Abstract
Autism is diagnosed by three major symptom categories: unusual reciprocal social interactions, impaired communication, and repetitive behaviors with restricted interests. Direct social approach in mice has strong face validity to simple social approach behaviors in humans, which are frequently impaired in autism. This unit presents a basic protocol for a standardized, high-throughput social approach test for assaying mouse sociability. Our automated three-chambered social approach task quantifies direct social approach behaviors when a subject mouse is presented with the choice of spending time with either a novel mouse or a novel object. Sociability is defined as the subject mouse spending more time in the chamber containing the novel target mouse than in the chamber containing the inanimate novel object. The Basic Protocol describes procedures for testing one subject at a time in a single apparatus. A Support Protocol addresses data collection.
Keywords: autism, social behaviors, sociability, social approach, three-chambered task, mouse models, automated behavioral test
INTRODUCTION
Abnormal reciprocal social interactions, which include low interest in peers, difficulty in maintaining social interaction, and failure to communicate effectively using nonverbal facial and gestural expressions, are central to the diagnosis of autism spectrum disorders (American Psychiatric Association, 1994; Lord et al., 2006; Lord and Bishop, 2009). Mouse models with strong face validity to the diagnostic symptoms provide essential tools for studying basic genetic and environmental causes of autism, and for developing treatment strategies. Mus musculus is a social species that exhibits a rich repertoire of social behaviors. Social behaviors in mice are important for forming bonds, foraging, defending territories, avoiding predators, and reproducing successfully (D’Amato and Populin, 1987; Laviola and Terranova, 1998; Moles and D’Amato, 2000; Branchi et al., 2001; Hurst et al., 2001; Miczek et al., 2001; Winslow, 2003; Kavaliers et al., 2006; Crawley, 2007a,b; Panksepp et al., 2007; Arakawa et al., 2008). Mice have a natural tendency to approach and investigate unfamiliar conspecifics, much like the way a person would greet a stranger. A person with autism often avoids interacting with a stranger, either withdrawing when approached or remaining aloof. Therefore, low social approach in mice represents an endophenotype with a reasonable analogy to the types of social deficits that characterize autism (Crawley et al., 2007; Silverman et al., 2010b; Yang et al., 2011b). We have developed equipment and methods that provide a relatively rapid screen for impaired sociability in mouse models of autism (Crawley, 2004; Duncan et al., 2004; Nadler et al., 2004; DeLorey et al., 2008; Jamain et al., 2008; Ryan et al., 2008; Nakatani et al., 2009; Page et al., 2009; Radyushkin et al., 2009; Yang et al., 2009,2011a; Ehninger et al., 2010; Silverman et al., 2010b; Defensor et al., 2011; Hamilton et al., 2011; Silverman et al., 2011). The Basic Protocol in this unit presents methods for testing mouse social approach behaviors using our automated three-chambered social approach task (Nadler et al., 2004; Moy et al., 2007, 2008b; Yang et al., 2009, 2011a; Silverman et al., 2010a,b). The Support Protocol addresses data collection.
AUTOMATED THREE-CHAMBERED SOCIAL APPROACH TASK
BASIC PROTOCOL
The automated three-chambered social approach task was developed in 2004 and has been widely employed as a standard test for assaying sociability in mice. Sociability is defined as the subject mouse spending more time in the chamber containing the novel mouse than in the chamber containing the inanimate novel object. A second corroborative measure is time spent sniffing the novel mouse versus time spent sniffing the novel object, which measures direct social interactions. Number of transitions across chambers offers a built-in control measure of exploratory locomotion (Crawley, 2007b, 2008; McFarlane et al., 2008; Moy et al., 2008b, 2009; Yang et al., 2009, 2011a; Silverman et al., 2010a,b). See the Support Protocol for information about collecting these data.
The three-chambered apparatus was originally designed by Nadler, Moy, Dold, Trang, Simmons, Perez, Young, Barbaro, Piven, Magnuson, and Crawley at the University of North Carolina and National Institute of Mental Health (Nadler et al., 2004). The apparatus and software are commercially manufactured and available from Dold Labs and Engineering. Similar but not identical equipment is commercially available from other sources such as Ugo Basile and Stoelting.
Materials
Subject mice: e.g., C57BL/6J (B6) and FVB.129P2-Pde6b+ Tyrc-ch/AntJ (FVB/AntJ) adult mice (The Jackson Laboratory) or C57BL/6N and FVB/NJ (e.g., Charles River Laboratories or Taconic Farms) between 8 weeks and 6 months of age (see Critical Parameters)
Target mice (novel mice): e.g., 129/SvImJ adult mice (The Jackson Laboratory) between 8 weeks and 6 months of age, preferably of the same sex and approximate body weight (within 5 g) as the subjects (see Critical Parameters)
Odorless mild dish soap and long-handled dish sponge
70% (v/v) ethanol in labeled spray bottle
Tap water in labeled spray bottle
Test room, with minimal cues visible to the subject
Two (or more) gooseneck desk lamps with incandescent 75-watt light bulbs
Lux meter (Fisher Scientific)
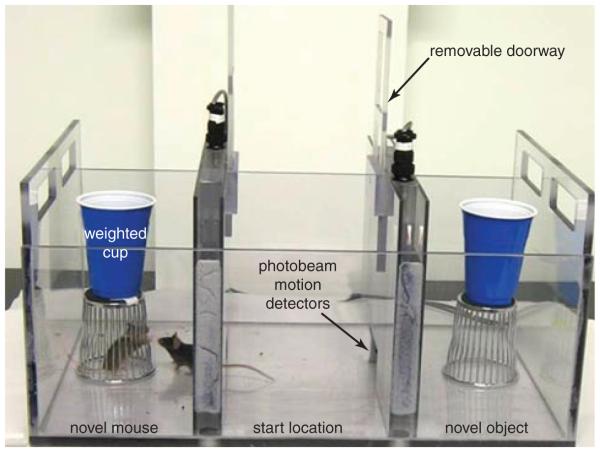
Automated three-chambered social test apparatus: e.g., Crawley automated three-chambered social approach apparatus for mice, hardware and software [(Fig. 8.26.1; Dold Labs and Engineering; (830)560-1471, doldglabeng@earthlink.net, doldonics@comcast.net)]; Ugo Basile, cat. no. 46503; Stoelting, cat. no. 60450
Figure 8.26.1.
