Abstract
Neurodevelopmental disorders such as autism and fragile X syndrome were long thought to be medically untreatable, on the assumption that brain dysfunctions were immutably hardwired before diagnosis. Recent revelations that many cases of autism are caused by mutations in genes that control the ongoing formation and maturation of synapses have challenged this dogma. Antagonists of metabotropic glutamate receptor subtype 5 (mGluR5), which modulate excitatory neurotransmission, are in clinical trials for fragile X syndrome, a major genetic cause of intellectual disabilities. About 30% of patients with fragile X syndrome meet the diagnostic criteria for autism. Reasoning by analogy, we considered the mGluR5 receptor as a potential target for intervention in autism. We used BTBR T+tf/J (BTBR) mice, an established model with robust behavioral phenotypes relevant to the three diagnostic behavioral symptoms of autism—unusual social interactions, impaired communication, and repetitive behaviors—to probe the efficacy of a selective negative allosteric modulator of the mGluR5 receptor, GRN-529. GRN-529 reduced repetitive behaviors in three cohorts of BTBR mice at doses that did not induce sedation in control assays of open field locomotion. In addition, the same nonsedating doses reduced the spontaneous stereotyped jumping that characterizes a second inbred strain of mice, C58/J. Further, GRN-529 partially reversed the striking lack of sociability in BTBR mice on some parameters of social approach and reciprocal social interactions. These findings raise the possibility that a single targeted pharmacological intervention may alleviate multiple diagnostic behavioral symptoms of autism.
INTRODUCTION
Autism spectrum disorders affect an estimated 1% of the population (1–4). Intensive behavioral therapy is currently the only effective treatment for the three diagnostic symptoms: qualitative impairment in social interaction, deficits in communication, and stereotyped repetitive behaviors with restricted interests (5–10). To date, only two drugs have been approved by the U.S. Food and Drug Administration for use in patients diagnosed with autism. These two agents, Risperdal and Abilify (11), do not target the core symptoms, but rather treat a cluster of associated symptoms referred to as irritability. Given the high financial and emotional burden to the families and educational and health care systems, affordable treatments for the core diagnostic symptoms of autism represent a severe unmet medical need.
Mouse models of autism spectrum disorders can serve as tools to evaluate the therapeutically relevant efficacy of experimental agents. To incorporate construct validity for the various genetic mutations identified in small numbers of people with autism (12–21), these mutations have been generated in mice (22, 23). Behavioral phenotypes with face validity for some of the diagnostic symptoms of autism have been reported in some of these mutant mouse models (24–47). Experimental interventions, working through diverse genetic and pharmacological mechanisms, rescue subsets of behavioral abnormalities in these mouse models (31, 43, 48–52). Discovery of elevated metabotropic glutamate receptor subtype 5 (mGluR5)–mediated signaling and protein synthesis in fragile X knockout mice (53–56) provided the rationale for testing mGluR5 antagonists in ongoing fragile X clinical trials. A large subset of cases of fragile X meet the diagnostic criteria for autism (57, 58). Because the primary symptoms of autism and fragile X differ in qualitative features, and mouse models of autism and fragile X display sharply divergent phenotypes, testing mGluR5 antagonists in specific assays of mouse behavioral phenotypes that optimize relevance to the diagnostic symptoms of autism would be most informative for translational goals.
Several naturally occurring inbred strains of mice display behavioral features that recapitulate diagnostic symptoms of neurodevelopmental disorders (59–70). These inbred strains are genetically homogeneous and commercially available, maximizing their feasibility as translational tools in medications development (71–73). Inbred strains with robust behavioral phenotypes relevant to the defining symptoms of autism, but without identified genetic mutations, are analogous to individuals with autism for whom the responsible genetic factor(s) remains unknown; at present, this is more than 75% of the cases of autism (21). In addition, inbred strains can incorporate multiple background genes that influence their behavioral deficits, allowing evaluation of two-hit and multiple-hit hypotheses of autism spectrum disorders.
BTBR T+tf/J (BTBR) is a commercially available inbred strain of mouse that displays behavioral phenotypes relevant to all three diagnostic symptoms of autism (60–62, 64, 66, 68–70, 72, 74–78). BTBR engages in low levels of reciprocal social interactions as juveniles and adults, minimal social approach by both males and females, and low levels of ultrasonic vocalizations in response to social olfactory cues and during reciprocal social interactions compared to other standard inbred strains such as C57BL/6J (B6) and FVB (60–62, 64, 66, 68–70, 74, 76–78). Social interaction and communication deficits in BTBR represent face validity to the first and second diagnostic symptoms of autism, respectively. Long bouts of repetitive self-grooming are the third major characteristic of BTBR, representing face validity to the third diagnostic symptom of autism, spontaneous stereotyped and repetitive patterns of behavior. Absence of the corpus callosum connecting the right and left cortical hemispheres in BTBR (79) is reminiscent of a small subset of individuals with autism who are acallosal (80, 81) and may be a sign of more global abnormalities in white matter connectivity in this mouse strain. Experimentally induced postnatal lesions of the corpus callosum in B6 mice, however, did not recapitulate the social deficits, or the repetitive self-grooming, that characterize BTBR (82). C58/J (C58) is another commercially available inbred strain of mouse that displays high levels of stereotyped vertical jumping behaviors, relevant to the motor stereotypies of the third diagnostic symptom category of autism (65, 67). Unlike mouse models based on identified genetic mutations, the background genes responsible for autism-relevant behavioral traits in these inbred strains of mice remain under investigation (64, 83–85).
The robustness and reproducibility of autism-relevant phenotypes in BTBR and C58 provide an attractive translational platform for the preclinical evaluation of intervention therapies (72, 73). A treatment that attenuates different forms of repetitive behaviors in two different inbred strains is likely to generalize across a range of repetitive behaviors and potentially to generalize across species. Here, we test the hypothesis that a selective negative allosteric modulator of the mGluR5 receptor, GRN-529, will ameliorate autism-relevant behavioral abnormalities in mouse models of autism.
RESULTS
GRN-529 brain penetration and mGluR5 receptor occupancy
We measured the plasma and brain exposure levels of GRN-529 (Fig. 1, B to D) and the relationship between unbound brain levels of GRN-529 and mGluR5 occupancy (Fig. 1F) after systemic administration. The relationship between GRN-529 brain exposure and mGluR5 occupancy was similar in B6, BTBR, and C58 mouse strains (Fig. 1F). A 30- to 60-min timeframe was chosen for occupancy and behavioral experiments because a 2-hour time course of plasma and brain exposure after GRN-529 administration revealed that peak concentrations occurred between 0 and 60 min in B6 mice (Fig. 1E).
Fig. 1.
