7 =36.22, P <0.001; −/−, F1,7 =30.00, P <0.001. (g, h) Paris cohort males preference for social novelty. Chamber time: +/+, F1,10 =5.35, P <0.05; +/−, F1,14 =2.68, NS; −/−, F1,13 =7.31, P <0.05. Sniff time: +/+, F1,10 =3.02, NS; +/−, F1,14 =9.01, P <0.01; −/−, F1,13 =0.49, NS. Similar results were found in female mice tested at the two sites (Figure S3).
Figure 4. Normal sociability in adult male Nlgn4 mice tested in the automated three-chambered social approach task.
(a, b) Bethesda cohort tested using the LBN method; +/+, N =7; +/−, N =12; −/− N =13. (c, d) Paris cohort tested using the Paris method: +/+, N =11; +/−, N =15; −/−, N =14, (e, f) Bethesda cohort tested using method described in Jamain et al. (2008): +/+, N =9; +/−, N =8; −/−, N =8. (g, h) Preference for social novelty: +/+, N =11; +/−, N =15; −/−, N =14. (a, b, e, f) *P <0.05, comparison between the novel mouse side and the novel object side; (c, d) *P <0.05, comparison between the novel mouse side and the empty cup side; (g, h) *P <0.05, comparison between the familiar mouse side and the unfamiliar target mouse side.
No evidence of impairments in adult reciprocal social interactions and concomitant ultrasonic vocalizations
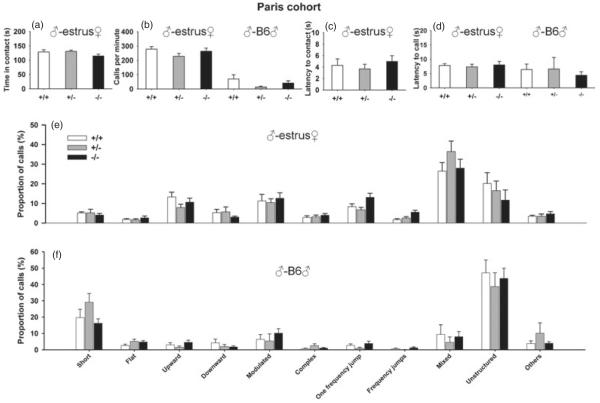
In the male–female social interaction test, no significant differences were found between wild-type and Nlgn4−/− mice or between wild-type and Nlgn4+/− mice on time in contact with the female (+/+ vs. −/−: W =47, P =0.47; +/+ vs. +/−: W =16.5, P =0.94; Fig. 5a), call rate (+/+ vs. −/−: W =37, P =0.93; +/+ vs. +/−: W =33, P =0.10; Fig. 5b), the latency to first contact with the female (+/+ vs. −/−: W =33, P =0.66; +/+ vs. +/−: W =19, P =0.88; Fig. 5c) and the latency to emit the first call (+/+ vs. −/−: W =42, P =0.79; +/+ vs. +/−: W =24, P =0.73; Fig. 5d). Analysis of the vocal repertoire revealed no significant differences in the distribution of calls among the 11 call categories (Figs 5e). In all three genotypes, the call repertoire consisted of 28–35% mixed calls, 15–20% unstructured calls, 10–15% one-frequency-jump, 8–15% upward calls, 8–12% modulated calls and small numbers of flat, complex, multiple-frequency-jumps calls.
Figure 5. Normal social and vocal behaviors in adult male Nlgn4 mice tested in the male–female social interaction test and the male–male resident–intruder test (Paris cohort).
(a) Time spent in contact between the male subject mouse and the estrus B6 female mouse. (b) Calling rate in the male–female social interaction test (left) and in the resident–intruder test. (c) Latency to the first contact between the Nlgn4 male and the estrus B6 female. (d) Latency to emit the first ultrasonic vocalization in the male–female social interaction test (left) and in the resident (Nlgn4 male)–intruder (B6 male) test (Sanders et al.). (e) Distribution of the different call types emitted during the 3-min male–female interaction test. (f) Distribution of ultrasonic vocalizations among the eleven different call types during the 4-min male–male interaction test; no significant difference occurred across genotypes. +/+, N = 7; +/−, N = 6; −/−, N = 11.