The social approach apparatus is a rectangular three-chambered box. Each chamber measures 20 cm (length) × 40.5 cm (width) × 22 cm (height). Dividing walls are made from clear Plexiglas, with small openings (10-cm width × 5-cm height) that allow access into each chamber. The center chamber is the start location. The weighted cup on top of the wire cup prevents climbing. The empty wire cup in the right chamber is the novel object. Photobeams are embedded across each doorway. An automated photobeam detector registers time spent in each chamber and number of transitions. This social approach apparatus and the accompanying software program are designed and manufactured by Dold Labs and Engineering.
Computer (Dell desktop, or similar PC) with software provided by the manufacturer of the automated three-chambered social test apparatus (Dold Labs and Engineering)
Automated video tracking systems, optional (e.g., see www.noldus.com/animal-behavior-research/solutions/research-small-lab-animals/sociability-test; Page et al., 2009)
Heavy-duty utility paper towels
Cameras (preferably CCTV security cameras, e.g., Panasonic WV-CP280) with mounting bracket
DVD recorder (if recording with CCTV cameras) TV monitor (if recording with CCTV cameras) Video cables
BNC and RCA connectors
Blank DVDs
Standard mouse group-housing cages
Marking pen (dark), and (if preferred) paw tattoos, ear punches, ear tags, or subcutaneous transponders
Paper tube or small cup (for transporting mouse)
Soft facial tissues
3.8-cm bottom diameter, rust-proof/rust-resistant, noncorrosive, steel wire pencil cups (e.g., see http://www.kitchen-plus.com; Fig. 8.26.1)
Holding area: dedicated room or quiet area near the testing room
Index cards and broad tip markers
Stopwatches without beepers or with beepers silenced (See Fig. 8.26.2)
Figure 8.26.2.
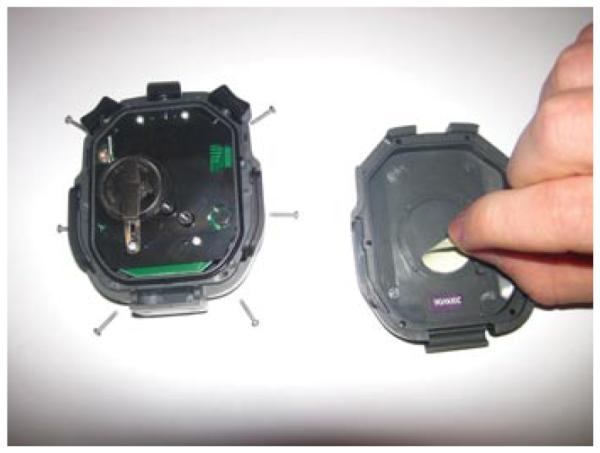
Stopwatches that emit a beeping sound can be silenced by removing an internal metal part from the inner surface of the back panel.
Plastic drinking cups filled with small heavy objects (to place on top of the inverted wire pencil cups)
NOTE: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must follow officially approved procedures for the care and use of laboratory animals.
Perform pretest preparations
-
1. Set up the room environment to minimize visual, olfactory, and auditory cues.
It is critical to conduct the three-chambered task in a room environment that is symmetrically balanced. The goal is to avoid introducing an innate side preference. Salient room cues, uneven illumination, and lingering odors could cause the subject to prefer certain parts of the apparatus. Therefore, it is important to keep the room setup plain and symmetrical, and the lighting even.
To minimize room cues that are visible to the subject, place the three-chambered apparatus on a table, and store supplies under the table or on utility carts that are lower than the table. What the subject sees when it is in the apparatus should be just the ceiling and plain walls. If for practical reasons there is no way to make the room plain and symmetrical, a simple solution is an environmental chamber, or a portable enclosure to shield the apparatus. Use odor-resistant materials such as Plexiglas or acrylic plastic. In addition to balancing visual cues, caution needs to be taken to avoid auditory distractions. It is advisable to conduct the experiment in a quiet room.
When first setting up a new testing room, side preferences are evaluated during the habituation phase of the task (steps 15 and 16). Corrections in room configuration and lighting are then made, until the group of mice displays no side preference.
-
2. Adjust the illumination, keeping the lighting dim and even, especially in different parts of the apparatus. For example, position two gooseneck desk lamps, one on each side of the room. For large rooms, place one light in each of the four corners of the room. Turn the lamp shades upward so that the bulbs are facing the ceiling. Never point the light source directly towards the apparatus.
NOTE: Standard fluorescent ceiling lights tend to be too bright for most behavioral experiments, including the three-chambered task.
-
3. Use a hand-held lux meter to check lighting in the three chambers of the automated three-chambered apparatus.
The two side chambers should read 5 to 6 lux. The difference between the two chambers should be < 1 to2 lux.
-
4. Before testing the first subject each day, wipe down the bottom and side walls of the clean apparatus as you would between tests with different animals (steps 29 to 32), first with paper towels moistened with 70% ethanol and water, and then with paper towels moistened with tap water. Dry with paper towels, and allow to air dry for 5 min before testing.
This procedure ensures that the odors in the apparatus are the same across subject mice. The apparatus should always be thoroughly washed with soap and water at the end of the day’s testing (see steps 33 and 34) and kept clean and dry when not in use.
-
5. Set up the video recording device with the following essentials:
CCTV or other analog or digital camera
Mounting bracket
DVD recorder
TV monitor
Video cables
BNC and RCA connectors
Blank DVDs.
Video recording of the experimental session is a good way to permit later comprehensive analysis.
Alternatively, videos could be digitized with a digital video converter and saved to a high-capacity computer drive, thus eliminating the need for a DVR and DVDs.
-
6. Minimize visual obstruction that could interfere with video analysis by placing the video camera above the apparatus. In addition, make sure there are no loose cables or wires dangling anywhere.
Coiled cables and wires may distract the mouse.
Prepare animals for the experiment
-
7. When animals arrive in the facility, transfer them to standard cages immediately. Do not mix adult males from different shipping compartments in the same cage.
Group housing can consist of two, three, or four mice per cage.
