Abstract
Emerging evidence indicates impairments in somatosensory function may be a major contributor to motor dysfunction associated with neurologic injury or disorders. However, the neuroanatomical substrates underlying the connection between aberrant sensory input and ineffective motor output are still under investigation. The primary somatosensory cortex (S1) plays a critical role in processing afferent somatosensory input and contributes to the integration of sensory and motor signals necessary for skilled movement. Neuroimaging and neurostimulation approaches provide unique opportunities to non-invasively study S1 structure and function including connectivity with other cortical regions. These research techniques have begun to illuminate casual contributions of abnormal S1 activity and connectivity to motor dysfunction and poorer recovery of motor function in neurologic patient populations. This review synthesizes recent evidence illustrating the role of S1 in motor control, motor learning and functional recovery with an emphasis on how information from these investigations may be exploited to inform stroke rehabilitation to reduce motor dysfunction and improve therapeutic outcomes.
Keywords: Primary somatosensory cortex, Rehabilitation, Motor control, Motor learning, Neuroimaging, Noninvasive brain stimulation, Stroke
1. Introduction
The planning, execution, and control of motor behaviors is a complex neural process in part dependent on correct sampling of multiple sensory modalities from the body periphery (e.g., somatosensation, vestibular, etc.) and external environment (e.g., vision, hearing, etc.) (Hummelsheim et al., 1988; Riemann and Lephart, 2002; Wolpert et al., 2013; Zarzecki et al., 1978). Without correct processing and translation of sensory input, both before and during movement, motor outputs are abnormal and/or inaccurate. Thus, there is a tight link between sensory processing and movement production. Accordingly, emerging evidence suggests abnormal processing of somatosensory information by the primary somatosensory cortex (S1) contributes to deficits seen in neurological disorders typically classified by motor dysfunction (e.g. stroke, Parkinson's disease, dystonia, ataxia, etc.) (Elbert et al., 1998; Hummelsheim et al., 1988; Jacobs et al., 2012; Konczak and Abbruzzese, 2013; Rub et al., 2003; Wolpert et al., 2013).
There is a growing body of literature regarding the effects of altered S1 function on M1 activity and the control of movement. Increased M1 excitability has been noted in animal models of neurological conditions involving S1 damage, such as stroke (Harrison et al., 2013; Winship and Murphy, 2009) and idiopathic dystonia (Domenech et al., 2013). Focal lesions to sensorimotor areas, similar to injuries resulting from stroke, have resulted in difficulty with a battery of motor behavioral tasks assessing gross motor function and reflexes in rats (Gerlai et al., 2000; Kleim et al., 2007; McIntosh et al., 1996), and impaired fine motor skills involving small objects in monkeys (Brinkman et al., 1985; Hikosaka et al., 1985).
Motor deficits observed after S1 lesions may not always be due to difficulty with executing motor commands but rather attributed to disrupted learning of new motor tasks, as motor deficits are attenuated if the task had been learned prior to S1 injury (Pavlides et al., 1993; Sakamoto et al., 1989, 1987). Another phenomenon that could affect motor function is the alteration of somatosensory maps within S1. Studies in rodents have found a shift in the sensory map after experimentally-induced stroke that results in an overlap with a portion of the motor representation where the neurons originally devoted to encode exclusively motor commands take on a small role in sensory processing, reducing their capacity for involvement in the motor system (Harrison et al., 2013; Winship and Murphy, 2009).
In the following sections, the importance of S1 to motor function will be considered using theoretical models, neuroimaging approaches, non-invasive neural stimulation technologies, and combined neuroimaging–neurostimulation paradigms. Finally, future clinical implications of a comprehensive understanding of the relationship between motor functioning and S1 structure, function, and connectivity will be discussed.
2. Modeling the role of S1 in sensorimotor integration
The balance between sensory input and motor output is essential for efficiently acting within the environment. For example, when grasping a previously visualized object, first the visual information about the object's location is identified based on input from the retina (e.g. Becke et al., 2015). Then the information integrated with the (currently available) visual and/or somatosensory information about the location and configuration of the body. During movement, somatosensory input from the primary effector(s) also is transmitted to the motor system in order to fine-tune the movement (e.g. Blakemore et al., 1998; Wolpert et al., 1995). For successful motor execution of most tasks, real-time somatosensory feedback must be encoded and provided to the motor system through integrative loops for a precise motor control (see also Perruchoud et al., 2014).
Nevertheless, the basic mechanisms, anatomo-functional neural underpinnings, and rehabilitation of sensorimotor function are still under investigation. In particular, current models of S1 function lack precision in defining the multifaceted role in processing afferent sensory information and regulating efferent motor commands of this cortical region. This section will review the available data on the anatomo-functional role of S1 in motor control, aiming at describing the reciprocal influence between somatosensory information and motor commands.
Two main features of S1 function deserve particular attention. First, S1 can drive movements in coordination with or independent of M1 activity. Converging evidence from animal research shows that rich fiber pathways interconnect S1 and M1 (Donoghue and Parham, 1983; Veinante and Deschenes, 2003; White and DeAmicis, 1977). These cortico-cortical connections are considered to modulate the relationship between sensory and motor components of sensorimotor processes (Petreanu et al., 2009; Xu et al., 2012). Recent theorizations about the directionality of such an exchange between S1 and M1 emphasize the dominant (probably disinhibitory) role of M1 over S1, both in rodents (Lee et al., 2013) and humans (Gandolla et al., 2014). In accordance with this view, animal research showed that lesions of S1 are associated with increased excitability of M1 (Domenech et al., 2013; Harrison et al., 2013). Furthermore, clinical observations in humans report increased peripheral somatosensory inflow facilitates functional reorganization of M1 (Hamdy et al., 1998) and that non-invasive stimulation of S1 induces shorter latencies to initiate movements (Meehan et al., 2011). These findings support a continuous mutual communication between sensory inflow and motor outflow (Kleinfeld et al., 2006; Lee et al., 2008). Other evidence conversely shows that S1 can drive motor commands without the intervention of M1. In particular, the behavioral outcome in response to a specific somatosensory stimulus, further associated with the earliest recorded cortical activity (in S1), can be triggered also by the stimulation of the same S1 subregion with latencies shorter than those of the motor region evoking the same movement, even when the motor region is pharmacologically inactivated (Matyas et al., 2010). In the same vein, motor deficits are less prominent if a particular movement is learned prior to a lesion of S1 (Sakamoto et al., 1989) and movement execution improves following the administration of S1-facilitating drugs (McIntosh et al., 1996).
The second important feature of S1 is that it is interconnected with other primary sensory cortices (e.g. visual and auditory; V1 and A1, respectively) and with subcortical structures encoding different sensory modalities. Unlike conventional views of the primary sensory cortices as unisensory regions, different perspectives propose that multisensory integration processes begin to take place in these regions prior to moving on to secondary association areas (Driver and Noesselt, 2008). The neural underpinnings of such crossmodal integration may be provided by the cortico-cortical connections between S1 and V1, described both in primates (Cappe and Barone, 2005) and humans (Ro et al., 2013), as well as by the modulation of human S1 activity in response to non-corresponding stimulation (Liang et al., 2013), e.g. acoustic (Murray et al., 2005) and visual information (Meyer et al., 2011). In addition, subcortico-cortical connections transmit information about different sensory modalities to non-matching primary sensory areas (Henschke et al., 2014).
In light of these findings, how can S1 contributions to movement control be modeled? In accordance with the multisensory nature of S1, initially multimodal sensory input must be combined with actual intentions and previous knowledge in order to initiate movements (Genewein and Braun, 2012). Current theoretical conceptualizations propose the existence of two internal movement prediction components. The first component can be defined as a “forward” model used by the nervous system to predict the behavioral outcome of a given motor command generated by M1 (Desmurget et al., 2009). The forward model is based on a copy of the motor command generated in M1, defined as an “efference copy” that, instead of being sent to the periphery, is to be processed by parietal regions (Sirigu et al., 1996). Simultaneously, the forward model contributes information to a so-called “feedforward model” used to anticipate the sensory consequence of the movement itself (Wolpert and Ghahramani, 2000). The feedforward model combines together the actual sensory consequences associated with an executed motor command and the sensory component of the predicted motor outcome (based on the forward model) to provide information on the potential mismatch between expected and real bodily states during the movement. In this way both the actual sensory information and the motor outcome are compared to the expected sensory consequences and the real movement, respectively. As a result of these recalibration mechanisms, the potential mismatch between the actual and predicted sensorimotor states can be used to update subsequent motor commands and may be used as an error signal facilitate motor learning.