In the male–male resident–intruder test, no significant differences were found on call rate (Fig. 5b; +/+ vs. −/−: W =44.5, P =0.62; +/+ vs. +/−: W =32, P =0.14) or the latency to call (Fig. 5d; +/+ vs. −/−: W =50, P =0.32; +/+ vs. +/−: W =22, P =0.95). Analysis of the vocal repertoire revealed no significant differences in the distribution of calls among the 11 call categories. In all three genotypes, the call repertoire consisted of approximately 40% of unstructured calls, 20–30% short calls, 5–10% modulated calls, 5–10% mixed calls and small numbers of complex and multiple frequency jumps calls (Fig. 5f). Similar results were found in a small sample of females tested in the female-female resident–intruder test (Figure S4).
No significant genotype differences in repetitive self-grooming
Time spent in self-grooming was quantified in a 10-min test in an empty cage. No significant genotype differences were found in either males (F2,32 = 0.89, NS; Fig. 6a) or females (F2,31 = 2.33, NS; Fig. 6b).
Figure 6. Repetitive self-grooming in male and female Nlgn4 mice of the Bethesda cohort.
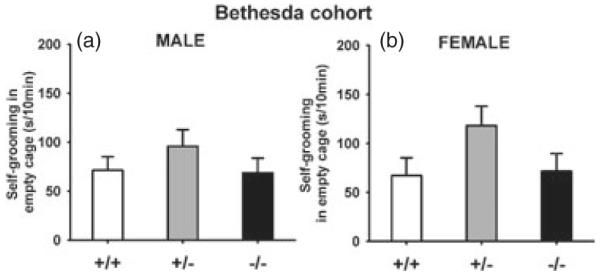
No genotype differences were detected in a 10-min test session. Nlgn4+/− mice showed a trend of spending more time in self-grooming as compared to wild-type mice, for both males and females. (a) Male: +/+, N =11; +/−, N =16; −/−, N =8; (b) Female: +/+, N =13; +/−, N =12; −/−, N =9.
Absence of significant genotype differences in anxiety-related and exploratory behaviors
On the elevated plus-maze, no significant genotype differences were found in the percentage of time spent in the open arms (F2,38 =0.73, NS; Fig. 7a) and open arm entries (F2,38 =0.68, NS; Fig. 7b). A significant genotype effect was found on total arm entries (F2, 38 =3.89, P <0.05; Fig. 7c). Post hoc comparisons revealed that Nlgn4+/− males made more total arm entries than wild-type controls (P <0.05). In the light ↔ dark exploration test, no significant genotype differences were found on number of transitions (F2,33 =0.40, NS; Fig. 7d), time spent in the dark compartment (F2,33 =0.70, NS; Fig. 7e) or latency to enter the dark compartment (F2,33 =1.44, NS; Fig. 7f). Similar results were found in females (Figure S5).
Figure 7. Normal anxiety-like behaviors in male Nlgn4 mice of the Bethesda cohort.