It is best not to mix adult males from different shipping compartments since unfamiliar adult males could be very aggressive toward one another (see Critical Parameters).
-
8. Allow the animals to acclimate to the facility for at least 1week before testing them, if using mice purchased from a commercial supplier.
We have obtained similar results in mice bred in-house and tested between 8 weeks and 6 months of age (Moy et al., 2004).
-
9. Mark subject mice in advance for identification, using dark pen markings on the tail or fur, paw tattoos, ear punches, ear tags, or subcutaneous transponders.
All of these marking methods are equally effective (see Ayadi et al., 2011).
Those who conduct the experiment should be able to interpret the identifying marks correctly, but should be blind to genotype and/or treatment information.
Train target mice
-
10. Habituate target mice (those to be used as novel mice) to the inverted wire cup and Plexiglas chamber in advance of experiments. For each training session, place the target mouse in a clean wire pencil cup and invert the cup into one side chamber. Repeat for a total of either three 10-min sessions or two 15-min sessions.
It is important that all mice used as novel target mice behave in a consistent and docile manner (i.e., with minimal exhibition of aberrant behaviors, agitation, or signs of aggression).
Multiple animals can be trained at the same time, provided adjacent cups are separated by several inches of space.
The small size of the wire cup (3.8-cm bottom diameter) ensures that the subject is always close to the novel mouse when it (the subject) moves around the cup. The space between the bars of the cup is large enough to permit visual, olfactory, acoustic, and some tactile signals to transmit through, yet small enough to prevent direct physical interactions.
-
11. IMPORTANT: Do not walk away while training target mice. Watch for behaviors that are potentially disruptive, such as bar-biting, excessive self-grooming, circling, or clinging to the side bars with all four paws.
In most animals, these behaviors decline quickly as training progresses. Animals that continue to show high levels of one or more of these behaviors after 30 min of training should not be used as a novel mouse. Approximately 75% of animals usually pass the training criteria.
Trained mice should be used only as novel mice and for nothing else.
Trained mice can be used many times over several weeks, and twice a day, but refresh your stock of novel mice every 2 months, or more often if the experimental load is heavy.
12. To avoid cross-contamination, label each cup with its dedicated function (e.g., ♂ novel mouse, ♂ novel object, ♀ novel mouse, and ♀ novel object). Keep cups for different uses in separate stacks.
Acclimate subject mice
The following steps (i.e., acclimation and phases I, II, and III) take place on the same day.
-
13. Transport animals in their home cages from the facility housing room to the behavioral testing area. Allow subject mice to rest for at least 1 hr after transportation, to acclimate to the room and help the animals recover from mild stress induced by transportation. Keep subject mice in a dedicated room or a quiet area nearby for as long as it takes to finish all experiments for the day.
IMPORTANT NOTE: Do NOT keep subject mice and target mice in the same room at any time. It is advisable to keep subject mice in the dedicated room or quiet area mentioned above, and target mice in the corridor outside the experiment room. If subject mice and target mice are housed in the same facility housing room, make sure that their cages are placed far apart, preferably on different racks.
Phase I: Habituate subject mice to the center chamber
This phase is the first 10-min session of the actual experiment. See the Support Protocol for information about data collection in the experiment.
-
14. Close the two doorways in the chamber, walk to the holding area where subjects are kept, carefully pick up a subject mouse from a cage, preferably using a small container (e.g., a small cardboard tube or paper cup), and transport it to the experimental testing room.
When taking one subject mouse out for testing, be careful to avoid disturbing the mice that remain in the cage. Do not chase animals around with your hand, but gently pick up any mouse that happens to come near. It is OK to transport the subject in a paper tube, in a cup, on your lab coat sleeve, or in a clean cage.
IMPORTANT: Do NOT dangle the subject by its tail when walking towards the apparatus, as hanging by the tail is a stressor for mice.
-
15. With the two doorways closed, place the subject in the center chamber. Move to a corner where you are unlikely to be seen by the subject mouse. Remain quiet during the 10-min period.
It is usually not necessary to video record this habituation session. Remote monitoring with the observer out of the testing room is an option.
Phase II: Habituate mice to all three chambers
16. To video record this phase, prepare an information card that shows date, time, test session, and animal identification or code.
-
17. Press the record button on the recording device, flash the information card to the camera for a few seconds, put down the card, open the doorways gently, and start the social approach equipment. Remain quiet in the corner, far side of the room, or outside the room.
IMPORTANT: In our laboratory, we conduct the habituation phase by placing the subject mouse in an entirely empty apparatus, with no empty wire cups present. This ensures that the test session offers the subject mouse a choice between a novel mouse and a novel object, NOT a choice between a novel mouse and a familiar object.
-
18. After 10 min, stop the session, and stop video recording.
Chamber time data (see Support Protocol) from Phase II provide read-outs that confirm whether room cues are well balanced. One needs to confirm a lack of side-preference (i.e., that subjects spend approximately the same amount of time in the two side chambers) before proceeding with more experiments.
Phase III: Test for sociability
-
19. Confine the subject to the center chamber before the novel object and the novel mouse are introduced to the side chambers. Use one hand to guide the subject to the center chamber and gently shut both doorways.
Be patient. Do not pick the animal up by the tail or otherwise force it to the center chamber.
20. Using clean gloves, take a clean empty wire cup, and place it bottom up, in one side chamber, ideally in the middle of the chamber, between the doorway and the side wall, as shown in Figure 8.26.1.
21. Take a wire cup dedicated to holding the novel mouse, walk to a cage holding the target (novel) mice, pick out one mouse, place it in the cup, and close the cage lid.
22. Walk back to the apparatus with the novel mouse in hand, and invert the cup into the other side chamber.
23. Take two weighted plastic drinking cups, and place one on top of each of the two inverted wire cups to prevent subject mice from standing on the top.
-
24. Start video recording, and immediately open both doorways simultaneously. Return to the corner or leave the room quickly and quietly.
To avoid contaminating the novel object with animal odors, always position the novel object before the novel mouse. If the novel mouse clings to the wires as you invert the cup, gently pull its tail to help it come down.