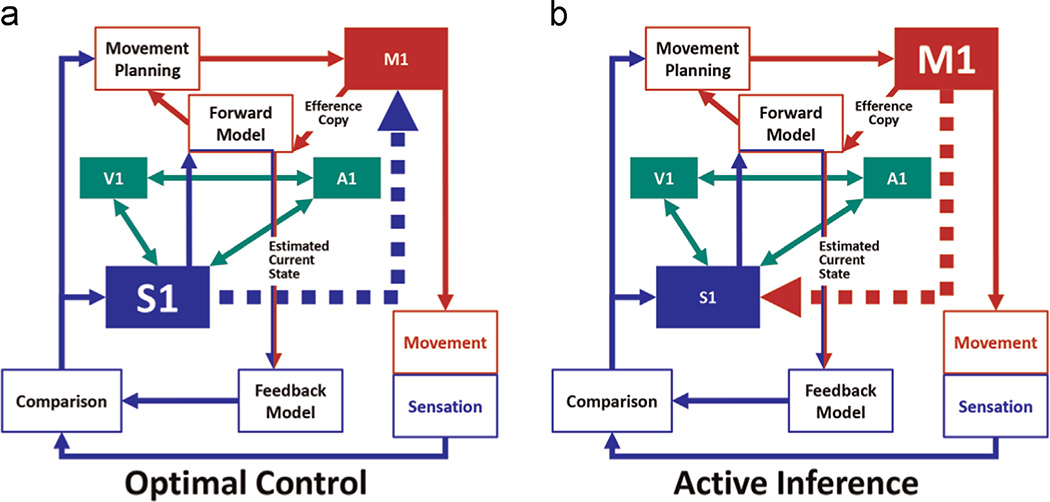
Two different options may explain the reciprocal role the sensory and motor components of such a complex interaction (Fig. 1). The "optimal control" theory postulates that the motor command contains purely motor information (Wolpert et al., 1995) and M1 only generates the movement (Wolpert and Kawato, 1998). In this view, the motor command contains purely motor information and the motor command is context-independent (Fig. 1a). The alternative "active inference" theory proposes that, instead of being uniquely motor, the motor command also contains information used to predict the sensory consequences of the triggered movement (Fig. 1b; Adams et al., 2013). According to this view, motor commands are context-dependent and modulate activity in S1. In other words, M1 activity has a direct effect on S1 activity both in terms of a facilitation of the M1-S1 connections and stronger S1 self-inhibition (in order to diminish sensitivity to unrelated information), which has been recently demonstrated in the human brain (Gandolla et al., 2014).
Fig. 1.
Theoretical model of information exchange between primary somatosensory (S1) and motor (M1) regions. According to the "optimal control" theory (a) S1 modulates M1 activity. According to the "active inference" theory (b), M1 modulates S1 activity. In addition, S1 exchanges and integrates information to and from other primary sensory areas, such as visual (V1) and auditory (A1).
How can these two perspectives be combined? It can indeed be hypothesized that the recruitment of one model or the other model depends on movement complexity. During simple movements, less reliance on sensory information is required and the system can rely on the optimal control model. On the other hand, increasing movement complexity would necessitate additional sensory information in order to successfully adapt the movement to the increased requirements of the task and environment, resulting in a greater potential of recruiting the active inference model.
Altogether, this body of evidence suggests that S1 is far from being an exclusively somatosensory processing area, but rather it is involved in merging and exchanging multimodal information through cortico–subcortical connections in order to fine tune sensations and movements in close cooperation with the motor cortex. Furthermore, the reviewed data highlight information flow between S1 and M1 changes in terms of directionality and quantity, suggesting that, rather than begin fixed, the relative weight of S1 and M1 contributions to movement execution normally vary according to context-dependent requirements. Advances in modeling the contributions of S1 to movement have provided a better understanding of the complex relationships underlying normal movement production. This improved understanding can now used to inform the study of the structural and functional substrates underlying abnormal movement in various neurologic conditions.
3. Imaging structural and functional differences in S1 after stroke
Recent development of advanced neuroimaging techniques has provided profound insights into the behavioral significance of structural and functional characteristics of the healthy and damaged brain. Bidirectional changes in brain structure and function underlie alterations in motor behavior. The clinical significance of examining the links between S1 structure and sensorimotor function is supported by evidence showing that approximately one-half of stroke patients in rehabilitation suffer from sensory discrimination impairments in the paretic hand (Carey and Matyas, 2011), and that integration of tactile afferent signals with motor commands is crucial for the performance of purposeful movements (Classen et al., 2000).
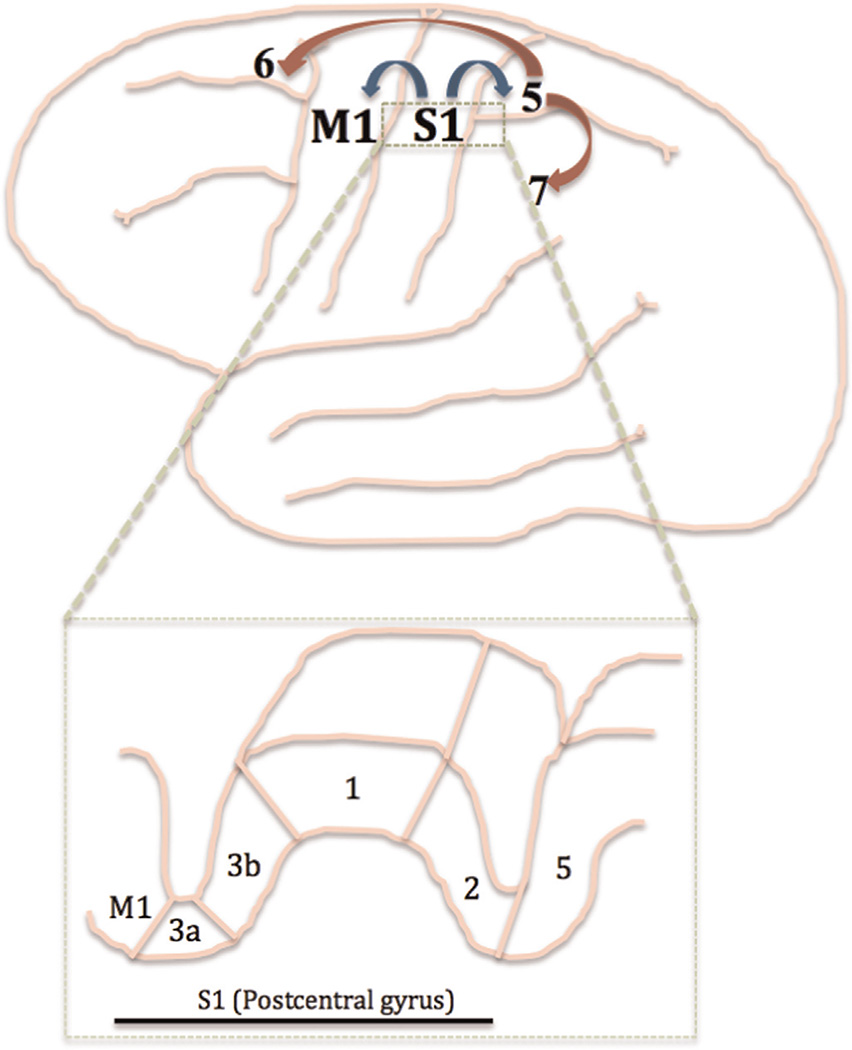
Cytoarchitectically, S1 is housed within the postcentral gyrus, composed of 4 subareas: Broca's Areas 3a, 3b, 1, and 2 (Jacobs et al., 2012; Jones et al., 1978; Rizzolatti and Kalaska, 2013; Vogt and Pandya, 1978) [Fig. 2]. Afferent signals from cutaneous stimulation are transmitted first to area 3b (sometime referred to as ‘S1 proper’ (Kaas, 1983)), and then to the other areas of S1, as well as to M1, supplementary motor and premotor cortices, and somatosensory association areas (Brodmann's areas 5 and 7) (Canedo, 1997; Ghosh et al., 1987; Jones et al., 1978; Pons and Kaas, 1986; Vogt and Pandya, 1978). Studies have highlighted the potential importance of area 3a on influencing motor activity, as it receives inputs from group I muscle afferents and contributes axons to descending motor pathways (Canedo, 1997; Ghosh et al., 1987; Zarzecki et al., 1978). The somatosensory association areas, located in posterior parietal cortices, also influence motor activity. These association areas receive input from neurons in S1, as well as from the visual and auditory systems, and project to the supplementary motor and premotor cortices. It has been theorized that the function of these association cortices is to integrate somatosensory information with other sensory modalities in order to create a multi-dimensional representation of the external environment and influence planned manipulation of objects (Andersen et al., 1997; Kandel, 2000; Pandya and Seltzer, 1982; Saper et al., 2000).
Fig. 2.
Projections between primary somatosensory (S1), motor (M1), and association cortices. Sensory information is projected directly from S1 to M1 and somatosensory association cortices (BA 5; blue arrows). Secondary projections occur from BA 5 to additional somatosensory cortices (BA 7) and premotor and supplementary motor cortices (BA 6; red arrows). Inset (dashed green box): cross-section of the cortex including M1, S1, and somatosensory association cortices. Cytoarchitecture of the subgroups of S1 (BA 3a, 3b, 1, and 2) is shown. Adapted from (Kandel et al., 2000; Saper et al., 2000). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
At a macrostructural level, a direct lesion to S1 or along the primary afferent sensory pathway is likely to result in some level of sensory dysfunction and, importantly, sensory impairments are usually paralleled by motor deficits (Taskin et al., 2006; Yamada et al., 2003). Often the resulting damage is not necessarily restricted to the local tissue damage at the primary lesion location. Microstructural brain injury can occur due to secondary degeneration. Using diffusion tensor imaging (DTI), alterations in white matter tissue properties have been found in non-lesioned brain areas (Borich et al., 2012; Lindberg et al., 2007). Structural properties of white matter, such as degree of myelination and axon diameter, influence the efficacy of signal transmission within the brain, thereby influencing functions associated with voluntary behavior (Seidl, 2014). As a result, post-stroke levels of impairment and motor recovery can be highly variable between individuals, and it is often difficult to parse out specific cause-and-effect relationships of brain structure and function with behavior.