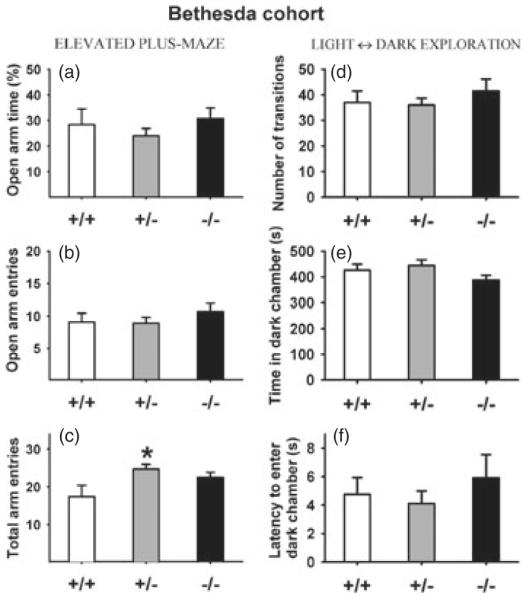
No genotype differences were detected in the elevated plus-maze test, on measures of (a) percentage open arm time, (b) number of open arm entries and (c) total number of entries into open + closed arms. +/+, N =12; +/−, N =16; −/−, N =13. No genotype differences were detected in the light ↔ dark exploration test, on measures of (d) number of transitions between compartments, (e) time spent in the dark chamber and (f) latency to enter the dark chamber. +/+, N =12; +/−, N =14; −/−, N =10.
In the open field test, no significant genotype differences were found on total distance traveled (Bethesda cohort males: F2,35 =0.27, NS; Paris cohort males: +/+ vs. +/−: W =26, P =0.53; +/+ vs. −/−: W =35, P =0.76; Fig. 8a,c; Bethesda cohort females: F2,35 =0.80, NS, data not shown) or time spent in the center zone (Bethesda cohort males: F2,35 =2.56, NS; Paris cohort males: +/+ vs. +/−: W =15, P =0.43; +/+ vs. −/−: W =24, P =0.46; Fig. 8b,d; Bethesda cohort females: F2,35 =0.55, NS, data not shown). In addition, no significant genotype differences were found in the Bethesda cohort, on vertical activity (males: F2,35 =0.15, NS, Table 1; females: F2,35 =2.57, NS, data not shown) and horizontal activity (males: F2,35 =0.38, NS, Table 1; females: F2,35 =1.04, NS, data not shown).
Figure 8. Normal open field explorations in Nlgn4 males.
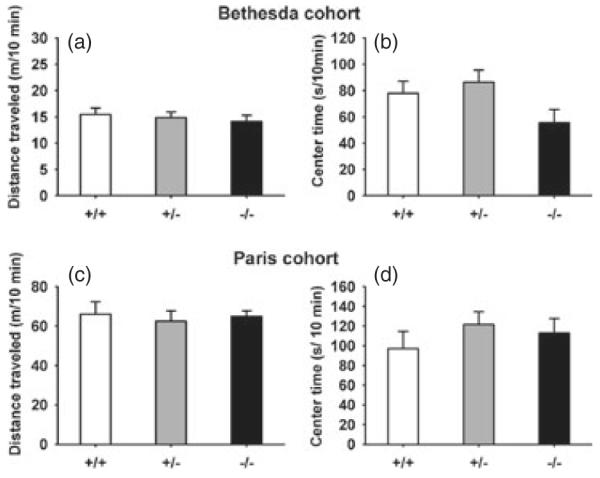
(a, b) Bethesda cohort. No genotype differences were found on (a) total distance traveled and (b) time spent in the center of a square open field in a 10-min test. +/+, N =12; +/−, N =16; −/−, N =10. (c, d) Paris cohort. No genotype differences were found on (c) total distance traveled and (d) time spent in the center of a circular arena in a 30-min test. +/+, N =7; +/−, N =6; −/−, N =9.
Table 1.