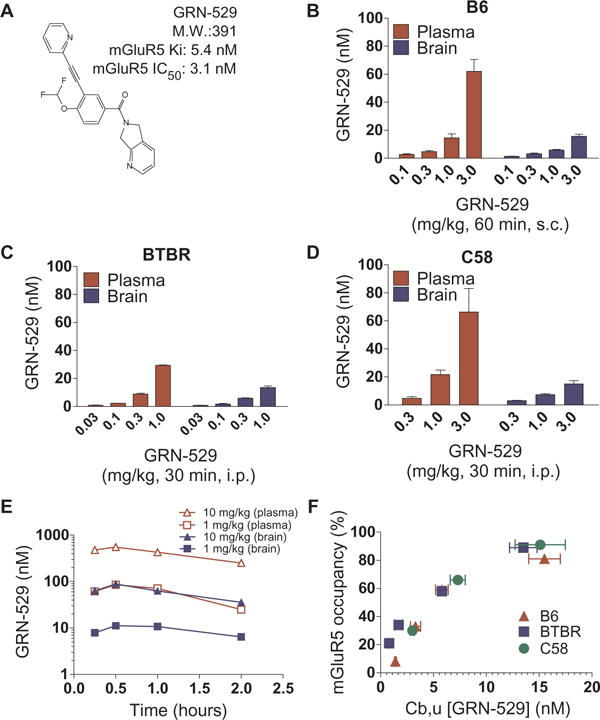
GRN-529 chemical structure, plasma and brain concentrations, and receptor occupancy in B6, BTBR, and C58 mice. (A) Chemical structure of GRN-529 and binding properties [Ki = 5.4 nM and median inhibitory concentration (IC50) = 3.1 nM] at rat mGluR5. (B to D) Unbound plasma and brain concentrations of GRN-529 30 or 60 min after systemic administration in B6, BTBR, and C58 mice. s.c., subcutaneously; i.p., intraperitoneally. (E) Time course of unbound plasma and brain concentrations (nM) of GRN-529 for 2 hours after systemic intraperitoneal administration in B6 mice. (F) Relationship of the concentration of unbound (Cb,u) GRN-529 concentrations (nM) and mGluR5 occupancy in brains from BTBR, B6, and C58 mice. n = 3 to 5 per dose and strain. Data are expressed as the mean for each group.
Amelioration of repetitive and stereotyped behaviors
GRN-529 and the prototypic mGluR5 antagonist 2-methyl-6-(phenylethynyl)pyridine (MPEP) reduced the high levels of repetitive self-grooming that characterizes the BTBR strain. Consistent with previous reports (62, 64, 69, 72, 74), BTBR mice treated with vehicle engaged in much longer bouts of self-grooming than did B6 (Fig. 2 and fig. S1). In three replications by two laboratories, acute administration of GRN-529 significantly reduced repetitive self-grooming scores in BTBR (Fig. 2, B and D, and fig. S1F) at doses that achieved 50 to 90% receptor occupancy (Fig. 1F). 3-((2-Methyl-4-thiazolyl)ethynyl)pyridine (MTEP), another standard mGluR5 antagonist, similarly reduced repetitive self-grooming in BTBR at some doses (fig. S1D) and similarly had no effect in B6 mice (fig. S1C), consistent with current and previous findings with the less selective mGluR5 antagonist MPEP (fig. S1, A and B) (72).
Fig. 2.
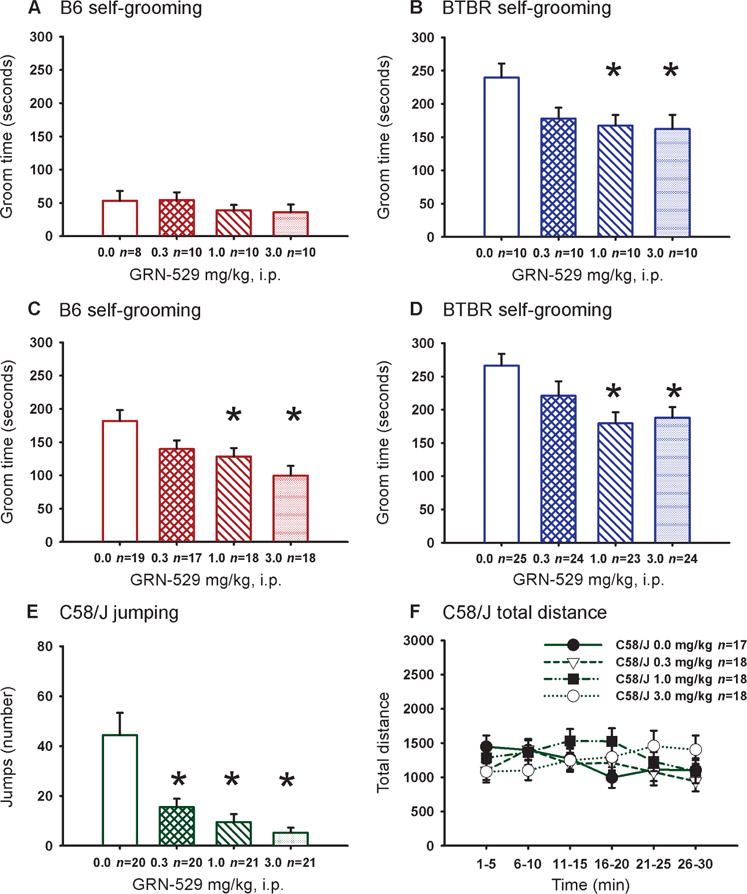
Effect of GRN-529 on repetitive self-grooming in BTBR and stereotyped jumping in C58 mice. Cumulative time spent self-grooming by BTBR and B6 mice was scored over a 10-min session in a clean, closed, empty cage after a 10-min acclimation period. Observations of stereotyped jumping behavior in C58 mice were quantified for a period of 10 min. GRN-529 was tested in two independent laboratory environments across three cohorts. (A) B6 mice did not display any significant differences in the amount of time spent self-grooming after treatment with vehicle (10% Tween 80/saline) or GRN-529 at doses of 0.3, 1.0, or 3.0 mg/kg intraperitoneally (n = 8 to 10 per dose, cohort 1, tested at NIMH, *P < 0.05 versus vehicle). (B) BTBR displayed significant reductions in their innately high levels of repetitive self-grooming after treatment with GRN-529 at doses of 1.0 and 3.0 mg/kg (n = 11 to 14 per dose, cohort 1, tested at NIMH, *P < 0.05 versus vehicle). (C) B6 mice displayed significant reductions in the amount of time spent self-grooming after treatment with GRN-529 at doses of 1.0 and 3.0 mg/kg compared to vehicle (cohort 2, tested at Pfizer). (D) BTBR again displayed significant reductions in high levels of repetitive self-grooming after treatment with GRN-529 at doses of 1.0 and 3.0 mg/kg intraperitoneally (n = 17 to 25 per dose for each strain, cohort 2, tested at Pfizer, *P < 0.05 versus vehicle). (E) Stereotyped vertical jumping in C58 mice was significantly reduced after GRN-529 administration at doses of 0.3, 1.0, and 3.0 mg/kg intraperitoneally versus vehicle (*P < 0.05, tested at Pfizer). (F) No adverse or sedating effects on the general activity of C58 mice were observed during open field locomotion (P > 0.05, tested at Pfizer).
C58 mice displayed high levels of stereotyped vertical jumping (Fig. 2E), consistent with previous findings (67). GRN-529 dose-dependently reduced jumping in C58, almost completely abolishing this repetitive behavior at 3.0 mg/kg, a dose that achieved ~90% occupancy (Fig. 1F). The effects in C58 were not attributable to reduced locomotion or sedation (Fig. 2F and fig. S6).