Commonly, white matter tissue properties within the posterior limb of the internal capsule (PLIC) are altered after stroke (Werring et al., 2000). Reports of abnormal ipsi- or contralesional PLIC tissue properties have been associated with greater levels of physical impairment (Borich et al., 2012; Qiu et al., 2011; Stinear et al., 2007), reduced motor learning (Borich et al., 2013; Stinear et al., 2007), lower levels of global motor function (Stinear, et al., 2007), and poorer hand dexterity (Borich et al., 2012; Schaechter et al., 2009). These changes may be partially explained by reduced transmission of sensory input via the PLIC, in addition to impaired motor output. Borstad and colleagues (2012) examined sensory component of the superior thalamic radiation (sSTR), which is upstream of the PLIC and includes all of the afferent connections of S1 (Wakana et al., 2004) in participants with chronic stroke. A strong correlation between the ipsi- and contralesional asymmetry of sSTR integrity and sensory function was observed, such that individuals with a larger asymmetry performed poorer on a measure of sensory discrimination with their paretic hand (Borstad et al., 2012). These findings are in line with a study in children with congenital hemiplegia showing the status of sensorimotor thalamic projections were more significantly correlated with paretic hand function than corticospinal tract connections (Rose et al., 2011). Despite recent experimental evidence, there remains a paucity of data evaluating the behavioral significance of changes in somatosensory tract structure in response to neurologic conditions.
Another white matter pathway commonly studied in individuals with stroke is the corpus callosum (CC), the largest commissural tract in the brain that connects homologous cortical regions of each hemisphere. The ability to produce skilled and coordinated movements relies on the dynamic interactions between the two hemispheres. The CC has a critical role in maintaining an appropriate balance of inter-hemispheric activity, which can be disrupted after stroke (Gupta et al., 2006; Perez and Cohen, 2008) and has been linked to motor dysfunction (Jang 2010; Lindenberg et al., 2012). The CC can be divided into functionally and anatomically distinct segments according to the cortico-cortical tracts that pass through it connecting homologous regions between each hemisphere ([Fling et al., 2011, Hofer and Frahm, 2006]). Overall, previous studies have focused almost exclusively on the transcallosal segment that connects the two primary motor cortices (M1–M1), whereas studies of the sensory segment (S1–S1) are sparse. Borich and colleagues (2012) reported the microstructural integrity of CC sensory fibers, but not CC motor fibers, was reduced in individuals with chronic stroke compared to healthy age and gender-matched controls. However, no significant correlation with motor function was observed (Borich et al., 2012). Based on these initial observations, further studies are necessary to better understand the functional significance of abnormal tissue properties of interhemispheric pathways after stroke and to verify the importance of S1 to S1 connections for motor function in this population.
An accumulating body of evidence suggests that, similar to the motor system, in healthy individuals the activation of S1 in one hemisphere modulates the activity of the contralateral S1. For example, functional magnetic resonance imaging (fMRI) studies conducted in monkeys (Lipton et al., 2006) and in humans (Blankenburg et al., 2008; Hlushchuk and Hari, 2006; Kastrup et al., 2008; Eickhoff et al., 2008; Klingner et al., 2011) describe a corresponding increase in activation in the contralateral S1, and transient decrease in activation in the ipsilateral S1 during peripheral hand stimulation. This decrease in ipsilateral S1 activation correlates with reduced sensory perception in the opposite hand (Kastrup et al., 2008). Similar patterns have emerged in electrophysiological studies in humans (Ragert et al., 2011; Brodie et al., 2014). However, considerations of how sensory networks change after stroke are highly dependent on the time point studied as brain function is altered not only with damage but also by recovery from damage. One common finding after unilateral stroke is a shift in activation from ipsilesional to contralesional sensorimotor areas (Murase et al., 2004; Nowak et al., 2009); resolution of this hemispheric imbalance is associated with sensorimotor recovery (Cramer, 2008; Rossini et al., 2007). This interhemispheric imbalance has been described specifically between the S1’s in individuals with chronic stroke; the larger the imbalance, the poorer motor task performance (Calautti et al., 2006). Resolution of the S1–S1 hemispheric imbalance has been reported in the acute phase post-stroke with recovery of sensory loss (L.M. Carey et al., 2002) in individuals with chronic stroke before and after skilled sensorimotor training (J.R. Carey et al., 2002; Schaechter et al., 2006) and following intensive treatment with neuromuscular electrical stimulation of the paretic forearm (Kimberley et al., 2004). These findings are in parallel to studies of laterality shifts in M1 with acute recovery (Zemke et al., 2003) and motor learning (Boyd et al., 2010; Calautti and Baron, 2003). An additional point to consider when addressing interhemispheric imbalances in S1 is the possible relationship between asymmetries in S1 anatomy and function with handedness, similar to lateralization. Although hemispheric asymmetries in S1 anatomy (Soros et al., 1999) and function (Jung et al., 2003, 2008) have been observed, it is currently unclear if these asymmetries are solely attributable to hand dominance.
Another common finding in fMRI experiments is a shift in primary sensorimotor activation towards the postcentral gyrus following stroke (Calautti et al., 2003; Cramer and Bastings, 2000; Laible et al., 2012; Pineiro et al., 2001; Schaechter et al., 2006). The behavioral significance of this posterior shift is elusive. Pineiro and colleagues proposed that it may potentially reflect an increased proprioceptive attentional process to offset motor impairment, or a recruitment of latent corticospinal fibers originating in S1 (Galea and Darian-Smith, 1994) to compensate for the limited output from M1 (Pineiro et al., 2001). Schaechter and colleagues (2006) reported an increase in ipsilesional S1 activation was correlated with increased cortical thickness (structural plasticity) in the same area, but these increases were not correlated with motor outcome in the sample studied (Schaechter et al., 2006). In a homogeneous group of patients with hand weakness but normal sensation, and no lesion within the S1, thalamus, or brainstem, a close relationship between improvements in hand function after constraint-induced movement therapy and increased peak changes in fMRI activation within the ipsilesional S1 was reported (Laible et al., 2012). Conversely, individuals with direct damage to the ventroposterior nucleus of the thalamus show reduced activation in the ipsilateral S1 (Taskin et al., 2006), and a negative correlation has been reported between touch discrimination and activation in ipsilesional S1, particularly after sub-cortical stroke (L.M. Carey et al., 2011). Thus, sensory network activity influences both sensory and motor function, and this activity appears to be closely related to therapy-induced gains in motor function seen after stroke.
4. Non-invasive brain stimulation (NIBS) targeting S1 to improve sensorimotor function after stroke
Normalization of hemispheric excitability after stroke has been associated with sensorimotor functional recovery (Cramer, 2008; Rossini et al., 2007) leading to experimental interventions to up- or down-regulate cortical activity in a targeted fashion in an effort to enhance functional recovery (Calautti and Baron, 2003). One approach to enhance motor function by modulating S1 excitability relies on stimulating the peripheral somatosensory system. Indeed, several studies have shown that pairing repetitive peripheral nerve stimulation of the paretic upper extremity with training enhances motor performance after stroke (Celnik et al., 2007; Conforto et al., 2010; Klaiput and Kitisomprayoonkul, 2009; Knutson et al., 2012; Wu et al., 2006). Furthermore, peripheral somatosensory stimulation can induce cortical reorganization of M1 (Hamdy et al., 1998). Together, these findings have prompted investigation into the use of NIBS techniques that can directly modulate S1 excitability and modify connections between S1 and other cortical areas, such as M1.
Transcranial magnetic stimulation (TMS) is a safe, painless, and non-invasive technique used to the alter electrical activity of the underlying brain tissue by electromagnetic induction using a stimulating coil at the surface of the skull (Hallett, 2000). When applied as a single pulse in healthy individuals, TMS over S1 transiently masks tactile sensation (Cohen et al., 1991; Hannula et al., 2005; Seyal et al., 1997) and disrupts sensorimotor performance (Meehan et al., 2008). Studies investigating paired pulse TMS over S1 demonstrate amplified masking of a tactile sensation with a sub-threshold conditioning stimulus (Koch et al., 2006), and decreased sensorimotor performance with a suprathreshold conditioning stimulus (Meehan et al., 2008). Essentially, these foundational studies confirmed linkages between S1 activity and somatosensory processing (Song et al., 2011) and reinforced the theoretical potential of S1 as a target to modify more complex sensorimotor behaviors. However, the behavioral consequences of S1 stimulation are more applicable when considering the longer-lasting modulatory effects of neuromodulatory forms of TMS.
Repetitive (r)TMS can be used to modulate local cortical excitability in a frequency and intensity-dependent manner (Maeda et al., 2000; Ridding and Ziemann, 2010; Siebner and Rothwell, 2003), for a period of time that outlasts the duration of stimulation (Chen et al., 2003). After stroke, high frequency (> 5 Hz) or low frequency (≤ 1 Hz) rTMS may be used to increase ipsilesional or decrease contralesional excitability respectively. Given recent evidence of functional S1–S1 connections mediated by the CC in the human brain (Brodie et al., 2014), theoretically either of these rTMS approaches could be used to reestablish the balance of interhemispheric excitability after stroke (Fregni and Pascual-Leone, 2007; Nowak et al., 2009). The majority of previous rTMS studies have focused on modulation of M1 excitability. However, S1 also possesses a high capacity for plastic change (Schaechter et al., 2006), and emerging studies suggest that rTMS targeting can modulate S1 excitability, sensory function and motor control.