IMPORANT NOTE: If sniff time is scored live by an investigator using stopwatches, make sure to use stopwatches that that do not beep. Silence beeping stopwatches by removing an internal metal part, as illustrated in Figure 8.26.2.
25. After 10 min, stop the video recording and stop the social approach session.
-
26. Use a paper tube or small cup to pick up the subject. Remove the novel mouse and mark its tail with permanent marker.
It is difficult and stressful to catch the subject by the tail. Picking up the mouse with a paper tube is much easier.
Each novel mouse can be used twice a day, with some break in between. Marking the target mice is a strategy to avoid using the same novel mouse twice in a row.
-
27. Record videotape/DVD and animal information in a notebook, including:
Sniff times (for scoring methods, see the Support Protocol)
Unusual mouse behavior (e.g., hyper- or hypo-locomotion, or jumping by the subject mouse during the experiment)
Signs of aggression or excessive repetitive behaviors
Environmental conditions (e.g., time of the day, room temperature, noises, unexpected interruptions, unusual incidents, and any testing errors).
These pieces of information are essential for matching chamber-time and sniff-time data for each subject.
-
28. After each subject from a given cage has been tested, put it together with other tested cagemates in a clean cage. After all animals from a given home cage have been tested, return them to their original home cage.
Care should be taken not to mix tested mice with untested cagemates, to avoid transmission of novel social odors that the tested mouse has acquired during the test session.
Phase IV: Test preference for social novelty (optional)
A fourth 10-min session can be added to measure preference for social novelty, although this is not as relevant to autism-like symptoms as sociability. After completion of the 10-min social approach test for sociability, a second novel mouse is placed inside the previously empty novel object cup, while the first novel mouse remains inside its cup. The subject mouse is then given 10 min to explore all three chambers. Preference for social novelty is defined as more time in the chamber with novel mouse 2 than time in the chamber with novel mouse 1, and more time spent sniffing novel mouse 2 than time spent sniffing novel mouse 1. Most mice prefer to spend more time near the completely unfamiliar novel mouse 2.
Preference for social novelty contains components of social recognition and social memory, which appear to be less relevant to the types of social deficits that define autism. Thus, the preference for social novelty test may be useful to investigate social recognition or social memory. For more information about specific methods for quantifying partner recognition and social memory, and as a control for the sensory ability of the subject mouse to discriminate social odors.
Perform post-test cleaning
Mice rely on olfactory cues to identify other animals. To accurately assess sociability in the three-chambered task, it is critical to clean the apparatus thoroughly between subjects and wash it at the end of the day. This is a point that cannot be overemphasized.
-
29. Remove visible fecal and urinary deposits using soft facial tissues to pick up visible deposits.
Soft tissue is absorbent and permits cleaning of hard-to-reach corners; therefore, it is preferred to the heavy-duty utility paper towels for picking up visible urinary spots and fecal boli.
-
30. Remove lingering odors by spraying a piece of heavy-duty utility paper towel with 70% ethanol until it is moistened and using it to wipe the lower part of the side walls and the floor surface, taking care to clean all corners. Use several pieces of paper towel to clean all three chambers, making sure to scrub the floor where the novel mouse sat. Wipe underneath the two removable dividing walls, as well as the frames of the two openings, places where mice tend to leave body odors and urinary marks.
Heavy-duty utility paper towels do not disintegrate when wetted with cleaning solutions, which is useful for scrubbing the box.
Mice rear against the wall frequently, but they usually cannot reach higher than 3 in., unless they jump. Therefore, it is not necessary to wipe the upper parts of the walls. Make sure to wipe the walls first and the bottom next; this will prevent bringing odors from the dirty bottom up to the relatively clean walls.
Mice tend to urinate near corners or close to the walls, so those spots need to be cleaned carefully.
31. After wiping the apparatus with 70% ethanol, wipe again with paper towels moistened with tap water to remove the lingering ethanol odor.
32. Use a piece of dry paper towel to absorb residual moisture, and air dry the apparatus for ~5 min before testing the next subject.
Clean equipment at the end of the day
-
33. Wash the apparatus with warm water and mild dish soap at the end of the day.
Remove the electronic parts, carry the apparatus to a large sink, and wash it with a long-handled dish sponge soaked with dish soap (e.g., Ivory liquid dish soap).
A flexible hose attached to the water faucet facilitates cleaning.
-
34. Rinse well with warm water. Dry the apparatus with clean cloth towels or paper towels, and place the apparatus on a clean flat surface to air dry it overnight.
Washing with soap and water is the best way to completely remove residual odors. Do not use scented dish soap or plastic dish scrubbers, which leave scratch marks. Use of a flexible hose will make washing the apparatus easier.
35. Thoroughly wash the cups after each use with odorless dish soap and a sponge, making sure to clean between the wires. Rinse cups with the warm water.
-
36. After all the cups have been washed, dry them with clean cloth towels or paper towels. Lay them on a flat surface, and let them air dry overnight. Do not stack cups until they are completely dry.
Lingering odors on the cups could mislead the subject and thereby significantly skew the results. Proper cleaning and storage of the cups is vital to the success of the experiments.
Water makes the wire cups rust quickly, so it is necessary to wipe them dry after washing. To increase efficiency, prepare clean cups in the experimental room or in a storage room nearby. Use a set of two clean cups for testing each subject. For example, a minimum of 16 cups should be prepared for 8 subjects.
Instead of cleaning the cups after each subject, keep all used cups in a large container and wash them all together after the last subject has been tested. Do not keep used cups in the testing room!
DATA COLLECTION
SUPPORT PROTOCOL
Automatically measuring entries and time spent in each chamber
The time spent in each chamber and the number of entries into the side chambers of the three-chambered box manufactured by Dold Labs and Engineering are automatically measured using the computer software provided by the manufacturer. The chamber has pairs of photobeam emitters and detectors embedded across each doorway. As the animal moves through the doorway, it sequentially breaks and unbreaks two infrared beams. An interface box with an embedded real-time controller monitors beam breaks and calculates transitions in and out of each chamber and time spent in each chamber. At the end of phase II and phase III, chamber time and transition data are automatically tallied by the software. The most recent version of the software saves data to the computer automatically. Video tracking systems have also been used to score this task. Interested readers can find more information in Page et al. (2009), Kaidanovich-Beilin et al. (2011), and at http://www.noldus.com/animal-behavior-research/solutions/research-small-lab-animals/sociability-test.