Complete statistical analyses of all behavioral experiments appear in the Supplementary Materials.
Improvement in social behaviors
Two parameters of social behavior were scored in our automated three-chambered social approach task (86) as described (72, 87). B6 control mice spent more time in the side chamber containing a novel mouse than in the side chamber containing a novel object, meeting the definition of normal sociability in this task, as extensively reported (22, 62, 64, 72–74, 87–89). GRN-529 did not affect the sociability in B6 controls at any dose (Fig. 3, A and C). BTBR mice displayed lack of sociability, defined as not spending more time in the side chamber with the novel mouse than in the side chamber with the novel object, as reported (22, 62, 64, 72–74, 87). GRN-529 reversed sociability deficits in BTBR at a dose of 3.0 mg/kg, as measured by time in the chamber (Fig. 3D). For time spent sniffing the novel mouse versus the novel object, which is a more precise and sensitive measure of true social interaction (87, 90), GRN-529 reversed the deficit in BTBR, again with no detrimental effect on B6 sociability (Fig. 3, A and C). BTBR mice spent significantly more time sniffing the novel mouse than the novel object after doses of 0.3, 1.0, and 3.0 mg/kg (Fig. 3B). The number of entries between chambers, an internal control for general exploration, was not significantly affected by the lower doses of GRN-529 in either strain, but elevated numbers of entries in both strains appeared at a dose of 3.0 mg/kg (Fig. 3, E and F). A second cohort displayed a similar pattern of responses (fig. S2).
Fig. 3.
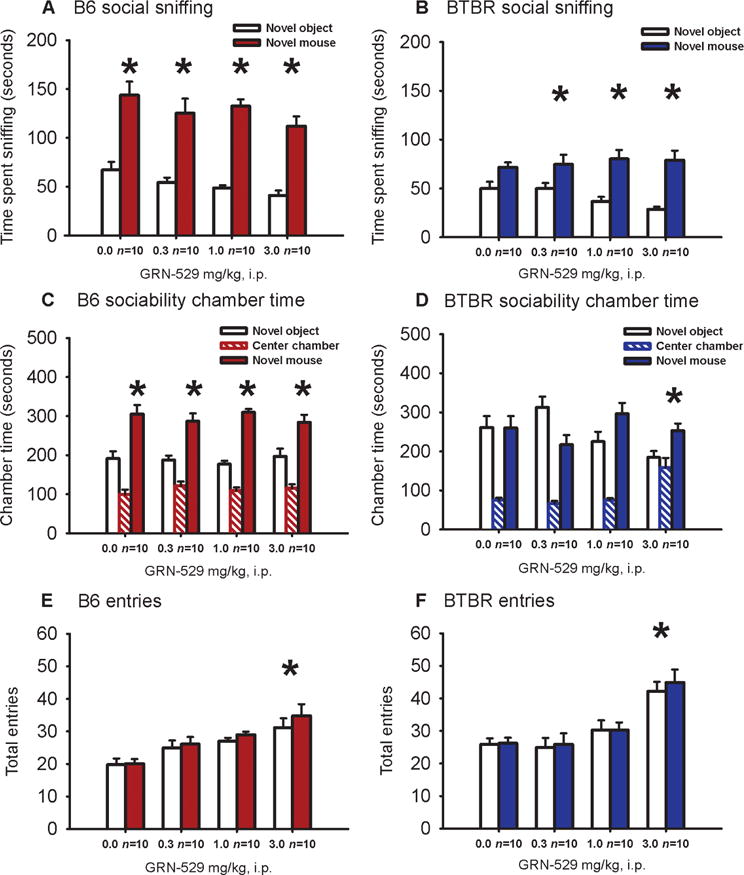
Effect of GRN-529 on social approach in adult BTBR mice. Social approach was assessed in an automated photocell-equipped three-chambered arena with observer scoring of direct sniffing interactions from videotapes of the social approach. (A) B6 mice displayed sociability on the more sensitive parameter, time spent sniffing the novel mouse compared to time spent sniffing the novel object, at each dose of GRN-529 and vehicle. (B) BTBR exhibited its characteristic lack of sociability on the sniff parameter after vehicle administration. BTBR treated with a single acute dose of GRN-529, 0.3, 1.0, or 3.0 mg/kg intraperitoneally, exhibited significant sociability on the sniff time parameter. (C) The B6 control strain displayed normal sociability, defined as spending more time in the chamber with the novel mouse than in the chamber with the novel object, after a single intraperitoneal dose of vehicle (10% Tween 80/saline) or GRN-529 at doses of 0.3, 1.0, and 3 mg/kg. (D) BTBR exhibited its characteristic lack of sociability, that is, did not spend more time in the novel mouse chamber than in the novel object chamber, after treatment with vehicle or the two lower doses of GRN-529. At the highest dose, 3.0 mg/kg, BTBR displayed significant sociability. (E and F) B6 (E) and BTBR (F) displayed a greater number of entries into the side chambers after treatment with GRN-529 at the highest dose, 3.0 mg/kg intraperitoneally, indicating a general increase of exploratory activity during the social approach task at that dose. *P < 0.05, novel mouse versus novel object in (A) to (D); *P < 0.05 versus vehicle in (E) and (F). n = 10 per dose for each strain, cohort 1, assayed at NIMH. See fig. S2 for replicated findings in cohort 2.
Multiple parameters of social behaviors were scored during a freely moving, dyadic reciprocal social interaction test in adult B6 and BTBR mice treated with vehicle or the most effective dose of GRN-529 in the social approach test, 3.0 mg/kg. These detailed interactive social parameters were collected with 129/SvImJ mice as stimulus partners, chosen for their inherently low spontaneous locomotion and aggression, as reported (60, 61, 91). During the reciprocal interaction test, B6 mice consistently exhibited high levels of nose-to-nose sniffing, front approach, and total time spent in social contact (Fig. 4, A and C, and fig. S3A), whereas BTBR mice displayed lack of sociability on these standard parameters (Fig. 4, B and D, and fig. S3B), consistent with previous reports (60, 78, 91). GRN-529 increased sociability in BTBR on some of these parameters, particularly on the most sensitive measures, nose-to-nose sniffing (Fig. 4B) and total time spent in social contact (Fig. 4D), while having no effect in B6 controls (Fig. 4, A and C, and fig. S3, A, C, and E). GRN-529 did not affect general exploratory locomotion in B6 and BTBR mice during the reciprocal interaction session (fig. S3, G to J). Further, during the freely moving, dyadic reciprocal interaction task, BTBR mice treated with vehicle engaged in much longer bouts of self-grooming and repetitive digging than did B6 mice (Fig. 4, E to H). Acute administration of GRN-529 significantly reduced these spontaneous repetitive behaviors within a social context (Fig. 4, F and H).
Fig. 4.