High frequency (≥ 5 Hz) rTMS applied over M1 increases cortical excitability, as measured by motor evoked potentials (MEPs) (Peinemann et al., 2004). Similarly when applied over S1, 5 Hz rTMS induces sustained increases in cortical excitability, indicated by larger SEPs in healthy individuals (Ragert et al., 2004). Similar effects have also been observed with intermittent theta burst stimulation (iTBS) (Huang et al., 2005), an excitatory form of patterned rTMS that results in longer-lasting effects with shorter stimulation durations compared to simple rTMS paradigms (Staines and Bolton, 2013). When applied over S1 in healthy individuals, iTBS increases SEP amplitudes (Katayama and Rothwell, 2007; Premji et al., 2010), but has not be shown to modulate M1 excitability (Katayama and Rothwell, 2007). Changes in sensation, which can be indexed both in sensory function and behavior, have been observed after excitatory rTMS including gains in spatial acuity (Ragert et al., 2003; Tegenthoff et al., 2005) and frequency discrimination (Pleger et al., 2006) of the hand. Following 5 Hz rTMS over the finger representation in S1, Tegenthoff and colleagues (2005) observed and expansion in the finger representation in healthy individuals that was correlated with improvements in tactile perception. Using fMRI, reorganization of activity sensorimotor network activity patterns within S1 and M1 were demonstrated following 5 Hz rTMS over S1 that lasted for up to 120 min following stimulation (Pleger et al., 2006) suggesting both local and remote changes can result from neuromodulation of S1.
The potential for rTMS of S1 to not only improve somatosensation but also enhance connectivity with other nodes within the sensorimotor network (e.g. M1) has important implications for motor learning. To induce persistent change in sensorimotor function, learning is required. Thus, motor learning is considered the basis of neurorehabilitation (Krakauer, 2006). Ragert and colleagues (2003) showed enhanced perceptual learning following repeated applications of 5 Hz rTMS over S1 in healthy individuals; however tactile discrimination was tested over several sessions on the same day of stimulation. When participants were re-tested 2 weeks later, their discrimination thresholds were at baseline levels (Ragert et al., 2003). Similarly, Karim and colleagues (2006) reported learning of a spatial discrimination task, but not of a frequency discrimination task, was facilitated following the application of 15 Hz rTMS over S1; yet again, all sensory testing was conducted on the same day of stimulation (Karim et al., 2006). Without significant improvements observed at a no-rTMS retention test, it is not currently possible to conclude that long-term memory consolidation and improved sensory function result from rTMS over S1. This highlights the need for study designs to incorporate delayed retention tests to define the persistent impact of NIBS over S1 (Boyd and Linsdell, 2009; Dayan and Cohen, 2011; Robertson et al., 2004).
Recently, Brodie and colleagues (2014) applied 5 Hz rTMS over ipsilesional S1 in individuals with chronic stroke followed immediately by motor skill practice of a serial visuomotor targeting task (Brodie et al., 2014). The intervention was repeated daily for 5 days. Individuals who received rTMS over S1 showed a generalized improvement of skill performance across training that persisted at a no-rTMS retention test at 24 h following the last practice session. Motor learning was associated with significant improvements in spatial acuity but not in upper extremity motor function or manual dexterity. Yet, to date, these findings have not been extended to determine whether pairing 1 Hz rTMS over S1 with neurorehabilitation might enhance clinically meaningful outcomes.
When applied at low frequencies (≤ 1 Hz), rTMS applied over M1 decreases motor cortex excitability (Chen et al., 1997). However, a number of reports of low frequency rTMS over S1 have not found a significant depression of SEP amplitudes in healthy individuals (Enomoto et al., 2001; Ogawa et al., 2004; Restuccia et al., 2007; Satow et al., 2003). Instead, alterations in high-frequency oscillations, which represent changes in localized activity of intracortical inhibitory interneurons, have been observed (Katayama et al., 2010; Ogawa et al., 2004; Restuccia et al., 2007). However Ishikawa and colleagues (2007) reported inhibitory (c) TBS over S1 suppressed SEP amplitudes from the stimulated S1 for at least 13 minutes after the stimulation period. This suppression occurred in the absence of changes in M1 excitability bilaterally (Ishikawa et al., 2007). In contrast, Zapallow and colleagues (2013) showed that cTBS over S1 increases intracortical inhibition between M1s for 45–60 min following stimulation in young healthy adults, providing one potential mechanism by which S1 may influence M1 activity and basal motor control (Zapallow et al., 2013).
The ability to transiently depress cortical activity within S1 of healthy individuals provides insights into the potential contributions of sensory dysfunction to sensorimotor impairment after stroke. For example, Vidoni and colleagues (2010) used 1 Hz rTMS over S1 as a ‘virtual lesion’ in healthy adults prior motor skill practice over two days. During training and at a no-rTMS retention test, improvements in tracking performance were diminished in the stimulation group compared to a sham stimulation control group (Vidoni et al., 2010). Thus disrupting S1 activity prior to skill practice reduced motor skill learning, further supporting a critical role of somatosensory information processing to motor function.
In individuals with unilateral stroke, it is possible that down-regulation of specific areas within the contralesional hemisphere may alter interhemispheric competition, thereby reducing inhibition of the ipsilesional hemisphere mediated by the contralesional side (Fregni and Pascual-Leone, 2007; Nowak et al., 2009). Meehan and colleagues (2011) showed that cTBS over contralesional M1 or over S1 paired with skill practice enhanced skill learning compared to practice alone. However, cTBS over contralesional M1 resulted in greater changes in velocity and acceleration, whereas cTBS over contralesional S1 resulted in faster time to initiate movement and in lower cumulative magnitude of each movement (Meehan et al., 2011). Contralesional S1 stimulation also induced substantial improvements in upper extremity motor function (Meehan et al., 2011). Taken together, neuromodulatory TMS targeting S1 can modulate both sensory and motor performance and, when applied over multiple sessions, can improve motor learning in both healthy individuals and patients with stroke. This NIBS approach is an intriguing option to further investigate potential clinical applications aimed at enhancing sensorimotor function.
Transcranial direct stimulation (tDCS) is another method that enables the non-invasive manipulation of cortical excitability. During tDCS a low intensity current is run between two large surface scalp electrodes; the effects depend on current polarity. In the motor system, anodal tDCS over the motor cortex increases cortical excitability as measured by MEPs, cathodal tDCS has the opposite effect (Nitsche and Paulus, 2000). The spatial resolution of tDCS is significantly poorer than that of TMS, and as a result it is difficult to precisely target specific cortical areas such as M1 and/or S1. Nevertheless, studies have examined the effects of tDCS protocols on S1 excitability. The data characterizing the effect of anodal tDCS over the motor cortex is mixed; one study reported significant increases in SEP amplitude (Matsunaga, 2004) while another failed to observe any effect (Dieckhofer et al., 2006). Similar mixed results have been reported for the effects of anodal tDCS over S1 on somatosensation (Ragert et al., 2008; Rogalewski et al., 2004), Cathodal tDCS over S1 reduced SEP amplitudes (Dieckhofer et al., 2006), and impaired tactile frequency discrimination (Rogalewski et al., 2004). Cathodal tDCS over the motor cortex area has not been shown to affect SEPs (Matsunaga, 2004). Overall, current evidence is inconsistent regarding the efficacy of tDCS protocols to modify S1 excitability due to a paucity of studies and heterogeneous results. Limitations of tDCS (e.g. difficulty in target localization, inability to identify stimulation intensities across individuals, and differences in simulation parameters across studies) may explain these inconsistent findings. Therefore, it is possible that improvements in standardization of tDCS protocols will result in a better understanding of the potential of tDCS approaches to modulate S1 activity to support motor function and recovery.
Although, NIBS over S1 is a promising approach to modulate sensorimotor activity and motor function, targeting S1 is associated with a number of challenges. It is more difficult to target this cortical region due to the lack of observable evoked peripheral responses during stimulation in comparison to targeting M1. While some researchers identify the hand representation in S1 by shifting the TMS coil ~2 cm posteriorly from the M1 hotspot, or using the international 10–20 system to visually approximate the location of S1, improved localization approaches are now available. Stereotaxic neuronavigation utilizes structural MRI data to identify and target non-motor cortical regions based on known anatomical location. FMRI-based activation maps can also be used to identify a stimulation target based on functional activity rather than anatomy.
Defining appropriate stimulation intensities for S1 is another challenge. All rTMS protocols discussed calculated S1 stimulation intensities using a percentage of the resting or active motor thresholds – measures of M1 excitability which may or may not be related to S1 excitability. Future work is needed to identify optimal stimulation protocols specifically for S1. At this point, due to lack of consistency between methods, results have been variable. Nevertheless, evidence of the behavioral consequences of S1 stimulation continues to accumulate, supporting the notion that S1 is integral to sensorimotor control and learning and may be a viable target for clinical applications of NIBS. It is important to note that, despite encouraging mechanistic investigations, a large-scale randomized clinical trial evaluating the efficacy of NIBS targeting of S1 to improve motor function after stroke has yet to be conducted.
5. Combining TMS with neuroimaging to study effective connectivity after stroke
The correlative nature of neuroimaging techniques limits empirical characterization of causal interactions between behavior with brain structure and function. By using TMS to stimulate a cortical region of interest during a behavior of interest, it is possible to study causal influences of the stimulated region on task performance. However, the brain is comprised of intricate and complex neuronal networks that are dynamically modifiable (Sporns et al., 2004) thus complicating the interpretation of TMS-based results. It is not clear if the observed change in behavior is solely due to stimulation of the targeted cortical region or if it is a result of interactions within functional neural networks that may also be influenced by structural network organization. Neuroimaging can be performed before, during or after TMS to non-invasively map the spatiotemporal dynamics of TMS-induced cortical activation (Siebner et al., 2009). For example, it is now common to use frameless stereotactic neuronavigation using previously acquired structural MRI data to spatially localize the individualized stimulation site for each participant to enable reproducible targeting within and between TMS sessions (Bashir et al., 2011; Julkunen et al., 2009). Combined TMS-neuroimaging can also be used to refine neuromodulation approaches by individualizing stimulation parameters based on characteristics of brain network structure and function. For example, cortical activation patterns associated with somatosensory discrimination have been mapped after stroke using fMRI (Carey et al., 2011). These task-based activation maps could used to personalize (r)TMS delivery based on each participant’s unique cortical activity patterns.