Normal general health, neurological reflexes, grip strength, locomotor activities and gait functions were seen in all genotypes of adult males tested at the Bethesda site. The data display the absence of genotype differences in general health measures, pain sensitivity, rotarod motor performance and open field exploratory activities for the Bethesda cohort
| Genotypes | +/+ (N =10) | +/− (N =16) | −/− (N =12) | P value |
|---|---|---|---|---|
| Fur condition (3 pt scale) | 2 | 2 | 2 | — |
| Bald patches (%) | 0 | 6.25 | 8.33 | 0.68 |
| Missing whiskers (%) | 10 | 6.25 | 8.33 | 0.94 |
| Piloerection (%) | 10 | 0 | 0 | 0.90 |
| Body tone (3 pt scale) | 2 | 2 | 2 | — |
| Limb tone (3 pt scale) | 2 | 2 | 2 | — |
| Physical abnormalities (%) | 0 | 6.25 | 8.33 | 0.68 |
| Body weight (grams) | 32.1±1.20 | 35.4±.81 | 32.8±1.10 | 0.57 |
| Body Temperature (°C) | 36.1±.28 | 36.6±.20 | 37.0±.23 | 0.56 |
| Transfer freezing (%) | 20 | 18.75 | 16.67 | 0.98 |
| Wild running (%) | 0 | 0 | 0 | — |
| Stereotypies (%) | 10 | 6.25 | 6.25 | 0.58 |
| Exploration (3 pt scale) | 2 | 2 | 2 | — |
| Trunk curl (%) | 50 | 56.3 | 58.3 | 0.93 |
| Wire hang (latency sec) | 50.3±3.72 | 48.2±4.3 | 45.2±6.21 | 0.79 |
| Forepaw reach (%) | 100 | 100 | 100 | — |
| Righting reflex (%) | 100 | 81.25 | 83.33 | 0.37 |
| Corneal (%) | 100 | 100 | 91.67 | 0.35 |
| Pinna (%) | 100 | 100 | 100 | — |
| Vibrissae (%) | 90 | 100 | 100 | 0.25 |
| Auditory startle (%) | 100 | 100 | 100 | — |
| Struggle/vocalization (%) | 10 | 0 | 0 | 0.90 |
| Dowel biting | 0.30±0.15 | 0.38±0.13 | 0.25±0.13 | 0.79 |
| Pain sensitivity | N =12 | N =16 | N =8 | |
| Hotplate latency | 4.90±0.35 | 4.45±0.30 | 5.38±0.50 | 0.24 |
| Tail flick latency | 1.63±0.04 | 1.64±0.10 | 1.81±0.10 | 0.41 |
| Rotarod motor coordination | N =11 | N =14 | N =8 | |
| Trial 1 | 123.8±18.2 | 111.3±17.2 | 119.3±33.4 | 0.91 |
| Trial 2 | 170.4±21.6 | 175.5±15.8 | 147.6±29.4 | 0.65 |
| Trial 3 | 206.7±14.6 | 179.2±14.9 | 191.6±34.8 | 0.59 |
| Open field exploration | ||||
| Total distance travelled | 1562.9±152.3 | 1424.9±132.8 | 1437.5±176.6 | 0.76 |
| Horizontal activity | 2865.7±249.0 | 2913.4±200.2 | 2799.5±331.2 | 0.69 |
| Vertical activity | 69.9±12.0 | 67.6±6.9 | 74.3±10.9 | 0.86 |
Absence of significant genotype differences in general health, neurological reflexes, pain sensitivity and rotarod motor coordination
Adult male mice were evaluated for general health and neurological reflexes between 10 and 16 weeks of age (Table 1). The three genotypes scored similarly on measures of body weight, neurological reflexes, motor functions including open field activity, wire hang and gait and responsivity to handling. No balding patches were observed in mice evaluated during this age range. Observations of home cage behaviors showed no abnormalities in general activity, group huddling and nesting. No excessive aggressive behaviors were observed in adult males. No significant genotype differences were found on hotplate (F2,64 = 0.50, NS) and tail flick (F2,64 = 1.20, NS) tests of pain sensitivity. No significant genotype differences were found in rotarod performance (F2,30 = 0.17, NS).
Discussion
In this study, we report the absence of genotype differences among later generations of Nlgn4 wild-type, heterozygous and null mutant mice on measures of physical characteristics, early developmental milestones, exploratory locomotion and anxiety-like behaviors (Table S1). Unexpectedly, results from our two new cohorts did not detect deficits in social approach, social interactions and adult ultrasonic vocalizations which had been reported in the original study of an earlier generation of the same line of Nlgn4 knockout mice (Jamain et al. 2008). The complete absence of significant genotype differences in social and vocalization behaviors in both the Bethesda and Paris cohorts was unpredicted. To understand the cause of discrepancies between our studies and the Jamain study, we carefully examined genetic, methodological and environmental differences between the two studies.