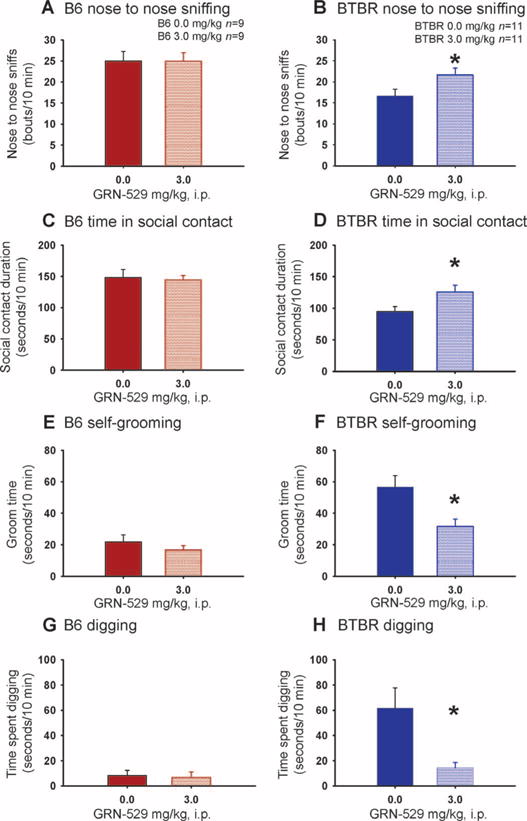
Effect of GRN-529 on dyadic reciprocal social interactions in BTBR mice. Social interactions were digitally recorded in dyads of mice in the Noldus PhenoTyper 3000 arena. Coded videos were subsequently scored by an observer uninformed of the treatment condition using Noldus Observer 8.0XT software. (A) The B6 control strain displayed normal sociability, as illustrated by high levels of nose-to-nose sniffing with the 129/SvImJ partner stimulus mouse, after a single intraperitoneal dose of vehicle (10% Tween 80/saline) or GRN-529 (3.0 mg/kg). (B) BTBR treated with vehicle exhibited its characteristic low sociability, displaying fewer bouts of nose-to-nose sniffing with the 129/SvImJ partner stimulus mouse. GRN-529 (3.0 mg/kg) increased nose-to-nose sniffing bouts in the BTBR. (C) B6 displayed high sociability on the parameter, time spent in social contact after GRN-529 or vehicle. (D) BTBR exhibited its characteristic low sociability on time spent in social contact after vehicle administration. BTBR treated with a single acute dose of GRN-529 (3.0 mg/kg) exhibited increased time in social contact. (E) Cumulative time spent self-grooming was calculated during the 10-min reciprocal social interaction test session. B6 mice treated with either vehicle or GRN-529 (3.0 mg/kg) did not display any significant differences in the amount of time spent self-grooming during the session. (F) BTBR treated with GRN-529 (3.0 mg/kg) displayed significant reductions in their high levels of repetitive self-grooming versus BTBR treated with vehicle. (G) Cumulative time spent digging in the arena floor bedding during the social task was calculated during the 10-min test session. B6 mice treated with either vehicle or GRN-529 (3.0 mg/kg) displayed similar time spent digging during the session. (H) BTBR treated with GRN-529 (3.0 mg/kg) displayed significant reductions in their high levels of repetitive digging behavior versus BTBR treated with vehicle. n = 9 to 11 per treatment group, GRN-529 (3.0 mg/kg) and vehicle, for each strain, *P < 0.05 versus vehicle.
Absence of sedation
Reductions in repetitive self-grooming and stereotyped jumping such as those that we saw could be caused by sedative actions of a pharmacological agent. Conversely, increased numbers of entries in the social approach apparatus could be caused by stimulant actions of a pharmacological agent. To directly detect nonspecific actions of GRN-529 on general activity, we tested the same doses at the 30-min time point in B6, BTBR, and C58 mice on open field locomotion. No evidence of sedation was detected in any strain at any dose during a 30-min test session (Fig. 5 and figs. S4 to S6). Higher total distances traveled were seen in both strains at a dose of 3.0 mg/kg, and higher vertical and center time scores were seen in BTBR at the higher doses in one cohort tested at Pfizer, indicating a moderate increase in general exploratory locomotion. No increases in total distance traveled were observed in C58 mice at any dose of GRN-529 tested (Fig. 2F). No qualitatively unusual behaviors were observed after GRN-529 treatments during any of the grooming, social, or open field test sessions.
Fig. 5.
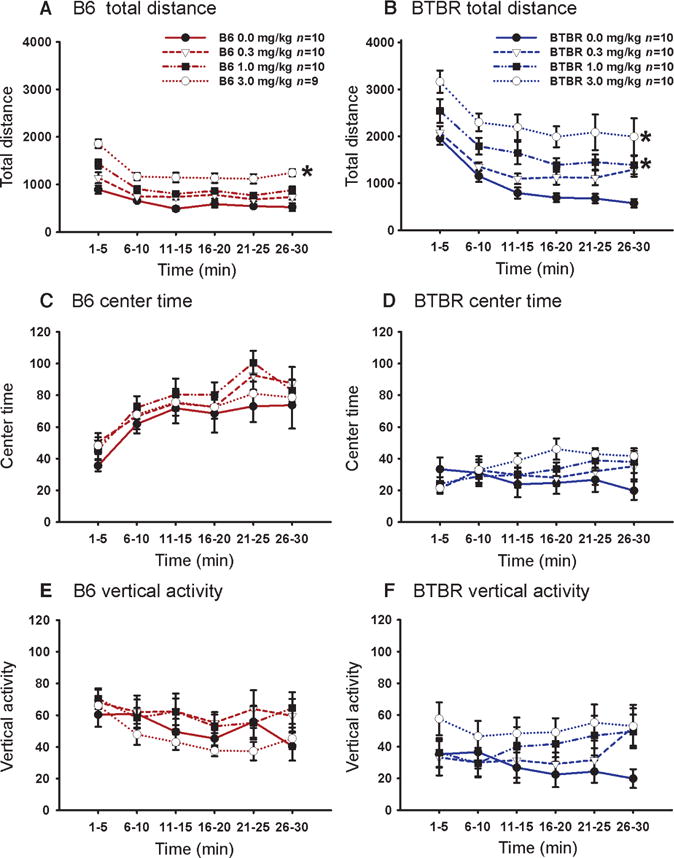
Effect of GRN-529 on open field locomotion at doses that reversed repetitive and social deficits. Exploratory locomotion was assayed in a standard automated open field arena, in 5-min time bins across a 30-min session after the identical GRN-529 treatments. (A) B6 displayed a significant increase in total distance traversed after GRN-529 at the highest dose, 3.0 mg/kg, intraperitoneally, compared to vehicle. (B and C) GRN-529 administration had no significant effect on (B) time spent in the center of the arena or (C) vertical activity in B6 tested at NIMH. n = 9 to 10 per dose. (D) BTBR displayed significant increases in total distance traversed after GRN-529 at doses of 1.0 and 3.0 mg/kg intraperitoneally compared to vehicle, indicating increased exploratory activity. (E and F) GRN-529 administration had no significant effect on (E) time spent in the center of the arena or (F) vertical activity in BTBR. *P < 0.05, n = 10 per dose, cohort 1 tested at NIMH. See fig. S4 for open field results replicated at NIMH and fig. S5 for open field results replicated at Pfizer. See fig. S6 for additional open field parameters in C58 mice.