Scoring sniff time
Time spent sniffing the wire cup containing the novel mouse and time spent sniffing the novel empty wire cup can be scored either live or from recorded experiments, using the same basic methods. When scoring a testing session live, care needs to be taken to avoid making noises that could disrupt ongoing behaviors in the subject mouse. The experimenter should sit at least 2 meters away from the apparatus, facing the center chamber directly, with equal views to both side chambers, and proceed as follows: (1) Hold two silenced stopwatches (see the Basic Protocol, step 25 annotation, and Fig. 8.26.2), one in each hand, for scoring time spent sniffing each cup. (2) Watching the subject carefully, start the timer as soon as the mouse begins sniffing, and stop the timer promptly when the mouse stops sniffing. The subject mouse is considered to be sniffing the cup when its nose is directed to the cup and the distance between the nose tip and wire bars is 1 cm or less. Remember to write down the cumulative time spent sniffing before clearing the timer for the next session.
If test sessions are to be recorded for later scoring, make sure that both cups and the circular region surrounding each cup are clearly visible on the screen. An ideal strategy for achieving this is to videotape from above, with the camera mounted ~1.5 meter above the center chamber. To ensure accuracy, it is advisable for two people to practice with the same videos until inter-rater reliability is 95% or higher.
COMMENTARY
Background Information
Because autism is diagnosed purely based on behavioral symptoms, it follows that a mouse model of autism must have strong face validity to clinical symptoms. The three-chambered task was based on similar equipment in the behavioral neuroscience literature, modified to measure simple social approach in mice. While normally developing children prefer to play with friends, children with autism often do not approach other children, but remain alone in a corner, playing with a toy. The three-chambered task simulates the playground scenario by asking the subject mouse, “Do you prefer to spend time with another mouse or with an inanimate object?” Owing to its strong face validity and reliability, the three-chambered task has become one of the most widely used behavioral tests for studying autism-relevant phenotypes in inbred strains and transgenic mouse lines. In recent years, the three-chambered task has been used to investigate environmental and genetic hypotheses relevant to autism (Crawley et al., 2007; Yang et al., 2007a,b, 2008, 2009; Chadman et al., 2008; DeLorey et al., 2008; Jamain et al., 2008; Molina et al., 2008; Matsuo et al., 2009; Nakatani et al., 2009; Page et al., 2009; Zhou et al., 2009; Zhao et al., 2010 Silverman et al., 2011) and to test efficacies of pharmacological and behavioral treatments (Silverman et al., 2010a; Chadman, 2011; Yang et al., 2011a).
Employing multiple units of the three-chambered apparatus simultaneously offers high-throughput, simple, automated, and standardized measures of direct social approach behaviors. Although fewer complex and nuanced differences in reciprocal social interaction are captured, the task is suitable as a first-line screening assay for autism-like phenotypes.
Critical Parameters
Subject mice characteristics
Subject mice are group housed mice between 8 weeks and 6 months of age. They do not have to be experimentally naïve. With the exception of highly stressful tests (e.g., learned helplessness, fear conditioning, Morris water maze), mice having experiences in many other low-stress tests (e.g., developmental milestones, juvenile social interaction, elevated plus-maze, open field exploration, light ↔ dark exploration, general health battery) usually display normal sociability scores in the three-chambered task (Crawley et al., 2007; Chadman et al., 2008; Yang et al., 2009). Retesting the same subjects in the three-chambered task appears to be unproblematic (Moy et al., 2004), provided that there is a break of several weeks between the initial test and the retest.
Sexual experiences and social isolation have strong influences on social behaviors in adult mice (Rawleigh et al., 1993; Koike et al., 2009; Bouet et al., 2011; Kercmar et al., 2011). To avoid these confounding factors, sexually naïve and group housed mice are preferred for this task. Episodic aggressive behaviors in the home cage are not unusual in adult male mice. It is better to avoid testing males that are unusually aggressive or submissive, since their social approach behaviors might be confounded by aggression or social anxiety.
Target mice (novel mouse) characteristics
Target mice are group-housed 129/SvImJ adults between 8 weeks and 6 months of age, preferably of the same sex as the subjects. Low activity and low aggression in this strain ensure that the target mouse sits quietly during the test session and that all of the approach is initiated by the subject mouse. Other inactive strains (e.g., AJ, LP/J, 129/SvJae) could be used instead of 129/SvImJ. In our experience (Yang and Silverman, unpub. observ.), sociability scores are usually not affected by the novel mouse’s strain and/or sex. However, we prefer inactive strains because they continue to sit quietly in the apparatus after repeated use, and over a long period of time. A same-sex novel mouse is used to avoid variability related to estrous cycles. The novel mouse could be younger or older than the subject, as long as both animals are within the age range of 2 to 6 months and their body weights are within 5 grams of each other.
Chamber time and sniff time
The single most important parameter of the three-chambered task is time spent in the two side chambers. Time spent sniffing the novel mouse and the novel object provides a corroborative and more specific measure of social investigation. The comparison between the two sides determines whether a group of animals displays sociability or not. Time spent in the center chamber is shown in graphs for illustrative purpose only, and should NOT be included in the statistical analysis for sociability. Repeated Measures ANOVA or Student’s t test is used to compare time spent in the chamber containing the novel mouse and time spent in the chamber containing the novel object, as well as time spent sniffing the novel mouse and time spent sniffing the novel object. A simple unpaired t test is appropriate for comparing chamber time or sniff time within a single group. Repeated Measures ANOVA is more appropriate for large experiments that have multiple groups, and when sociability needs to be analyzed within each group, e.g., within each drug dose when several doses are tested. This is because Repeated Measure ANOVA avoids the type I error associated with multiple t tests. If the ANOVA or t test result is statistically significant, sociability is present. If the result is not significant, sociability is absent. Sociability is a yes-or-no phenotype, obtained by comparing two sides within each group. Sociability is NOT a graded parameter for quantitatively comparing chamber times across groups. More finegrained reciprocal interaction tests are needed for quantitative comparisons of social behaviors across groups.