Mapping reorganization of white and gray matter tissue and structural networks in stroke can also be performed prior to TMS. A recent report described smaller volumes of white matter underlying ipsilesional S1 predicted less motor task improvement following an intervention pairing high-frequency rTMS over the ipsilesional S1 followed by motor training of the paretic arm in individuals with chronic stroke (Brodie et al., 2014). However, there is currently a paucity of data combining neuroimaging with TMS to characterize S1 excitability as well as the structural and functional connections between S1 and M1. With the introduction of navigated TMS using structural MRI data, it is now possible to reproducibly target any cortical region of interest. However, it is not possible to use TMS alone to evoke a measurable response in S1, which limits the current understanding of how S1 excitability may be modulated by NIBS or task practice to support motor function in health or disease.
In contrast to performing imaging before or after NIBS, functional neuroimaging can be performed during TMS to evaluate immediate spatiotemporal cortical network dynamics of TMS-induced responses (Ilmoniemi et al., 1997). This approach remains methodologically challenging due to technical aspects associated with acquiring functional imaging data in the harsh TMS environment (Ilmoniemi et al., 2010; Sato et al., 2015). Concurrent TMS- neuroimaging can uniquely investigate causal information flow through functional neural networks mediated by excitatory and inhibitory connections (Bortoletto et al., 2015). Yet, to date, no studies have been published using concurrent TMS-neuroimaging in individuals with stroke, nor have studies used concurrent approaches to study local cortical excitability and regional connectivity in response to stimulation of S1 in general. This knowledge gap suggests there are substantial opportunities to improve our understanding of the neurobiological mechanisms of cortical reorganization both after stroke and in response to rehabilitation interventions as well as further elaborate the salient interactions between S1 and M1 that underlie human sensorimotor control.
6. Clinical implications and conclusions
Advances in neuroimaging and neurostimulation research are rapidly expanding our understanding of the role of the sensory system in the recovery from stroke. Moving forward the challenge will be to exploit our understanding of the role(s) of the sensory system in motor recovery to formulate novel therapeutic interventions. Critically, S1 is heavily connected with ipsilateral M1 as well as with the sensory association areas of the parietal cortex. In addition, it is now clear that the two sensory cortices are both neuroanatomically and functionally linked, such that they may mutually inhibit one another (Brodie et al., 2014; Ragert et al., 2011). These extensive connections enable S1 to influence not only voluntary movements, but perhaps more importantly, motor learning. Indeed, S1 has a central role in theoretical conceptualizations of motor learning such as the internal model (Ito, 2000). The internal model posits that output from M1 is directly affected by input from S1, and that with task practice this relationship enables sensory information to refine the emerging motor plan (Hwang and Shadmehr, 2005; Nowak et al., 2004; Thoroughman and Shadmehr, 1999). This theoretical model is supported by findings from an experiemnt where rTMS was used to disrupt S1 function (Vidoni et al., 2010). Altering sensory function of healthy individuals with 1Hz rTMS over S1 results in more errors and slower movements during physical practice; importantly these changes persist at a no-rTMS retention test. These data indicate that learning a new motor task is influenced by sensory input, regardless of the accuracy of this information.
It is clear that the nervous system is continually updating based on the afferent information (Wei and Kording, 2009). Impaired somatosensation during task practice leads to the development of an inaccurate internal model or motor plan and, in turn, degrades motor learning. These data have important implications for people with centrally impaired sensation, such as what commonly occurs after stroke, as they suggest that it is imperative to design novel therapies that focus on remediation of sensory processing deficits. It is also important to consider the cognitive aspects associated with sensorimotor control as movement planning, strategy and selection will exert and influence on the sensorimotor interactions discussed in detail in this review. Similar to sensory dysfunction observed in typical motor-based neurologic disorders, many of these conditions also present with cognitive dysfunction that will influence motor control and motor learning associated with the recovery of function.
Future work needs to focus on gaining a clearer understanding of the neuroanatomy of sensory connectivity in both the damaged and healthy brain. To date it remains unclear what proportion of the CST carries ascending sensory information. Similarly, it is only recently that interhemispheric sensory to sensory connectivity has begun to be explored (Brodie et al., 2014; Ragert et al., 2011). Little information currently exists that characterizes how brain damage, such as stroke, affects connectivity between brain regions. Further, it is not known how patterns of recovery after stroke may impact the flow of sensory information within the brain. Without this information it will be difficult to design effective therapeutics that seek to shape trajectories of recovery following brain damage.
The present review clearly supports the concept that somatosensation, particularly central sensory processing, is crucial for both motor learning in healthy adults and motor recovery after brain damage. We have demonstrated the intricate connections and functions of the sensory system, as they are understood to date. The data presented here also suggest that sensation is a necessary consideration in motor rehabilitation. These findings have implications for both learning theory and rehabilitation medicine, in particular regarding the importance of developing novel rehabilitation approaches to enhancing recovery of sensory loss after stroke. As discussed, future work should consider the impact of pairing interventions such as non-invasive brain stimulation over S1 or peripheral sensory stimulation with neurorehabilitation. In addition, it is clear that because of the complexity of the central sensory system that studies employing multimodal imaging and behavioral mapping approaches will yield the most useful data as we continue to discover more about the role(s) of somatosensation in recovery from brain damage.
Acknowledgments
LAB is a Canada Research Chair and receives support from the Michael Smith Foundation for Health Research (MSFHR) (Grant no. CI-SCH-01796).
References
- Adams RA, Shipp S, Friston KJ. Predictions not commands: active inference in the motor system. Brain Struct. Funct. 2013;218:611–643. doi: 10.1007/s00429-012-0475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Bradley DC, Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu. Rev. Neurosci. 1997;20:303–330. doi: 10.1146/annurev.neuro.20.1.303. [DOI] [PubMed] [Google Scholar]
- Bashir S, Edwards D, Pascual-Leone A. Neuronavigation increases the physiologic and behavioral effects of low-frequency rTMS of primary motor cortex in healthy subjects. Brain Topogr. 2011;24:54–64. doi: 10.1007/s10548-010-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becke A, Muller N, Vellage A, Schoenfeld MA, Hopf JM. Neural sources of visual working memory maintenance in human parietal and ventral extra-striate visual cortex. NeuroImage. 2015;110:78–86. doi: 10.1016/j.neuroimage.2015.01.059. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat. Neurosci. 1998;1:635–640. doi: 10.1038/2870. [DOI] [PubMed] [Google Scholar]
- Blankenburg F, Ruff CC, Bestmann S, Bjoertomt O, Eshel N, Josephs O, Weiskopf N, Driver J. Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS-fMRI. Journal of Neuroscience. 2008;28:13202–13208. doi: 10.1523/JNEUROSCI.3043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borich MR, Mang C, Boyd LA. Both projection and commissural pathways are disrupted in individuals with chronic stroke: investigating microstructural white matter correlates of motor recovery. BMC Neurosci. 2012;13:107. doi: 10.1186/1471-2202-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borich MR, Brown KE, Boyd LA. Motor skill learning is associated with diffusion characteristics of white matter in individuals with chronic stroke. J. Neurol. Phys. Ther. 2013 doi: 10.1097/NPT.0b013e3182a3d353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borstad A, Schmalbrock P, Choi S, Nichols-Larsen DS. Neural correlates supporting sensory discrimination after left hemisphere stroke. Brain Res. 2012;1460:78–87. doi: 10.1016/j.brainres.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoletto M, Veniero D, Thut G, Miniussi C. The contribution of TMS-EEG coregistration in the exploration of the human cortical connectome. Neurosci. Biobehav. Rev. 2015;49c:114–124. doi: 10.1016/j.neubiorev.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Linsdell MA. Excitatory repetitive transcranial magnetic stimulation to left dorsal premotor cortex enhances motor consolidation of new skills. BMC Neurosci. 2009;10:72. doi: 10.1186/1471-2202-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd LA, Vidoni ED, Wessel BD. Motor learning after stroke: is skill acquisition a prerequisite for contralesional neuroplastic change? Neurosci. Lett. 2010;482:21–25. doi: 10.1016/j.neulet.2010.06.082. [DOI] [PubMed] [Google Scholar]