DISCUSSION
Autism is a behaviorally diagnosed, lifetime neurodevelopmental disorder. Biological abnormalities have been reported in eye tracking, neuroanatomical pathway connectivity, brain regional volumes, cortical activation during social and communication tasks as measured with functional magnetic resonance imaging and magnetoencephalography, serotonin levels, and other biological assays, but not with sufficient consistency for these to constitute uniform diagnostic biomarkers (92–94). Therefore, therapeutic efficacy is currently evaluated by improvement in the diagnostic behavioral symptoms (11, 50, 95). Compelling neuropharmacological targets for autism remain to be identified. Target strategies draw from therapeutics under investigation for other neurodevelopmental disorders and hypotheses emerging from mutations in synaptic genes identified in small numbers of individuals with autism spectrum disorders (43, 49, 52). One such strategy is modulation of glutamatergic neurotransmission through the mGluR5 receptor (96), which is under investigation for the treatment of fragile X (55).
Using the BTBR mouse model, which recapitulates endophenotypic analogies to the diagnostic social deficits, impaired communication, and repetitive behavioral symptoms of autism, we evaluated GRN-529, a compound with high specificity for the mGluR5 receptor. As demonstrated by Hughes and colleagues (97), in competition binding experiments, GRN-529 competes for [3H]MPEP binding at mGluR5 with high affinity [inhibition constant (Ki) = 5.4 ± 0.43 nM], antagonizes glutamate-induced increases in calcium signaling, but does not directly bind to the orthosteric binding site or affect the affinity of glutamate for this site, consistent with negative allosteric modulation (97). Using pharmaco-kinetic and ex vivo receptor occupancy studies, we demonstrated brain exposure and target engagement for GRN-529 across the efficacious dose range (0.3 to 3.0 mg/kg) in B6, BTBR, and C58 mice. Treatment with GRN-529 at these doses reversed the social approach deficits in BTBR on two standardized mouse assays for sociability. In particular, the more ethologically meaningful parameters of time engaged in interactive sniffing of a novel mouse versus time spent sniffing a novel object during social approach, bouts of nose-to-nose sniffing and time in social contact during reciprocal interactions, were restored in BTBR by the acute pharmacological intervention, which had no effect in the normal control B6 strain.
We discovered strong reductions in the repetitive self-grooming phenotype in the BTBR mouse model of autism after GRN-529 treatment, replicated in three cohorts of mice at two research sites. Magnitudes of reduction were consistent with a previous report with the prototypic mGluR5 antagonist MPEP, which required higher doses and is known to also act at N-methyl-D-aspartate (NMDA) receptors (72). Parallel attenuation of self-grooming in BTBR was confirmed at the high dose of MPEP, and for the more brain penetrant and selective mGluR5 analog MTEP, although its dose-response curve was nonlinear. Further, GRN-529 markedly reduced a stereotyped behavior in another inbred strain, vertical jumping in C58.
Lower self-grooming and jumping scores in the treated mice were not the result of overall behavioral sedation, because open field activity was not reduced by GRN-529 at the same doses, time point, and route of administration. Small increases in open field scores, and on number of chamber entries in the three-chambered apparatus, were detected in B6 and BTBR mice after the higher doses of GRN-529, although exploratory locomotion was not significantly affected by GRN-529 in B6 and BTBR mice engaged in social interaction in the PhenoTyper arena. Total distance traversed in the open field was not affected by GRN-529 in C58, did not reach the range considered hyperactive for B6, and did not mimic the qualitative type of fast, unstructured activity that characterizes rodent responses to psychostimulants. Nevertheless, it remains conceptually possible that increased general exploration could contribute to increased sociability. Further, these results may suggest that mGluR5 treatment could prove helpful for the subset of individuals with autism and attention deficit hyperactivity disorder.
Our results show that negative allosteric modulation of the mGluR5 receptor improves social interactions, reduces high levels of repetitive behaviors in BTBR, and reduces stereotyped behaviors in C58, relevant to the first and third diagnostic symptoms of autism. Early clinical indications of beneficial actions of mGluR5 antagonists in fragile X syndrome make this class of therapeutic targets of particular interest (55). The present preclinical findings on reversal of features relevant to autism in two mouse models convey promise for the mGluR5 strategy as a therapeutic intervention for two core diagnostic symptoms of autism.
MATERIALS AND METHODS
Adult male and female C57BL/6J (B6) and BTBR T+tf/J (BTBR) mice tested at the National Institute of Mental Health (NIMH) in Bethesda, Maryland, were bred from adult pairs originally purchased from The Jackson Laboratory (JAX). B6, BTBR, and C58/J (C58) tested at Pfizer in Groton, Connecticut, were purchased as adults from JAX. Behavioral parameters were scored with automated equipment or from digital videotapes by investigators uninformed of treatment. To further ensure absence of unconscious bias by the raters, we recorded identities of subject mice from paw tattoos only after the behavioral test session ended. All procedures were approved by the NIMH and the Pfizer Inc. Animal Care and Use Committees. Complete behavioral and biochemical methods appear in the Supplementary Materials.
Mice were injected with GRN-529 and killed at the time points indicated for pharmacokinetic and ex vivo receptor occupancy assays (Fig. 1, B to F). Plasma and forebrain samples were collected for analysis of drug concentrations by liquid chromatography–mass spectrometry, and of receptor occupancy by binding of 1 nM [3H]MPEPy, similar to the procedures used by Hughes et al. (97). Results from the pharmacokinetic, ex vivo receptor occupancy and/or previous behavioral assays in B6, BTBR, and C58 mice were used to select the doses and posttreatment interval for the present behavioral studies. Complete methods and results appear in the Supplementary Materials.
Repetitive self-grooming was scored from digital videotapes with methods previously published (22, 62, 64, 72, 74, 89) and described in the Supplementary Materials. Briefly, each subject mouse was placed in a bare, empty cage for a 10-min habituation session and then video was recorded for a 10-min test session. Cumulative time spent self-grooming was scored from the videos using a high-accuracy Traceable stopwatch (Thomas Scientific) with the auditory component silenced.
Sociability in the automated three-chambered apparatus developed by our group (86) was conducted as published (22, 32, 62, 64, 72, 74, 87, 89) and described in the Supplementary Materials. Briefly, each subject mouse was placed in the bare, empty three-chambered apparatus for a 10-min habituation session. A novel object, an inverted wire cup, was then placed in one side chamber, and a novel mouse was placed inside a second inverted wire cup in the other side chamber. The subject mouse was given a 10-min test session, offering the choice of spending time in the vicinity of a novel social partner or a novel inanimate object. Time in each chamber, representing proximity to a social partner, was scored by the automated software. Time spent in directly sniffing the novel mouse, representing actual reciprocal social interactions, was subsequently scored from digital videos of the sessions by an observer with a stopwatch.
Reciprocal social interaction was tested in adult B6 and BTBR subject mice during a 10-min session in a Noldus PhenoTyper 3000 arena, as described (91). 129/SvImJ mice were used as interaction stimulus partners to evaluate social behavior in response to social cues from a uniform stimulus mouse during a 10-min session between freely moving dyads (60, 64, 91). Standard interaction parameters, time spent in repetitive behaviors, and arena exploration were simultaneously scored as published (91) and described in the Supplementary Materials.
Open field locomotor activity was evaluated in a standard AccuScan photocell-equipped open field over a 30-min test session, using methods previously published (32, 72, 89, 98) and described in the Supplementary Materials. Automated parameters including total distance, vertical activity, and center time were generated by the VersaMax software.