First, we rigorously checked genotyping and data analysis to rule out errors. Nlgn4 genotyping was routinely performed in both laboratories using protocols directly based on the original publication (Jamain et al. 2008). Accuracy of PCR genotyping was verified by Western blot performed in the Max Planck Institute (N. Brose, personal communication). Authors from the original study rigorously checked the original behavioral data (Jamain et al. 2008) and ruled out any problem in the original data analysis (T. Bourgeron et al., personal communication).
Second, we carefully examined genetic mutation, breeding strategy, housing condition and genetic background in the two studies. All mice used in this study originated from the same mutated mouse line (Jamain et al. 2008). As Figure 1 showed, het × het breeding was used to generate subjects in the Jamain study. The same breeding strategy was used to generate the Bethesda cohort and the Paris cohort. Subjects used in this study were reared in mixed-genotype cages, with no other environmental enrichment except for a piece of paper tissue, similarly to mice from the Jamain study (Ehrenreich H., personal communication). While the Nlgn4 mutation is identical in all cohorts, the genetic background of the mouse strains might be slightly different in later generations. In the original study, the Nlgn4−/− were generated on a 129P2/OlaHsd background and backcrossed onto the C57BL/6 J background for six generations before behavioral experiments were started (Jamain et al. 2008). In this study, the Bethesda cohort had one additional backcross and the Paris cohort had two additional backcrosses than the mice used in the Jamain study. It is unlikely that the absence of social deficits in this study is attributable to the number of backcrosses, because the Bethesda cohort and the Paris cohort had different numbers of backcrosses, yet results from these two cohorts were highly similar. However, we cannot exclude the possibility that additional susceptibility alleles within the 129P2/OlaHsd genome could have been present in the early generations of Nlgn4−/− mice and lost through genetic drift in the later generations. There are approximately 4.6 million sequences of single nucleotide polymorphisms between the two mouse strains 129P2/OlaHsd and C57BL/6 J (Keane et al. 2011). Because of the difference in backcrossing the expected percentage of 129P2/OlaHsd genome still present in the Nlgn4−/− mice is 1.56% for the Jamain study (six backcrosses), 0.78% in the Bethesda cohort (seven backcrosses) and 0.39% in the Paris cohort (eight backcrosses).
Third, methodological differences were carefully compared. For the three-chambered task, two slightly different protocols were used to test the Bethesda cohort and the Paris cohort, and both were different from the protocol used in the Jamain study (Table S2). As shown in Fig. 4, highly similar results were found in the Bethesda cohort and the Paris cohort, despite differences in testing procedures. In addition, a separate batch of males of the Bethesda cohort was tested in the three-chambered apparatus using a protocol identical to that described in the original study (Jamain et al. 2008). Results showed no trend of social deficits in any genotype, suggesting that slight methodological differences are unlikely to account for the large discrepancy between our studies and the Jamain study. The male–female social interaction test was performed using methods very similar to those described in the original study (Jamain et al. 2008). However, unlike in the Jamain study, our study did not indicate a reduction in ultrasonic vocalizations in Nlgn4−/− males in the presence of an estrus female. In this study, same-sex social interaction tests were conducted by pairing a subject mouse and an age-matched B6 partner mouse. This method is commonly used to study reciprocal social interactions in mouse models of autism (Schmeisser et al. 2012; Yang et al. 2009, 2012). In the Jamain study, each subject was paired with a partner of the same genotype. It is possible that pairing mutant with mutant may show differences not observed in interactions between mutant and B6. Our studies and the Jamain study also differ in the order of testing. It is possible that certain phenotypes are more sensitive to testing order than others. Notably, while order of testing was not identical in the Paris and Bethesda labs, both labs reported normal sociability of Nlgn4−/− mice.