- Brinkman J, Colebatch JG, Porter R, York DH. Responses of precentral cells during cooling of post-central cortex in conscious monkeys. J. Physiol. 1985;368:611–625. doi: 10.1113/jphysiol.1985.sp015879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie SM, Borich MR, Boyd LA. Impact of 5-Hz rTMS over the primary sensory cortex is related to white matter volume in individuals with chronic stroke. Eur. J. Neurosci. 2014;40:3405–3412. doi: 10.1111/ejn.12717. [DOI] [PubMed] [Google Scholar]
- Brodie SM, Villamayor A, Borich MR, Boyd LA. Exploring the specific time course of interhemispheric inhibition between the human primary sensory cortices. J. Neurophysiol. 2014;112:1470–1476. doi: 10.1152/jn.00074.2014. [DOI] [PubMed] [Google Scholar]
- Brodie SM, Meehan S, Borich MR, Boyd LA. 5 Hz repetitive transcranial magnetic stimulation over the ipsilesional sensory cortex enhances motor learning after stroke. Front. Hum. Neurosci. 2014;8:143. doi: 10.3389/fnhum.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults – a review. Stroke. 2003;34:1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- Calautti C, Leroy F, Guincestre JY, Baron JC. Displacement of primary sensorimotor cortex activation after subcortical stroke: a longitudinal PET study with clinical correlation. NeuroImage. 2003;19:1650–1654. doi: 10.1016/s1053-8119(03)00205-2. [DOI] [PubMed] [Google Scholar]
- Calautti C, Naccarato M, Jones PS, Sharma N, Day DD, Carpenter AT, Bullmore ET, Warburton EA, Baron JC. The relationship between motor deficit and hemisphere activation balance after stroke: a 3T fMRI study. NeuroImage. 2006;34:322–331. doi: 10.1016/j.neuroimage.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Canedo A. Primary motor cortex influences on the descending and ascending systems. Prog. Neurobiol. 1997;51:287–335. doi: 10.1016/s0301-0082(96)00058-5. [DOI] [PubMed] [Google Scholar]
- Cappe C, Barone P. Heteromodal connections supporting multisensory integration at low levels of cortical processing in the monkey. Eur. J. Neurosci. 2005;22:2886–2902. doi: 10.1111/j.1460-9568.2005.04462.x. [DOI] [PubMed] [Google Scholar]
- Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, Ugurbil K. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- Carey LM, Matyas TA. Frequency of discriminative sensory loss in the hand after stroke in a rehabilitation setting. J. Rehabil. Med. 2011;43:257–263. doi: 10.2340/16501977-0662. [DOI] [PubMed] [Google Scholar]
- Carey LM, Abbott DF, Puce A, Jackson GD, Syngeniotis A, Donnan GA. Reemergence of activation with poststroke somatosensory recovery: a serial fMRI case study. Neurology. 2002;59:749–752. doi: 10.1212/wnl.59.5.749. [DOI] [PubMed] [Google Scholar]
- Carey LM, Abbott DF, Harvey MR, Puce A, Seitz RJ, Donnan GA. Relationship between touch impairment and brain activation after lesions of subcortical and cortical somatosensory regions. Neurorehabil. Neural Repair. 2011;25:443–457. doi: 10.1177/1545968310395777. [DOI] [PubMed] [Google Scholar]
- Celnik P, Hummel F, Harris-Love M, Wolk R, Cohen LG. Somatosensory stimulation enhances the effects of training functional hand tasks in patients with chronic stroke. Arch. Phys. Med. Rehabil. 2007;88:1369–1376. doi: 10.1016/j.apmr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Chen W-H, Mima T, Siebner HR, Oga T, Hara H, Satow T, Begum T, Nagamine T, Shibasaki H. Low-frequency rTMS over lateral premotor cortex induces lasting changes in regional activation and functional coupling of cortical motor areas. Clin. Neurophysiol. 2003;114:1628–1637. doi: 10.1016/s1388-2457(03)00063-4. [DOI] [PubMed] [Google Scholar]
- Classen J, Steinfelder B, Liepert J, Stefan K, Celnik P, Cohen LG, Hess A, Kunesch E, Chen R, Benecke R, Hallett M. Cutaneomotor integration in humans is somatotopically organized at various levels of the nervous system and is task dependent. Exp. Brain Res. 2000;130:48–59. doi: 10.1007/s002210050005. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Bandinelli S, Sato S, Kufta C, Hallett M. Attenuation in detection of somatosensory stimuli by transcranial magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 1991;81:366–376. doi: 10.1016/0168-5597(91)90026-t. [DOI] [PubMed] [Google Scholar]
- Conforto AB, Ferreiro KN, Tomasi C, dos Santos RL, Moreira VL, Marie SK, Baltieri SC, Scaff M, Cohen LG. Effects of somatosensory stimulation on motor function after subacute stroke. Neurorehabil. Neural Repair. 2010;24:263–272. doi: 10.1177/1545968309349946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann. Neurol. 2008;63:272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Bastings EP. Mapping clinically relevant plasticity after stroke. Neuropharmacology. 2000;39:842–851. doi: 10.1016/s0028-3908(99)00258-0. [DOI] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Reilly KT, Richard N, Szathmari A, Mottolese C, Sirigu A. Movement intention after parietal cortex stimulation in humans. Science. 2009;324:811–813. doi: 10.1126/science.1169896. [DOI] [PubMed] [Google Scholar]
- Dieckhofer A, Waberski TD, Nitsche M, Paulus W, Buchner H, Gobbele R. Transcranial direct current stimulation applied over the somatosensory cortex - differential effect on low and high frequency SEPs. Clin. Neurophysiol. 2006;117:2221–2227. doi: 10.1016/j.clinph.2006.07.136. [DOI] [PubMed] [Google Scholar]
- Domenech J, Barrios C, Tormos JM, Pascual-Leone A. Somatosensory cortectomy induces motor cortical hyperexcitability and scoliosis: an experimental study in developing rats. Spine J. 2013;13:938–946. doi: 10.1016/j.spinee.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Parham C. Afferent connections of the lateral agranular field of the rat motor cortex. J. Comp. Neurol. 1983;217:390–404. doi: 10.1002/cne.902170404. [DOI] [PubMed] [Google Scholar]
- Driver J, Noesselt T. Multisensory interplay reveals crossmodal influences on 'sensory-specific' brain regions, neural responses, and judgments. Neuron. 2008;57:11–23. doi: 10.1016/j.neuron.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C, Fink GR, Zilles K. Functional lateralization of face, hand, and trunk representation in anatomically defined human somatosensory areas. Cerebral Cortex. 2008;18:2820–2830. doi: 10.1093/cercor/bhn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert T, Candia V, Altenmuller E, Rau H, Sterr A, Rockstroh B, Pantev C, Taub E. Alteration of digital representations in somatosensory cortex in focal hand dystonia. Neuroreport. 1998;9:3571–3575. doi: 10.1097/00001756-199811160-00006. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Ugawa Y, Hanajima R, Yuasa K, Mochizuki H, Terao Y, Shiio Y, Furubayashi T, Iwata NK, Kanazawa I. Decreased sensory cortical excitability after 1 Hz rTMS over the ipsilateral primary motor cortex. Clin. Neurophysiol. 2001;112:2154–2158. doi: 10.1016/s1388-2457(01)00667-8. [DOI] [PubMed] [Google Scholar]
- Fling BW, Benson BL, Seidler RD. Transcallosal sensorimotor fiber tract structure-function relationships. Hum. Brain Mapp. 2011;34:384–395. doi: 10.1002/hbm.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat. Clin. Pract. Neurol. 2007;3:383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- Galea MP, Darian-Smith I. Multiple corticospinal neuron populations in the macaque monkey are specified by their unique cortical origins, spinal terminations, and connections. Cereb. Cortex. 1994;4:166–194. doi: 10.1093/cercor/4.2.166. [DOI] [PubMed] [Google Scholar]
- Gandolla M, Ferrante S, Molteni F, Guanziroli E, Frattini T, Martegani A, Ferrigno G, Friston K, Pedrocchi A, Ward NS. Re-thinking the role of motor cortex: context-sensitive motor outputs? NeuroImage. 2014;91:366–374. doi: 10.1016/j.neuroimage.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genewein T, Braun DA. A sensorimotor paradigm for Bayesian model selection. Front. Hum. Neurosci. 2012;6:291. doi: 10.3389/fnhum.2012.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, Thibodeaux H, Palmer JT, van Lookeren Campagne M, Van Bruggen N. Transient focal cerebral ischemia induces sensorimotor deficits in mice. Behav. brain Res. 2000;108:63–71. doi: 10.1016/s0166-4328(99)00130-8. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Brinkman C, Porter R. A quantitative study of the distribution of neurons projecting to the precentral motor cortex in the monkey (M. fascicularis) J. Comp. Neurol. 1987;259:424–444. doi: 10.1002/cne.902590309. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Saksena S, Hasan KM, Agarwal A, Haris M, Pandey CM, Narayana PA. Focal Wallerian degeneration of the corpus callosum in large middle cerebral artery stroke: serial diffusion tensor imaging. J. Magn. Reson. Imaging: JMRI. 2006;24:549–555. doi: 10.1002/jmri.20677. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat. Neurosci. 1998;1:64–68. doi: 10.1038/264. [DOI] [PubMed] [Google Scholar]
- Hannula H, Ylioja S, Pertovaara A, Korvenoja A, Ruohonen J, Ilmoniemi RJ, Carlson S. Somatotopic blocking of sensation with navigated transcranial magnetic stimulation of the primary somatosensory cortex. Hum. Brain Mapp. 2005;26:100–109. doi: 10.1002/hbm.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TC, Silasi G, Boyd JD, Murphy TH. Displacement of sensory maps and disorganization of motor cortex after targeted stroke in mice. Stroke. 2013;44:2300–2306. doi: 10.1161/STROKEAHA.113.001272. [DOI] [PubMed] [Google Scholar]
- Henschke JU, Noesselt T, Scheich H, Budinger E. Possible anatomical pathways for short-latency multisensory integration processes in primary sensory cortices. Brain Struct. Funct. 2014;220:955–977. doi: 10.1007/s00429-013-0694-4. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Tanaka M, Sakamoto M, Iwamura Y. Deficits in manipulative behaviors induced by local injections of muscimol in the first somatosensory cortex of the conscious monkey. Brain Res. 1985;325:375–380. doi: 10.1016/0006-8993(85)90344-0. [DOI] [PubMed] [Google Scholar]