Supplementary Material
Fig. S1. mGluR5 antagonists reduced repetitive self-grooming in B6 and BTBR.
Fig. S2. GRN-529 partially ameliorated social approach deficits in BTBR.
Fig. S3. GRN-529 partially ameliorated reciprocal interaction social deficits in BTBR without sedation or hyperactivation in BTBR.
Fig. S4. GRN-529 did not induce sedation at doses that reversed repetitive and social deficits in cohort 2 B6 and BTBR mice at NIMH.
Fig. S5. GRN-529 did not induce sedation in cohort 3 B6 and BTBR mice at doses that reversed repetitive behaviors in cohort 3 at Pfizer.
Fig. S6. GRN-529 did not induce sedation in C58 mice at Pfizer.
Acknowledgments
Funding: This work was supported by the NIMH Intramural Research Program (J.L.S., M.N.K., S.M.T., S.S.T., and J.N.C.) and Pfizer Global Research (D.G.S., S.J.S.R., D.K.B., D.L.S., K.F., and R.H.R.).
Footnotes
Author contributions: J.N.C., J.L.S., R.H.R., D.G.S., and S.J.S.R. designed the study; J.N.C., J.L.S., D.G.S., and S.J.S.R. wrote the paper; J.L.S., M.N.K., S.M.T., and S.S.T. conducted the behavioral experiments and statistical analyses at NIMH; S.J.S.R. and D.K.B. conducted the behavioral experiments and statistical analyses at Pfizer; D.L.S. and K.F. conducted the pharmacokinetics and receptor occupancy at Pfizer.
Competing interests: The authors declare that they have no competing interests. GRN-529 (PF-05212391) is published as international patent application publication number WO2010/124047.
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/4/131/131ra51/DC1
Material and Methods
REFERENCES AND NOTES
- 1.Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, Ghandour RM, Singh GK, Strickland BB, Trevathan E, van Dyck PC. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009;124:1395–1403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- 2.Kim BJ, Cheon WS, Oh HC, Kim JW, Park JD, Kim JG. Prevalence and risk factor of erosive esophagitis observed in Korean National Cancer Screening Program. J Korean Med Sci. 2011;26:642–646. doi: 10.3346/jkms.2011.26.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lord C. Epidemiology: How common is autism? Nature. 2011;474:166–168. doi: 10.1038/474166a. [DOI] [PubMed] [Google Scholar]
- 4.Pinto-Martin JA, Levy SE, Feldman JF, Lorenz JM, Paneth N, Whitaker AH. Prevalence of autism spectrum disorder in adolescents born weighing <2000 grams. Pediatrics. 2011;128:883–891. doi: 10.1542/peds.2010-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic Criteria from DSM-IV. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 6.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 7.Krasny L, Williams BJ, Provencal S, Ozonoff S. Social skills interventions for the autism spectrum: Essential ingredients and a model curriculum. Child Adolesc Psychiatr Clin N Am. 2003;12:107–122. doi: 10.1016/s1056-4993(02)00051-2. [DOI] [PubMed] [Google Scholar]
- 8.Landa RJ. Diagnosis of autism spectrum disorders in the first 3 years of life. Nat Clin Pract Neurol. 2008;4:138–147. doi: 10.1038/ncpneuro0731. [DOI] [PubMed] [Google Scholar]
- 9.Zwaigenbaum L, Bryson S, Lord C, Rogers S, Carter A, Carver L, Chawarska K, Constantino J, Dawson G, Dobkins K, Fein D, Iverson J, Klin A, Landa R, Messinger D, Ozonoff S, Sigman M, Stone W, Tager-Flusberg H, Yirmiya N. Clinical assessment and management of toddlers with suspected autism spectrum disorder: Insights from studies of high-risk infants. Pediatrics. 2009;123:1383–1391. doi: 10.1542/peds.2008-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Donaldson A, Varley J. Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics. 2010;125:e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McPheeters ML, Warren Z, Sathe N, Bruzek JL, Krishnaswami S, Jerome RN, Veenstra-Vanderweele J. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics. 2011;127:e1312–e1321. doi: 10.1542/peds.2011-0427. [DOI] [PubMed] [Google Scholar]
- 12.Jamain S, Quach H, Betancur C, Råstam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Paris Autism Research International Sibpair Study, Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abrahams BS, Geschwind DH. Advances in autism genetics: On the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, Nelson SF, Cantor RM, Geschwind DH. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brune CW, Korvatska E, Allen-Brady K, Cook EH, Jr, Dawson G, Devlin B, Estes A, Hennelly M, Hyman SL, McMahon WM, Munson J, Rodier PM, Schellenberg GD, Stodgell CJ, Coon H. Heterogeneous association between engrailed-2 and autism in the CPEA network. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:187–193. doi: 10.1002/ajmg.b.30585. [DOI] [PubMed] [Google Scholar]
- 16.Buxbaum JD. Multiple rare variants in the etiology of autism spectrum disorders. Dialogues Clin Neurosci. 2009;11:35–43. doi: 10.31887/DCNS.2009.11.1/jdbuxbaum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gauthier J, Spiegelman D, Piton A, Lafrenière RG, Laurent S, St-Onge J, Lapointe L, Hamdan FF, Cossette P, Mottron L, Fombonne E, Joober R, Marineau C, Drapeau P, Rouleau GA. Novel de novo SHANK3 mutation in autistic patients. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:421–424. doi: 10.1002/ajmg.b.30822. [DOI] [PubMed] [Google Scholar]
- 18.Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Wood S, Zhang H, Estes A, Brune CW, Bradfield JP, Imielinski M, Frackelton EC, Reichert J, Crawford EL, Munson J, Sleiman PM, Chiavacci R, Annaiah K, Thomas K, Hou C, Glaberson W, Flory J, Otieno F, Garris M, Soorya L, Klei L, Piven J, Meyer KJ, Anagnostou E, Sakurai T, Game RM, Rudd DS, Zurawiecki D, McDougle CJ, Davis LK, Miller J, Posey DJ, Michaels S, Kolevzon A, Silverman JM, Bernier R, Levy SE, Schultz RT, Dawson G, Owley T, McMahon WM, Wassink TH, Sweeney JA, Nurnberger JI, Coon H, Sutcliffe JS, Minshew NJ, Grant SF, Bucan M, Cook EH, Buxbaum JD, Devlin B, Schellenberg GD, Hakonarson H. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar RA, Marshall CR, Badner JA, Babatz TD, Mukamel Z, Aldinger KA, Sudi J, Brune CW, Goh G, Karamohamed S, Sutcliffe JS, Cook EH, Geschwind DH, Dobyns WB, Scherer SW, Christian SL. Association and mutation analyses of 16p11.2 autism candidate genes. PLoS One. 2009;4:e4582. doi: 10.1371/journal.pone.0004582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berkel S, Marshall CR, Weiss B, Howe J, Roeth R, Moog U, Endris V, Roberts W, Szatmari P, Pinto D, Bonin M, Riess A, Engels H, Sprengel R, Scherer SW, Rappold GA. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet. 2010;42:489–491. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- 21.Miles JH. Autism spectrum disorders—A genetics review. Genet Med. 2011;13:278–294. doi: 10.1097/GIM.0b013e3181ff67ba. [DOI] [PubMed] [Google Scholar]
- 22.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ey E, Leblond CS, Bourgeron T. Behavioral profiles of mouse models for autism spectrum disorders. Autism Res. 2011;4:5–16. doi: 10.1002/aur.175. [DOI] [PubMed] [Google Scholar]
- 24.Molina J, Carmona-Mora P, Chrast J, Krall PM, Canales CP, Lupski JR, Reymond A, Walz K. Abnormal social behaviors and altered gene expression rates in a mouse model for Potocki-Lupski syndrome. Hum Mol Genet. 2008;17:2486–2495. doi: 10.1093/hmg/ddn148. [DOI] [PubMed] [Google Scholar]