The automated three-chambered task was originally designed to investigate simple sociability deficits in mouse models of autism. Positive findings are then followed up with a separate, more complex analysis for reciprocal social interactions (e.g., scoring dyads of freely moving mice on graded, quantitative parameters such as follow, crawl over and under, nose-to-nose sniff, and nose-to-anogenital sniff). An example of a robust mouse model of autism that exhibits deficits in both the three-chambered task and the reciprocal social interaction test is the BTBR T+tf/J inbred strain (Yang et al., 2007b, 2009; McFarlane et al., 2008).
Number of transitions between chambers
The three-chambered task requires that the subject be able to move around and explore all three chambers. Inactivity is a major confounding factor that could interfere with the experiment and skew results. Number of transitions is an important built-in control parameter that could be used to measure exploratory locomotion. This variable could be compared across different groups to identify differences in activity. Animals with extremely low numbers of transitions during Phase II and Phase III could be excluded from the experiment. For an example of excluding inactive animals from data analysis, see Yang et al. (2009).
Troubleshooting
Chamber time is not recorded
In most cases, this is due to photocell malfunctioning, which happens when a photo sensor is blocked, e.g., by mouse hair, dirt, or bedding. To check for problems, turn on the machine and push a toy car through the doorways; monitor the time and transition numbers that occur on the screen as the toy car passes. Locate the problematic photocells, and clean the lens with moistened cotton-tipped swabs. Restart the machine after cleaning. This is usually all it takes to correct the error. If the problem continues, contact the equipment manufacturer.
Chamber time and sniff time give different results
These two parameters usually correlate positively with each other. However, there are occasions when chamber time is not significant but sniff time is significant. How do you decide whether the animals have displayed sociability or not? There is no simple answer to this quandary, but there are several things to consider: (1) double-check the video to see if sniff time has been scored accurately, and whether the animals exhibited any competing or unusual behaviors, e.g., excessive self-grooming, jumping, or rearing; (2) check the number of entries (in our experience, animals that are hyperactive or inactive tend to have low sniff time); and (3) repeat the experiment with a new cohort of mice. If the same results are found, we suggest drawing conclusions based on chamber time.
Mice exhibiting abnormal exploratory behaviors
Subtle environmental factors could have substantial influences on mouse behaviors. When inexplicable behaviors are observed, the first thing to do is to look into factors that could agitate mice. For example, cage changing could make mice aggressive and hyperactive, an inexperienced animal caretaker or experimenter might handle the mice inappropriately, noises and vibrations could distract the animals, odors that come through a ceiling vent could make the animals rear up and sniff the air frequently, or rats from other nearby laboratories could induce fear reactions in subject mice. This is by no means an exhaustive list. To get reliable results, one needs to be vigilant about potentially interfering factors and meticulous in controlling them.
Control mice do not display sociability
Mice used as controls in experiments are usually those that are known to display significant sociability. C57BL/6J, FVB/NJ, and FVB/Ant strains have been shown to exhibit high sociability in a number of published studies (Bolivar et al., 2007; Moy et al., 2007, 2008b; Silverman et al., 2010a,b; Yang et al., 2011a). It is therefore expected that these strains should show sociability in every laboratory. If a group of 8 to12 adult mice of one of these highly social strains fails to show sociability, it is almost certain that something is wrong. Procedural errors and environmental stressful factors are the most common sources of unusual results. Experiments should be put on hold until the control strain resumes normal sociability in a consistent manner. To monitor for occurrences of unusual results, always include a few mice from the control strains in your routine experiments. If the control mice behave the way they typically do, you can be more confident about your results.
For researchers who are testing genetic hypotheses of autism by using transgenic and knockout mice, the wildtype littermates are the correct control group. A lack of sociability in the wildtype group makes it difficult to interpret data from the mutant group. A critical factor to consider here is the genetic background of the mice. Because embryonic stem (ES) cells derived from 129 mice colonize germ lines efficiently, they are the most widely used cell lines for producing targeted mutants. Unfortunately, some 129 substrains are not ideal for behavioral experiments, owing to their inactivity, anatomical abnormality, low sociability, and/or high anxiety-like behaviors (Rodgers et al., 2002; Holmes et al., 2003; Moy et al., 2004, 2007). Transgenic mice that are on a 129 background or a mixed background with a substantial portion from a 129 strain are likely to behave differently from mice on a B6 background. Problems in general health, especially neurological reflexes, vision, olfaction, and locomotion can significantly affect behaviors in the social approach task. It is therefore important to evaluate animals for general health as described by Crawley (2007a, 2008) before testing them in the social approach task. Additional tests for anxiety-like phenotypes might be necessary to control for low general exploration and artifactually high amount of time spent in the center chamber. If the wildtypes do not display sociability due to genetic background issues, one solution is to backcross the mutant line with B6 for multiple generations until phenotypes of the wildtype controls are almost identical to those of B6.
Anticipated Results
Large strain differences in social behaviors exist among inbred mouse strains. This topic has been discussed at length elsewhere (Yang et al., 2011b). Among commonly used strains, multiple laboratories have consistently reported high sociability in B6 and FVB (FVB/NJ and FVB/Ant) strains and low sociability in BTBR and BALB/c strains (Brodkin et al., 2004; Bolivar et al., 2007; Panksepp and Lahvis, 2007; Moy et al., 2007, 2008a; McFarlane et al., 2008; Yang et al., 2007b, 2009, 2011a).
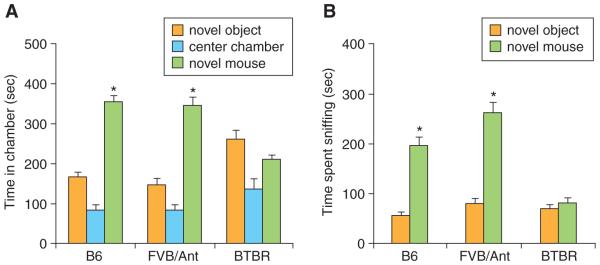
As Figure 8.26.3 illustrates, B6 and FVB/Ant mice spent significantly more time in the chamber containing the novel mouse than in the chamber containing the novel object, and more time sniffing the novel mouse than sniffing the novel object (indicated by asterisks on top of novel mouse bars), whereas BTBR mice spent similar time with both novel stimuli in either chamber. These strains with highly replicable sociability scores could be used as benchmarks in laboratories that conduct the three-chambered task routinely. Unusual behaviors in these strains often indicate methodological problems that require correction before proceeding with experiments.