- Hlushchuk Y, Hari R. Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. Journal of Neuroscience. 2006;26:5819–5824. doi: 10.1523/JNEUROSCI.5536-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited – comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Hummelsheim H, Bianchetti M, Wiesendanger M, Wiesendanger R. Sensory inputs to the agranular motor fields: a comparison between precentral, supplementary-motor and premotor areas in the monkey. Exp. Brain Res. 1988;69:289–298. doi: 10.1007/BF00247574. [DOI] [PubMed] [Google Scholar]
- Hwang EJ, Shadmehr R. Internal models of limb dynamics and the encoding of limb state. J. Neural Eng. 2005;2:S266–S278. doi: 10.1088/1741-2560/2/3/S09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Kicic D. Methodology for Combined TMS and EEG. Brain Topogr. 2010;22:233–248. doi: 10.1007/s10548-009-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Naatanen R, Katila T. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8:3537–3540. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Matsunaga K, Nakanishi R, Kawahira K, Murayama N, Tsuji S, Huang YZ, Rothwell JC. Effect of theta burst stimulation over the human sensorimotor cortex on motor and somatosensory evoked potentials. Clin. Neurophysiol. 2007;118:1033–1043. doi: 10.1016/j.clinph.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Ito M. Mechanisms of motor learning in the cerebellum. Brain Res. 2000;886:237–245. doi: 10.1016/s0006-8993(00)03142-5. [DOI] [PubMed] [Google Scholar]
- Jacobs M, Premji A, Nelson AJ. Plasticity-inducing TMS protocols to investigate somatosensory control of hand function. Neural Plast. 2012;2012:350574. doi: 10.1155/2012/350574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SH. Prediction of motor outcome for hemiparetic stroke patients using diffusion tensor imaging: A review. NeuroRehabil. 2010;27:367–372. doi: 10.3233/NRE-2010-0621. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Hendry SH. Intracortical connectivity of architectonic fields in the somatic sensory, motor and parietal cortex of monkeys. J. Comp. Neurol. 1978;181:291–347. doi: 10.1002/cne.901810206. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Saisanen L, Danner N, Niskanen E, Hukkanen T, Mervaala E, Kononen M. Comparison of navigated and non-navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. NeuroImage. 2009;44:790–795. doi: 10.1016/j.neuroimage.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Jung P, Baumgartner U, Magerl W, Treede RD. Hemispheric asymmetry of hand representation in human primary somatosensory cortex and handedness. Clin. Neurophysiol. 2008;119:2579–2586. doi: 10.1016/j.clinph.2008.04.300. [DOI] [PubMed] [Google Scholar]
- Jung P, Baumgartner U, Bauermann T, Magerl W, Gawehn J, Stoeter P, Treede RD. Asymmetry in the human primary somatosensory cortex and handedness. NeuroImage. 2003;19:913–923. doi: 10.1016/s1053-8119(03)00164-2. [DOI] [PubMed] [Google Scholar]
- Kaas JH. What, if anything, is SI? Organization of first somatosensory area of cortex. Physiol. Rev. 1983;63:206–231. doi: 10.1152/physrev.1983.63.1.206. [DOI] [PubMed] [Google Scholar]
- Kandel E, Schwartz J, Jessell T. Principles of Neural Science. 4th. New York: McGraw-Hill; 2000. [Google Scholar]
- Kandel ER. From nerve cells to cognition: the internal cellular representation required for perception and action. In: Kandel ER, Schwartz JH, Jessell T, editors. Principles of Neural Science. 4. New York: McGraw-Hill Companies; 2000. pp. 381–403. [Google Scholar]
- Karim AA, Schuler A, Hegner YL, Friedel E, Godde B. Facilitating effect of 15-Hz repetitive transcranial magnetic stimulation on tactile perceptual learning. J. Cogn. Neurosci. 2006;18:1577–1585. doi: 10.1162/jocn.2006.18.9.1577. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Baudewig J, Schnaudigel S, Huonker R, Becker L, Sohns JM, Dechent P, Klingner C, Witte OW. Behavioral correlates of negative BOLD signal changes in the primary somatosensory cortex. Neuroimage. 2008;41:1364–1371. doi: 10.1016/j.neuroimage.2008.03.049. [DOI] [PubMed] [Google Scholar]
- Katayama T, Rothwell JC. Modulation of somatosensory evoked potentials using transcranial magnetic intermittent theta burst stimulation. Clin. Neurophysiol. 2007;118:2506–2511. doi: 10.1016/j.clinph.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Katayama T, Suppa A, Rothwell JC. Somatosensory evoked potentials and high frequency oscillations are differently modulated by theta burst stimulation over primary somatosensory cortex in humans. Clin. Neurophysiol. 2010;121:2097–2103. doi: 10.1016/j.clinph.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey LL, Lojovich JM, Carey JR. Electrical stimulation driving functional improvements and cortical changes in subjects with stroke. Exp. Brain Res. 2004;154:450–460. doi: 10.1007/s00221-003-1695-y. [DOI] [PubMed] [Google Scholar]
- Klaiput A, Kitisomprayoonkul W. Increased pinch strength in acute and subacute stroke patients after simultaneous median and ulnar sensory stimulation. Neurorehabilit. Neural Repair. 2009;23:351–356. doi: 10.1177/1545968308324227. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Boychuk JA, Adkins DL. Rat models of upper extremity impairment in stroke. ILAR J. 2007;48:374–384. doi: 10.1093/ilar.48.4.374. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr. Opin. Neurobiol. 2006;16:435–444. doi: 10.1016/j.conb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Klingner CM, Huonker R, Flemming S, Hasler C, Brodoehl S, Preul C, Burmeister H, Kastrup A, Witte OW. Functional deactivations: multiple ipsilateral brain areas engaged in the processing of somatosensory information. Human Brain Mapping. 2011;32:127–140. doi: 10.1002/hbm.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson JS, Harley MY, Hisel TZ, Makowski NS, Fu MJ, Chae J. Contralaterally controlled functional electrical stimulation for stroke rehabilitation; Proceedings of the IEEE Conference Engineering Medical Biology Society; 2012. pp. 314–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Franca M, Albrecht UV, Caltagirone C, Rothwell JC. Effects of paired pulse TMS of primary somatosensory cortex on perception of a peripheral electrical stimulus. Exp. Brain Res. 2006;172:416–424. doi: 10.1007/s00221-006-0359-0. [DOI] [PubMed] [Google Scholar]
- Konczak J, Abbruzzese G. Focal dystonia in musicians: linking motor symptoms to somatosensory dysfunction. Front. Hum. Neurosci. 2013;7:297. doi: 10.3389/fnhum.2013.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr. Opin. Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- Laible M, Grieshammer S, Seidel G, Rijntjes M, Weiller C, Hamzei F. Association of activity changes in the primary sensory cortex with successful motor rehabilitation of the hand following stroke. Neurorehabil. Neural Repair. 2012;26:881–888. doi: 10.1177/1545968312437939. [DOI] [PubMed] [Google Scholar]
- Lee S, Carvell GE, Simons DJ. Motor modulation of afferent somatosensory circuits. Nat. Neurosci. 2008;11:1430–1438. doi: 10.1038/nn.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat. Neurosci. 2013;16:1662–1670. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Mouraux A, Hu L, Iannetti GD. Primary sensory cortices contain distinguishable spatial patterns of activity for each sense. Nat. Commun. 2013;4:1979. doi: 10.1038/ncomms2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg PG, Skejo PH, Rounis E, Nagy Z, Schmitz C, Wernegren H, Bring A, Engardt M, Forssberg H, Borg J. Wallerian degeneration of the corticofugal tracts in chronic stroke: a pilot study relating diffusion tensor imaging, transcranial magnetic stimulation, and hand function. Neurorehabil. Neural Repair. 2007;21:551–560. doi: 10.1177/1545968307301886. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Zhu LL, Ruber T, Schlaug G. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum. Brain Mapp. 2012;33:1040–1051. doi: 10.1002/hbm.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton ML, Fu KM, Branch CA, Schroeder CE. Ipsilateral hand input to area 3b revealed by converging hemodynamic and electrophysiological analyses in macaque monkeys. J. Neurosci. 2006;26:180–185. doi: 10.1523/JNEUROSCI.1073-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin. Neurophysiol. 2000;111:800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Matsunaga K. Effect of transcranial DC sensorimotor cortex stimulation on somatosensory evoked potentials in humans. Clin. Neurophysiol. 2004;115:456–460. doi: 10.1016/s1388-2457(03)00362-6. [DOI] [PubMed] [Google Scholar]
- Matyas F, Sreenivasan V, Marbach F, Wacongne C, Barsy B, Mateo C, Aronoff R, Petersen CC. Motor control by sensory cortex. Science. 2010;330:1240–1243. doi: 10.1126/science.1195797. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Smith DH, Voddi M, Perri BR, Stutzmann JM. Riluzole, a novel neuroprotective agent, attenuates both neurologic motor and cognitive dysfunction following experimental brain injury in the rat. J. Neurotrauma. 1996;13:767–780. doi: 10.1089/neu.1996.13.767. [DOI] [PubMed] [Google Scholar]
- Meehan SK, Legon W, Staines WR. Paired-pulse transcranial magnetic stimulation of primary somatosensory cortex differentially modulates perception and sensorimotor transformations. Neuroscience. 2008;157:424–431. doi: 10.1016/j.neuroscience.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Meehan SK, Dao E, Linsdell MA, Boyd LA. Continuous theta burst stimulation over the contralesional sensory and motor cortex enhances motor learning post-stroke. Neurosci. Lett. 2011;500:26–30. doi: 10.1016/j.neulet.2011.05.237. [DOI] [PubMed] [Google Scholar]