Overall, these results suggest that methodological differences between the two studies are unlikely to be the chief reason for the absence of autism-relevant phenotypes in later generations of mice tested in Bethesda and Paris.
Variability in behavioral readouts in similar knockout lines on different genetic backgrounds is a well-known phenomenon (Bernardet & Crusio 2006). Fmr1−/− mice mutated in the fragile X gene are a good example of phenotypic variability, with only the anxiety-like phenotype robustly replicating (Bernardet & Crusio 2006; Spencer et al. 2011). Mice with the Fmr1 mutation backcrossed onto different genetic backgrounds including B6, A/J, DBA/2 J, FVB/NJ (FVB), 129S1/SvImJ and CD-1 displayed diverse behavioral phenotypes (Spencer et al. 2011). Bernardet and Crusio similarly highlighted the variability of phenotypes of Fmr1−/− mice on different genetic backgrounds (B6, FVB, FVB × B6 hybrids; Bernardet & Crusio 2006). For example, spatial learning deficits in the Morris water maze were present in the FVB background but not in the B6 background. However, variance in the Fmr1−/− mouse behavior could not be explained solely by a difference in genetic background, because behavioral differences also occurred in a similar genetic background [e.g. rate of learning in the reversal learning phase of the Morris water maze (B6); escape latency in the visible platform condition in the water maze (B6); anxiety-related behaviors in the elevated plus-maze (FVB × B6); number of correct trials in the cross-shaped water maze (B6); escape latency in the Morris water maze (B6); contextual and cued fear conditioning (B6); open field activity (B6); auditory startle response (B6; FVB); Bernardet & Crusio 2006].
Difference in the epigenetic regulation of the synaptic genes might explain part of the variance. As suggested by Radyushkin et al. (2009), other NLGN or downstream proteins might compensate for the lack of Nlgn4. The rapid evolution of Nlgn4 in mice suggests that its function in the brain is under less stringent control than that of other NLGNs (Bolliger et al. 2008). The variability we have discovered in the behavioral phenotype of Nlgn4−/− mice is reminiscent of the variability between patients carrying mutations in NLGN4X. Indeed, even in the same family with a shared genetic background, individual carriers of NLGN4X mutations have different clinical diagnostics (Daoud et al. 2009; Jamain et al. 2003). Thus, mutation in a single NLGN might cause a broad range of cognitive disorders and/or susceptibility to personality and emotional disorders.
Changes in phenotype across generations within a line of mutant mice may occur more often than has been reported in the literature. Deficits in male–female social interactions were found in heterozygous male Shank3 mice in an early generation (Bozdagi et al. 2010) but not in later generations (Yang et al. 2012). Clear demonstrations of phenotypes lost or gained in later generations will be important to recognize. It may be possible to identify changes in the expression of downstream genes that confer protection from the consequences of the original mutation. Identification of altered expression of compensatory genes in mouse models may shed light on protective and susceptibility genes in human syndromes. In addition to aiding the understanding of multigenetic factors mediating phenotypes, discovery of protective genes could pave the way for therapeutic interventions.
Lastly, our results emphasize the need to replicate behavioral phenotypes in two or more cohorts of mice, in two or more laboratories. Gene × environment interactions are well known, and can influence the results obtained in any given experiments. Repeating experiments is a research tradition in all fields of science. Especially for behavioral assays, which are particularly sensitive to environmental perturbations, confidence in findings is increased when the same results are obtained in two or more cohorts of mice.
In summary, we have characterized the variability of Nlgn4−/− mice on social interactions and vocalizations in a social setting. Although we cannot disentangle potential causes of these differences, it is important to report this variability before considering future treatment protocols using these mutant mice. Genetically homogeneous inbred mouse strains and congenic lines of targeted mutations offer appealing strategies, but even when genetic background is similar, behavioral differences can persist, because of routine gene × environment interactions. This inter-individual variability is also apparent in humans carrying mutations in NLGN genes and more generally in synaptic genes. The identification of risk and protective alleles within the same subject is one of the main challenges for understanding the inheritance of ASD. To date, it is not clear how many loci regulate synapse formation, maintenance and homeostasis, nor how these variants interact with each other to modulate the risk for ASD (Toro et al. 2010). A better knowledge of these genetic interactions will be necessary to understand the complex inheritance pattern of ASD.