- 25.Moretti P, Zoghbi HY. MeCP2 dysfunction in Rett syndrome and related disorders. Curr Opin Genet Dev. 2006;16:276–281. doi: 10.1016/j.gde.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 26.DeLorey TM. GABRB3 gene deficient mice: A potential model of autism spectrum disorder. Int Rev Neurobiol. 2005;71:359–382. doi: 10.1016/s0074-7742(05)71015-1. [DOI] [PubMed] [Google Scholar]
- 27.Shu W, Cho JY, Jiang Y, Zhang M, Weisz D, Elder GA, Schmeidler J, De Gasperi R, Sosa MA, Rabidou D, Santucci AC, Perl D, Morrisey E, Buxbaum JD. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc Natl Acad Sci USA. 2005;102:9643–9648. doi: 10.1073/pnas.0503739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheh MA, Millonig JH, Roselli LM, Ming X, Jacobsen E, Kamdar S, Wagner GC. En2 knockout mice display neurobehavioral and neurochemical alterations relevant to autism spectrum disorder. Brain Res. 2006;1116:166–176. doi: 10.1016/j.brainres.2006.07.086. [DOI] [PubMed] [Google Scholar]
- 29.Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mineur YS, Huynh LX, Crusio WE. Social behavior deficits in the Fmr1 mutant mouse. Behav Brain Res. 2006;168:172–175. doi: 10.1016/j.bbr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci USA. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etherton MR, Blaiss CA, Powell CM, Südhof TC. Mouse neurexin-1α deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci USA. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, Tomonaga S, Watanabe Y, Chung YJ, Banerjee R, Iwamoto K, Kato T, Okazawa M, Yamauchi K, Tanda K, Takao K, Miyakawa T, Bradley A, Takumi T. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137:1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Page DT, Kuti OJ, Prestia C, Sur M. Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior. Proc Natl Acad Sci USA. 2009;106:1989–1994. doi: 10.1073/pnas.0804428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radyushkin K, Hammerschmidt K, Boretius S, Varoqueaux F, El-Kordi A, Ronnenberg A, Winter D, Frahm J, Fischer J, Brose N, Ehrenreich H. Neuroligin-3-deficient mice: Model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009;8:416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- 38.Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Katz AM, Scattoni ML, Harris MJ, Saxena R, Silverman JL, Crawley JN, Zhou Q, Hof PR, Buxbaum JD. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young DM, Schenk AK, Yang SB, Jan YN, Jan LY. Altered ultrasonic vocalizations in a tuberous sclerosis mouse model of autism. Proc Natl Acad Sci USA. 2010;107:11074–11079. doi: 10.1073/pnas.1005620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter MD, Shah CR, Muller CL, Crawley JN, Carneiro AM, Veenstra-Vanderweele J. Absence of preference for social novelty and increased grooming in integrin β3 knockout mice: Initial studies and future directions. Autism Res. 2011;4:57–67. doi: 10.1002/aur.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehninger D, Silva AJ. Increased levels of anxiety-related behaviors in a Tsc2 dominant negative transgenic mouse model of tuberous sclerosis. Behav Genet. 2011;41:357–363. doi: 10.1007/s10519-010-9398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, Golshani P, Trachtenberg JT, Peles E, Geschwind DH. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SE, Zhou YD, Zhang G, Jin Z, Stoppel DC, Anderson MP. Increased gene dosage of Ube3a results in autism traits and decreased glutamate synaptic transmission in mice. Sci Transl Med. 2011;3:103ra97. doi: 10.1126/scitranslmed.3002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, McCoy PA, Rodriguiz RM, Pan Y, Je HS, Roberts AC, Kim CJ, Berrios J, Colvin JS, Bousquet-Moore D, Lorenzo I, Wu G, Weinberg RJ, Ehlers MD, Philpot BD, Beaudet AL, Wetsel WC, Jiang YH. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet. 2011;20:3093–3108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wöhr M, Roullet FI, Hung AY, Sheng M, Crawley JN. Communication impairments in mice lacking Shank1: Reduced levels of ultrasonic vocalizations and scent marking behavior. PLoS One. 2011;6:e20631. doi: 10.1371/journal.pone.0020631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang M, Scattoni ML, Chadman KK, Silverman JM, Crawley JN. In: Behavioral Evaluation of Genetic Mouse Models of Autism. Amaral DG, Dawson G, Geschwind DH, editors. Oxford Univ Press; Oxford, UK: 2010. [Google Scholar]
- 48.Hayashi ML, Rao BS, Seo JS, Choi HS, Dolan BM, Choi SY, Chattarji S, Tonegawa S. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci USA. 2007;104:11489–11494. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ehninger D, Li W, Fox K, Stryker MP, Silva AJ. Reversing neurodevelopmental disorders in adults. Neuron. 2008;60:950–960. doi: 10.1016/j.neuron.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cobb S, Guy J, Bird A. Reversibility of functional deficits in experimental models of Rett syndrome. Biochem Soc Trans. 2010;38:498–506. doi: 10.1042/BST0380498. [DOI] [PubMed] [Google Scholar]
- 52.Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 54.Dölen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krueger DD, Bear MF. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med. 2011;62:411–429. doi: 10.1146/annurev-med-061109-134644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacquemont S, Curie A, des Portes V, Torrioli MG, Berry-Kravis E, Hagerman RJ, Ramos FJ, Cornish K, He Y, Paulding C, Neri G, Chen F, Hadjikhani N, Martinet D, Meyer J, Beckmann JS, Delange K, Brun A, Bussy G, Gasparini F, Hilse T, Floesser A, Branson J, Bilbe G, Johns D, Gomez-Mancilla B. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci Transl Med. 2011;3:64ra1. doi: 10.1126/scitranslmed.3001708. [DOI] [PubMed] [Google Scholar]
- 57.Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Jr, Roberts J, Mirrett P. Autistic behavior in children with fragile X syndrome: Prevalence, stability, and the impact of FMRP. Am J Med Genet A. 2006;140A:1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- 58.Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, Tassone F, Hagerman PJ, Herman H, Hagerman RJ. Autism profiles of males with fragile X syndrome. Am J Ment Retard. 2008;113:427–438. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 60.Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: Variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T + tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fairless AH, Dow HC, Toledo MM, Malkus KA, Edelmann M, Li H, Talbot K, Arnold SE, Abel T, Brodkin ES. Low sociability is associated with reduced size of the corpus callosum in the BALB/cJ inbred mouse strain. Brain Res. 2008;1230:211–217. doi: 10.1016/j.brainres.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 65.Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JW. Development of a mouse test for repetitive, restricted behaviors: Relevance to autism. Behav Brain Res. 2008;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ryan BC, Young NB, Crawley JN, Bodfish JW, Moy SS. Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain. Behav Brain Res. 2010;208:178–188. doi: 10.1016/j.bbr.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Defensor EB, Pearson BL, Pobbe RL, Bolivar VJ, Blanchard DC, Blanchard RJ. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behav Brain Res. 2011;217:302–308. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pearson BL, Pobbe RL, Defensor EB, Oasay L, Bolivar VJ, Blanchard DC, Blanchard RJ. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:228–235. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wöhr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wagner GC, Avena N, Kita T, Nakashima T, Fisher H, Halladay AK. Risperidone reduction of amphetamine-induced self-injurious behavior in mice. Neuropharmacology. 2004;46:700–708. doi: 10.1016/j.neuropharm.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res. 2011;4:17–27. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010;171:1197–1208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chadman KK. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacol Biochem Behav. 2011;97:586–594. doi: 10.1016/j.pbb.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 77.Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem. 2011;116:291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010;214:443–449. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wahlsten D, Metten P, Crabbe JC. Survey of 21 inbred mouse strains in two laboratories reveals that BTBR T/+ tf/tf has severely reduced hippocampal commissure and absent corpus callosum. Brain Res. 2003;971:47–54. doi: 10.1016/s0006-8993(03)02354-0. [DOI] [PubMed] [Google Scholar]
- 80.Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, Sherr EH. Agenesis of the corpus callosum: Genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- 81.Booth R, Wallace GL, Happé F. Connectivity and the corpus callosum in autism spectrum conditions: Insights from comparison of autism and callosal agenesis. Prog Brain Res. 2011;189:303–317. doi: 10.1016/B978-0-444-53884-0.00031-2. [DOI] [PubMed] [Google Scholar]
- 82.Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bolivar V, Solanki M, Du W, Day S, Manley K. Autism-Like Behaviors in Mice Are Modified by Genetic Background, Sex and Testing Protocol. Society for Neuroscience; Washington, DC: 2011. [Google Scholar]
- 84.Bothe G, Solanki M, Du W, Kusek G, Auerbach R, Manley K, Bolivar V. Genetic Investigations of Corpus Callosum Abnormalities in BTBR T+ tf/J Mice. Society for Neuroscience; Washington, DC: 2011. [Google Scholar]
- 85.Jones-Davis D, Yang M, Rider E, Sen S, Crawley J, Sherr E. Identification of Loci Associated with Autism-Relevant Behavioral Traits in the BTBR Strain of Mouse. Society for Neuroscience; Washington, DC: 2011. [Google Scholar]
- 86.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 87.Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Curr Protoc Neurosci. 2011;Chapter 8 doi: 10.1002/0471142301.ns0826s56. Unit 8.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brodkin ES. BALB/c mice: Low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 89.Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, Sheng M, Crawley JN. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fairless AH, Shah RY, Guthrie AJ, Li H, Brodkin ES. Deconstructing sociability, an autism-relevant phenotype, in mouse models. Anat Rec. 2011;294:1713–1725. doi: 10.1002/ar.21318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang M, Abrams DN, Zhang JY, Weber MD, Katz AM, Clarke AM, Silverman JL, Crawley JN. Low sociability in BTBR T+tf/J mice is independent of partner strain. Physiol Behav. 2012 doi: 10.1016/j.physbeh.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2009;374:1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bent S, Hendren RL. Improving the prediction of response to therapy in autism. Neurotherapeutics. 2010;7:232–240. doi: 10.1016/j.nurt.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Veenstra-Vanderweele J, Blakely RD. Networking in autism: Leveraging genetic, biomarker and model system findings in the search for new treatments. Neuropsychopharmacology. 2012;37:196–212. doi: 10.1038/npp.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. 2008;20:775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- 96.Gasparini F, Bilbe G, Gomez-Mancilla B, Spooren W. mGluR5 antagonists: Discovery, characterization and drug development. Curr Opin Drug Discov Devel. 2008;11:655–665. [PubMed] [Google Scholar]
- 97.Hughes Z, Neal SJ, Smith DL, Sukoff Rizzo SJ, Pulicicchio CM, Lotarski S, Lu S, Dwyer JM, Brennan J, Olsen M, Bender CN, Kouranova E, Andree TH, Harrison JE, Whiteside GT, Springer D, O’Neil SV, Leonard SK, Schechter LE, Dunlop J, Rosenzweig-Lipson S, Ring RH. Negative allosteric modulation of metabotropic glutamate receptor 5 results in broad spectrum activity relevant to treatment resistant depression. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 98.Bailey KR, Pavlova MN, Rohde AD, Hohmann JG, Crawley JN. Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plus-maze. Pharmacol Biochem Behav. 2007;86:8–20. doi: 10.1016/j.pbb.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anderson JJ, Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Roppe J, King C, Cosford ND, Varney MA. In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3-methoxy-5-(pyridin-2-ylethynyl)pyridine) Eur J Pharmacol. 2003;473:35–40. doi: 10.1016/s0014-2999(03)01935-6. [DOI] [PubMed] [Google Scholar]
- 100.Terranova ML, Laviola G. Scoring of social interactions and play in mice during adolescence. Curr Protocols Toxicol. 2005;26:13.10.1–13.10.11. doi: 10.1002/0471140856.tx1310s26. [DOI] [PubMed] [Google Scholar]
- 101.Terranova ML, Laviola G. Scoring of Social Interactions and Play in Mice During Adolescence. John Wiley & Sons Inc; Hoboken, NJ: 2005. [DOI] [PubMed] [Google Scholar]
- 102.Hamilton SM, Spencer CM, Harrison WR, Yuva-Paylor LA, Graham DF, Daza RA, Hevner RF, Overbeek PA, Paylor R. Multiple autism-like behaviors in a novel transgenic mouse model. Behav Brain Res. 2011;218:29–41. doi: 10.1016/j.bbr.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bangash MA, Park JM, Melnikova T, Wang D, Jeon SK, Lee D, Syeda S, Kim J, Kouser M, Schwartz J, Cui Y, Zhao X, Speed HE, Kee SE, Tu JC, Hu JH, Petralia RS, Linden DJ, Powell CM, Savonenko A, Xiao B, Worley PF. Enhanced polyubiquitination of Shank3 and NMDA receptor in a mouse model of autism. Cell. 2011;145:758–772. doi: 10.1016/j.cell.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. mGluR5 antagonists reduced repetitive self-grooming in B6 and BTBR.
Fig. S2. GRN-529 partially ameliorated social approach deficits in BTBR.
Fig. S3. GRN-529 partially ameliorated reciprocal interaction social deficits in BTBR without sedation or hyperactivation in BTBR.
Fig. S4. GRN-529 did not induce sedation at doses that reversed repetitive and social deficits in cohort 2 B6 and BTBR mice at NIMH.
Fig. S5. GRN-529 did not induce sedation in cohort 3 B6 and BTBR mice at doses that reversed repetitive behaviors in cohort 3 at Pfizer.
Fig. S6. GRN-529 did not induce sedation in C58 mice at Pfizer.