Figure 8.26.3.
Adult male C57BL/6J (B6) and FVB/AntJ mice displayed sociability, defined as spending more time in the chamber containing the novel mouse than in the chamber containing the novel object (A), and more time sniffing the novel mouse than sniffing the novel object (B). Adult male BTBR T+tf/J (BTBR) mice did not display sociability, spending similar amounts of time in the chamber containing the novel mouse and in the chamber containing the novel object (A), and similar amounts of time sniffing the novel mouse and sniffing the novel object (B). B6 (n = 12), FVB/Ant (n = 16) and BTBR (n = 12). The asterisks (∗) indicate p < 0.01 for the comparison between novel mouse and novel object. This figure is reproduced from Silverman et al. (2010b), with permission from the Nature Publishing Group. For the color version of this figure go to http://wwwcurrentprotocols.com/protocol/ns0826.
There is usually a positive and significant correlation between chamber time and sniff time (Fig. 8.26.4), meaning that chamber time alone might be sufficient for high-throughput screening. A lack of significant correlation between chamber time and sniff time might indicate methodological problems to be solved.
Figure 8.26.4.
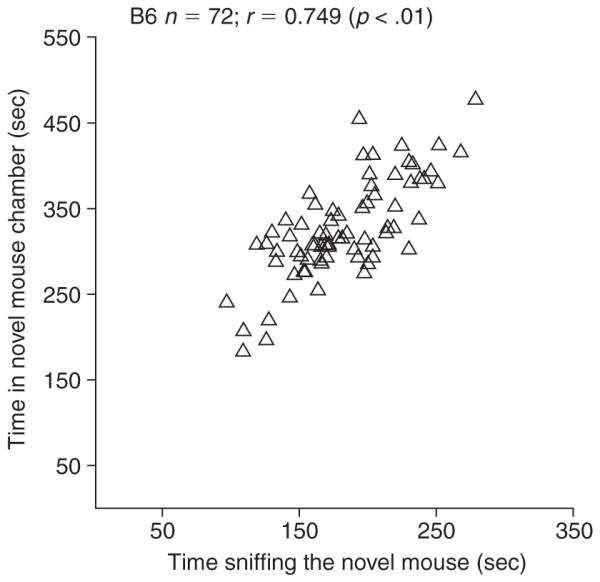
Significant correlation between time spent in chamber and sniff time in C57BL/6J adult male mice (n = 72; M. Yang, M.D. Weber, A.M. Clarke, and V. Zhodzishsky, unpub. observ.).
Time Considerations
The pretest acclimation of novel target mice takes ~1 hr. The social approach task, with its three 10-min sessions (Phase I, II, and III), plus intersession transitions and post-test cleaning, takes ~45 to 50 min to complete. It takes another 5 to 10 min to take notes, organize/transfer data, and reset machines. Altogether, this basic single-unit method takes one person 60 ± 10 min to finish for one mouse. It is reasonable to test six to eight mice a day. With a high-throughput setup using four or more boxes simultaneously, it is possible to finish six rounds, up to 24 subjects a day.
Acknowledgement
This work was supported by the National Institute of Mental Health Intramural Research Program MH02179-24.
Literature Cited
- Ahern TH, Modi ME, Burkett JP, Young LJ. Evaluation of two automated metrics for analyzing partner preference tests. J. Neurosci. Methods. 2009;182:180–188. doi: 10.1016/j.jneumeth.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Washington, D.C.: 1994. [Google Scholar]
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. A new test paradigm for social recognition evidenced by urinary scent marking behavior in C57BL/6J mice. Behav. Brain Res. 2008;190:97–104. doi: 10.1016/j.bbr.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayadi A, Ferrand G, Goncalves da Cruz I, Xavier Warot X. Mouse breeding and colony management. Curr. Protoc. Mouse Biol. 2011;1:239–264. doi: 10.1002/9780470942390.mo100214. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav. Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouet V, Lecrux B, Tran G, Freret T. Effect of preversus post-weaning environmental disturbances on social behaviour in mice. Neurosci. Lett. 2011;488:221–224. doi: 10.1016/j.neulet.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Branchi I, Santucci D, Alleva E. Ultrasonic vocalisation emitted by infant rodents: A tool for assessment of neurobehavioural development. Behav. Brain Res. 2001;125:49–56. doi: 10.1016/s0166-4328(01)00277-7. [DOI] [PubMed] [Google Scholar]
- Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Chadman KK. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacol. Biochem. Behav. 2011;97:586–594. doi: 10.1016/j.pbb.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment. Retard. Dev. Disabil. Res. Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- Crawley J. What’s Wrong With My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. Second John Wiley & Sons; Hoboken, New Jersey: 2007a. [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007b;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: Comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- D’Amato FR, Populin R. Mother-offspring interaction and pup development in genetically deaf mice. Behav. Genet. 1987;17:465–475. doi: 10.1007/BF01073113. [DOI] [PubMed] [Google Scholar]
- Defensor EB, Pearson BL, Pobbe RL, Bolivar VJ, Blanchard DC, Blanchard RJ. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+tf/J mice. Behav. Brain Res. 2011;217:302–308. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: A potential model of autism spectrum disorder. Behav. Brain Res. 2008;187:207–220. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Perez A, Eddy DM, Zinzow WM, Lieberman JA, Snouwaert JN, Koller BH. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav. Brain Res. 2004;153:507–519. doi: 10.1016/j.bbr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Sano Y, de Vries PJ, Dies K, Franz D, Geschwind DH, Kaur M, Lee YS, Li W, Lowe JK, Nakagawa JA, Sahin M, Smith K, Whittemore V, Silva AJ. Gestational immune activation and Tsc2 haploinsufficiency cooperate to disrupt fetal survival and may perturb social behavior in adult mice. Mol. Psychiatry. 2010 doi: 10.1038/mp.2010.115. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SM, Spencer CM, Harrison WR, Yuva-Paylor LA, Graham DF, Daza RA, Hevner RF, Overbeek PA, Paylor R. Multiple autism-like behaviors in a novel transgenic mouse model. Behav. Brain Res. 2011;218:29–41. doi: 10.1016/j.bbr.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Lit Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: The influence of genetic background. Genes Brain Behav. 2003;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DH, Cavaggioni A, Beynon RJ. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–634. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. U.S.A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, Woodgett JR. Assessment of social interaction behaviors. J. Vis. Exp. 2011;25:48. doi: 10.3791/2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E, Agmo A, Braun WJ, Colwell DD, Muglia LJ, Ogawa S, Pfaff DW. Inadvertent social information and the avoidance of parasitized male mice: A role for oxytocin. Proc. Natl. Acad. Sci. U.S.A. 2006;103:4293–4298. doi: 10.1073/pnas.0600410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kercmar J, Budefeld T, Grgurevic N, Tobet SA, Majdic G. Adolescent social isolation changes social recognition in adult mice. Behav. Brain Res. 2011;216:647–651. doi: 10.1016/j.bbr.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Ibi D, Mizoguchi H, Nagai T, Nitta A, Takuma K, Nabeshima T, Yoneda Y, Yamada K. Behavioral abnormality and pharmacologic response in social isolationreared mice. Behav. Brain Res. 2009;202:114–121. doi: 10.1016/j.bbr.2009.03.028. [DOI] [PubMed] [Google Scholar]