Supplementary Material
Acknowledgments
We are grateful to Professor Nils Brose at the Max Planck Institute for generously contributing breeding pairs of the Nlgn4 mice which were used to generate the mice in this study, and for encouraging our in-depth analyses of the behavioral consequences of Nlgn4 mutations in mice. We thank Sandrine Vandormael-Pournin (Génétique Fonctionnelle de la Souris, Institut Pasteur) for the protocol to detect oestrus status in female mice. M.Y., A.M.K., L.W., J.L.S. and J.N.C. were supported by the National Institute of Mental Health Intramural Research Program. E.E. was supported by Fondation de France, ANR FLEXNEURIM (ANR09BLAN034003), C.S.L., N.T., A.L.S. and T.B. by ANR (ANR-08-MNPS-037-01 - SynGen), Neuron-ERANET (EUHF-AUTISM), Fondation Orange and the Fondation FondaMentale. P.F. was supported by the Fondation Bettencourt-Schueller.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-V) Development. American Psychiatric Association; Washington, DC: 2011. [Google Scholar]
- Bernardet M, Crusio WE. Fmr1 KO mice as a possible model of autistic features. Scientific World Journal. 2006;6:1164–1176. doi: 10.1100/tsw.2006.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolliger MF, Pei J, Maxeiner S, Boucard AA, Grishin NV, Sudhof TC. Unusually rapid evolution of Neuroligin-4 in mice. Proc Natl Acad Sci U S A. 2008;105:6421–6426. doi: 10.1073/pnas.0801383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Bourgeron T, Jamain S, Granon S. Animal models of autism: focus on genetics models and behavioral paradigms. In: Fisch GS, Flint J, editors. Transgenic and Knockout Models of Neuropsychiatric Disorders. Human Press; Totowa, NJ: 2005. [Google Scholar]
- Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Scattoni ML, Harris MJ, Saxena R, Katz AM, Silverman JL, Zhou Q, Crawley JN, Hof PR, Buxbaum JD. Shank3 haploinsufficiency leads to altered synaptic development, transmission, and plasticity, as well as to social deficits. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Daoud H, Bonnet-Brilhault F, Vedrine S, Demattei MV, Vourc’h P, Bayou N, Andres CR, Barthelemy C, Laumonnier F, Briault S. Autism and nonsyndromic mental retardation associated with a de novo mutation in the NLGN4X gene promoter causing an increased expression level. Biol Psychiatry. 2009;66:906–910. doi: 10.1016/j.biopsych.2009.05.008. [DOI] [PubMed] [Google Scholar]
- El-Kordi A, Winkler D, Hammerschmidt K, Kastner A, Krueger D, Ronnenberg A, Ritter C, Jatho J, Radyushkin K, Bourgeron T, Fischer J, Brose N, Ehrenreich H. Development of an autism severity score for mice using Nlgn4 null mutants as a construct-valid model of heritable monogenic autism. Behav Brain Res. doi: 10.1016/j.bbr.2012.11.016. (in press) (in press) [DOI] [PubMed] [Google Scholar]
- Geschwind DH. Genetics of autism spectrum disorders. Trends Cogn Sci. 2011;15:409–416. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SR, Iossifov I, Levy D, Ronemus M, Wigler M, Vitkup D. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron. 2011;70:898–907. doi: 10.1016/j.neuron.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon M, Soykan T, Falkenburger B, Hammer M, Patrizi A, Schmidt KF, Sassoe-Pognetto M, Lowel S, Moser T, Taschenberger H, Brose N, Varoqueaux F. Neuroligin-4 is localized to glycinergic postsynapses and regulates inhibition in the retina. Proc Natl Acad Sci U S A. 2011;108:3053–3058. doi: 10.1073/pnas.1006946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Goodstadt L, Danecek P, et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477:289–294. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Raynaud M, Ronce N, Lemonnier E, Calvas P, Laudier B, Chelly J, Fryns JP, Ropers HH, Hamel BC, Andres C, Barthelemy C, Moraine C, Briault S. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson-Yuen A, Saldivar JS, Sommer S, Picker J. Familial deletion within NLGN4 associated with autism and Tourette syndrome. Eur J Hum Genet. 2008;16:614–618. doi: 10.1038/sj.ejhg.5202006. [DOI] [PubMed] [Google Scholar]
- Macarov M, Zeigler M, Newman JP, Strich D, Sury V, Tennenbaum A, Meiner V. Deletions of VCX-A and NLGN4: a variable phenotype including normal intellect. J Intellect Disabil. 2007;51:Res329–Res333. doi: 10.1111/j.1365-2788.2006.00880.x. [DOI] [PubMed] [Google Scholar]
- O’Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, Karakoc E, Mackenzie AP, Ng SB, Baker C, Rieder MJ, Nickerson DA, Bernier R, Fisher SE, Shendure J, Eichler EE. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2012a;44:471. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Roak BJ, Vives L, Girirajan S, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012b;485:246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, Klei L, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radyushkin K, Hammerschmidt K, Boretius S, Varoqueaux F, El-Kordi A, Ronnenberg A, Winter D, Frahm J, Fischer J, Brose N, Ehrenreich H. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009;8:416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- Robertson MM. The Gilles De La Tourette syndrome: the current status. Arch Dis Child Educ Pract Ed. 2012;97:166–175. doi: 10.1136/archdischild-2011-300585. [DOI] [PubMed] [Google Scholar]
- Sanders SJ, Ercan-Sencicek AG, Hus V, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Murtha MT, Gupta AR, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T +tf/J mouse model of autism. PLoS One. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser MJ, Ey E, Wegener S, Bockmann J, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–260. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Smith DG, Rizzo SJ, Karras MN, Turner SM, Tolu SS, Bryce DK, Smith DL, Fonseca K, Ring RH, Crawley JN. Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Sci Transl Med. 2012;4:131ra151. doi: 10.1126/scitranslmed.3003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, Sheng M, Crawley JN. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Hamilton SM, Thomas AM, Serysheva E, Yuva-Paylor LA, Paylor R. Modifying behavioral phenotypes in Fmr1KO mice: genetic background differences reveal autistic-like responses. Autism Res. 2011;4:40–56. doi: 10.1002/aur.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Konyukh M, Delorme R, Leblond C, Chaste P, Fauchereau F, Coleman M, Leboyer M, Gillberg C, Bourgeron T. Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends Genet. 2010;26:363–372. doi: 10.1016/j.tig.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ, Geschwind DH. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Oliveira G, Coutinho A, Yang C, Feng J, Katz C, Sram J, Bockholt A, Jones IR, Craddock N, Cook EH, Jr., Vicente A, Sommer SS. Analysis of the neuroligin 3 and 4 genes in autism and other neuropsychiatric patients. Mol Psychiatry. 2005;10:329–332. doi: 10.1038/sj.mp.4001629. [DOI] [PubMed] [Google Scholar]
- Yang M, Bozdagi O, Scattoni ML, Wöhr M, Roullet FI, Katz AM, Abrams DN, Kalikhman D, Simon H, Woldeyohannes L, Zhang JY, Harris MJ, Saxena R, Silverman JL, Buxbaum JD, Crawley JN. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J Neurosci. 2012;32:6525–6541. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley J. Automated three-chambered social approach task for mice. Curr Protoc Neurosci. 2011;8:26. doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T + tf/J mice are unchanged by cross-fostering with C57BL/6 J mothers. Int J Dev Neurosci. 2007;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.