- Laviola G, Terranova ML. The developmental psychobiology of behavioural plasticity in mice: The role of social experiences in the family unit. Neurosci. Biobehav. Rev. 1998;23:197–213. doi: 10.1016/s0149-7634(98)00021-9. [DOI] [PubMed] [Google Scholar]
- Lord C, Bishop SL. The autism spectrum: Definitions, assessment and diagnoses. Br. J. Hosp. Med. (London) 2009;70:132–135. doi: 10.12968/hmed.2009.70.3.40552. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to9 years of age. Arch. Gen. Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Tanda K, Nakanishi K, Yamasaki N, Toyama K, Takao K, Takeshima H, Miyakawa T. Comprehensive behavioral phenotyping of ryanodine receptor type 3 (RyR3) knockout mice: Decreased social contact duration in two social interaction tests. Front. Behav. Neurosci. 2009;3:3. doi: 10.3389/neuro.08.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav. Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- Moles A, D’Amato FR. Ultrasonic vocalization by female mice in the presence of a conspecific carrying food cues. Animal Behav. 2000;60:689–694. doi: 10.1006/anbe.2000.1504. [DOI] [PubMed] [Google Scholar]
- Molina J, Carmona-Mora P, Chrast J, Krall PM, Canales CP, Lupski JR, Reymond A, Walz K. Abnormal social behaviors and altered gene expression rates in a mouse model for Potocki-Lupski syndrome. Hum. Mol. Genet. 2008;17:2486–2495. doi: 10.1093/hmg/ddn148. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: An approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behav. Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JW. Development of a mouse test for repetitive, restricted behaviors: Relevance to autism. Behav. Brain Res. 2008a;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav. Brain Res. 2008b;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, D’Ercole AJ, Crawley JN, Magnuson TR, Lauder JM. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009;8:129–142. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, Tomonaga S, Watanabe Y, Chung YJ, Banerjee R, Iwamoto K, Kato T, Okazawa M, Yamauchi K, Tanda K, Takao K, Miyakawa T, Bradley A, Takumi T. Abnormal behavior in a chromosomeengineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137:1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page DT, Kuti OJ, Prestia C, Sur M. Haploinsufficiency for Pten and serotonin transporter cooperatively influences brain size and social behavior. Proc. Natl. Acad. Sci. U.S.A. 2009;106:1989–1994. doi: 10.1073/pnas.0804428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS ONE. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radyushkin K, Hammerschmidt K, Boretius S, Varoqueaux F, El-Kordi A, Ronnenberg A, Winter D, Frahm J, Fischer J, Brose N, Ehrenreich H. Neuroligin-3-deficient mice: Model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009;8:416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- Rawleigh JM, Kemble ED, Ostrem J. Differential effects of prior dominance or subordination experience on conspecific odor preferences in mice. Physiol. Behav. 1993;54:35–39. doi: 10.1016/0031-9384(93)90040-m. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Boullier E, Chatzimichalaki P, Cooper GD, Shorten A. Contrasting phenotypes of C57BL/6JOlaHsd, 129S2/SvHsd and 129/SvEv mice in two exploration-based tests of anxiety-related behaviour. Physiol. Behav. 2002;77:301–310. doi: 10.1016/s0031-9384(02)00856-9. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Young NB, Moy SS, Crawley JN. Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57BL/6J mice. Behav. Brain Res. 2008;193:235–242. doi: 10.1016/j.bbr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010a;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010b;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN. Low stress reactivity and neuroendocrine factors in the BTBR T(+)tf/J mouse model of autism. Neuroscience. 2010c;171:1197–1208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, Sheng M, Crawley JN. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT. Mouse social recognition and preference. Curr. Protoc. Neurosci. 2003;22:8.16.1–8.16.16. doi: 10.1002/0471142301.ns0816s22. [DOI] [PubMed] [Google Scholar]
- Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front. Behav. Neurosci. 2007a;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int. J. Dev. Neurosci. 2007b;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Weber MD, Crawley JN. Light phase testing of social behaviors: Not a problem. Front. Neurosci. 2008;2:186–191. doi: 10.3389/neuro.01.029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur. J. Neurosci. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res. 2011a;4:17–27. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Scattoni ML, Chadman CC, Silverman JL, Crawley JN. Behavioral evaluation of genetic mouse models of autism. In: Amaral D, Geschwind D, Dawson G, editors. Autism Spectrum Disorders. Oxford University Press; New York: 2011b. pp. 904–932. [Google Scholar]
- Zhao Y, Fung C, Shin D, Shin BC, Thamotharan S, Sankar R, Ehninger D, Silva A, Devaskar SU. Neuronal glucose transporter isoform 3 deficient mice demonstrate features of autism spectrum disorders. Mol. Psychiatry. 2010;15:286–299. doi: 10.1038/mp.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J. Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]