- Meyer K, Kaplan JT, Essex R, Damasio H, Damasio A. Seeing touch is correlated with content-specific activity in primary somatosensory cortex. Cereb. Cortex. 2011;21:2113–2121. doi: 10.1093/cercor/bhq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Murray MM, Molholm S, Michel CM, Heslenfeld DJ, Ritter W, Javitt DC, Schroeder CE, Foxe JJ. Grabbing your ear: rapid auditory-somatosensory multisensory interactions in low-level sensory cortices are not constrained by stimulus alignment. Cereb. Cortex. 2005;15:963–974. doi: 10.1093/cercor/bhh197. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak DA, Glasauer S, Hermsdorfer J. How predictive is grip force control in the complete absence of somatosensory feedback? Brain. 2004;127:182–192. doi: 10.1093/brain/awh016. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil. Neural Repair. 2009;23:641–656. doi: 10.1177/1545968309336661. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Ukai S, Shinosaki K, Yamamoto M, Kawaguchi S, Ishii R, Takeda M. Slow repetitive transcranial magnetic stimulation increases somatosensory high-frequency oscillations in humans. Neurosci. Lett. 2004;358:193–196. doi: 10.1016/j.neulet.2004.01.038. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. Association areas of the cerebral cortex. Trends Neurosci. 1982;5:386–390. [Google Scholar]
- Pavlides C, Miyashita E, Asanuma H. Projection from the sensory to the motor cortex is important in learning motor skills in the monkey. J. Neurophysiol. 1993;70:733–741. doi: 10.1152/jn.1993.70.2.733. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Reimer B, Loer C, Quartarone A, Munchau A, Conrad B, Siebner HR. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin. Neurophysiol. 2004;115:1519–1526. doi: 10.1016/j.clinph.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J. Neurosci. 2008;28:5631–5640. doi: 10.1523/JNEUROSCI.0093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perruchoud D, Murray MM, Lefebvre J, Ionta S. Focal dystonia and the Sensory-Motor Integrative Loop for Enacting (SMILE) Front. Hum. Neurosci. 2014;8:458. doi: 10.3389/fnhum.2014.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineiro R, Pendlebury S, Johansen-Berg H, Matthews PM. Functional MRI detects posterior shifts in primary sensorimotor cortex activation after stroke – evidence of local adaptive reorganization? Stroke. 2001;32:1134–1139. doi: 10.1161/01.str.32.5.1134. [DOI] [PubMed] [Google Scholar]
- Pleger B, Blankenburg F, Bestmann S, Ruff CC, Wiech K, Stephan KE, Friston KJ, Dolan RJ. Repetitive transcranial magnetic stimulation-induced changes in sensorimotor coupling parallel improvements of somatosensation in humans. J. Neurosci. 2006;26:1945–1952. doi: 10.1523/JNEUROSCI.4097-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons TP, Kaas JH. Corticocortical connections of area 2 of somatosensory cortex in macaque monkeys: a correlative anatomical and electrophysiological study. J. Comp. Neurol. 1986;248:313–335. doi: 10.1002/cne.902480303. [DOI] [PubMed] [Google Scholar]
- Premji A, Ziluk A, Nelson AJ. Bilateral somatosensory evoked potentials following intermittent theta-burst repetitive transcranial magnetic stimulation. BMC Neurosci. 2010;11:91. doi: 10.1186/1471-2202-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M, Darling WG, Morecraft RJ, Ni CC, Rajendra J, Butler AJ. White matter integrity is a stronger predictor of motor function than BOLD response in patients with stroke. Neurorehabil. Neural Repair. 2011;25:275–284. doi: 10.1177/1545968310389183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragert P, Vandermeeren Y, Camus M, Cohen LG. Improvement of spatial tactile acuity by transcranial direct current stimulation. Clin. Neurophysiol. 2008;119:805–811. doi: 10.1016/j.clinph.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragert P, Nierhaus T, Cohen LG, Villringer A. Interhemispheric interactions between the human primary somatosensory cortices. PLoS One. 2011;6:e16150. doi: 10.1371/journal.pone.0016150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragert P, Becker M, Tegenthoff M, Pleger B, Dinse HR. Sustained increase of somatosensory cortex excitability by 5 Hz repetitive transcranial magnetic stimulation studied by paired median nerve stimulation in humans. Neurosci. Lett. 2004;356:91–94. doi: 10.1016/j.neulet.2003.11.034. [DOI] [PubMed] [Google Scholar]
- Ragert P, Dinse HR, Pleger B, Wilimzig C, Frombach E, Schwenkreis P, Tegenthoff M. Combination of 5 Hz repetitive transcranial magnetic stimulation (rTMS) and tactile coactivation boosts tactile discrimination in humans. Neurosci. Lett. 2003;348:105–108. doi: 10.1016/s0304-3940(03)00745-6. [DOI] [PubMed] [Google Scholar]
- Restuccia D, Ulivelli M, De Capua A, Bartalini S, Rossi S. Modulation of high-frequency (600 Hz) somatosensory-evoked potentials after rTMS of the primary sensory cortex. Eur. J. Neurosci. 2007;26:2349–2358. doi: 10.1111/j.1460-9568.2007.05828.x. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. Physiol. 2010;588:2291–2304. doi: 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann BL, Lephart SM. The sensorimotor system, part II: the role of proprioception in motor control and functional joint stability. J. Athl. Train. 2002;37:80–84. [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Kalaska JF. Voluntary Movement: The Parietal and Premotor Cortex. In: Kandel ER, Schwartz JH, Jessell TM, Seigelbaum SA, Hudspeth AJ, editors. Principles of Neural Science. 5. New York: McGraw-Hill Companies; 2013. pp. 865–893. [Google Scholar]
- Ro T, Ellmore TM, Beauchamp MS. A neural link between feeling and hearing. Cereb. Cortex. 2013;23:1724–1730. doi: 10.1093/cercor/bhs166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, Miall RC. Current concepts in procedural consolidation. Nat. Rev. Neurosci. 2004;5:576–582. doi: 10.1038/nrn1426. [DOI] [PubMed] [Google Scholar]
- Rogalewski A, Breitenstein C, Nitsche MA, Paulus W, Knecht S. Transcranial direct current stimulation disrupts tactile perception. Eur. J. Neurosci. 2004;20:313–316. doi: 10.1111/j.0953-816X.2004.03450.x. [DOI] [PubMed] [Google Scholar]
- Rose S, Guzzetta A, Pannek K, Boyd R. MRI structural connectivity, disruption of primary sensorimotor pathways, and hand function in cerebral palsy. Brain Connect. 2011;1:309–316. doi: 10.1089/brain.2011.0034. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Altamura C, Ferreri F, Melgari JM, Tecchio F, Tombini M, Pasqualetti P, Vernieri F. Neuroimaging experimental studies on brain plasticity in recovery from stroke. Eur. Medicophys. 2007;43:241–254. [PubMed] [Google Scholar]
- Rub U, Schultz C, Del Tredici K, Gierga K, Reifenberger G, de Vos RA, Seifried C, Braak H, Auburger G. Anatomically based guidelines for systematic investigation of the central somatosensory system and their application to a spinocerebellar ataxia type 2 (SCA2) patient. Neuropathol. Appl. Neurobiol. 2003;29:418–433. doi: 10.1046/j.1365-2990.2003.00504.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Porter LL, Asanuma H. Long-lasting potentiation of synaptic potentials in the motor cortex produced by stimulation of the sensory cortex in the cat: a basis of motor learning. Brain Res. 1987;413:360–364. doi: 10.1016/0006-8993(87)91029-8. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Arissian K, Asanuma H. Functional role of the sensory cortex in learning motor skills in cats. Brain Res. 1989;503:258–264. doi: 10.1016/0006-8993(89)91672-7. [DOI] [PubMed] [Google Scholar]
- Saper CB, Iversen S, Frackowiak R. Integration of sensory and motor function: the association areas of the cerebral cortex and the cognitive capabilities of the brain. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4. New York: McGraw-Hill Companies; 2000. pp. 349–380. [Google Scholar]
- Sato S, Bergmann TO, Borich MR. Opportunities for concurrent transcranial magnetic stimulation and electroencephalography to characterize cortical activity in stroke. Front. Hum. Neurosci. 2015;9:250. doi: 10.3389/fnhum.2015.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satow T, Mima T, Yamamoto J, Oga T, Begum T, Aso T, Hashimoto N, Rothwell JC, Shibasaki H. Short-lasting impairment of tactile perception by 0.9Hz-rTMS of the sensorimotor cortex. Neurology. 2003;60:1045–1047. doi: 10.1212/01.wnl.0000052821.99580.d3. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Moore CI, Connell BD, Rosen BR, Dijkhuizen RM. Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain. 2006;129:2722–2733. doi: 10.1093/brain/awl214. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Fricker ZP, Perdue KL, Helmer KG, Vangel MG, Greve DN, Makris N. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum. Brain Mapp. 2009;30:3461–3474. doi: 10.1002/hbm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl AH. Regulation of conduction time along axons. Neuroscience. 2014;276:126–134. doi: 10.1016/j.neuroscience.2013.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyal M, Siddiqui I, Hundal NS. Suppression of spatial localization of a cutaneous stimulus following transcranial magnetic pulse stimulation of the sensorimotor cortex. Electroencephalogr. Clin. Neurophysiol. 1997;105:24–28. doi: 10.1016/s0924-980x(96)96090-7. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp. Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Bergmann TO, Bestmann S, Massimini M, Johansen-Berg H, Mochizuki H, Bohning DE, Boorman ED, Groppa S, Miniussi C, PascualLeone A, Huber R, Taylor PC, Ilmoniemi RJ, De Gennaro L, Strafella AP, Kahkonen S, Kloppel S, Frisoni GB, George MS, Hallett M, Brandt SA, Rushworth MF, Ziemann U, Rothwell JC, Ward N, Cohen LG, Baudewig J, Paus T, Ugawa Y, Rossini PM. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul. 2009;2:58–80. doi: 10.1016/j.brs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Duhamel JR, Cohen L, Pillon B, Dubois B, Agid Y. The mental representation of hand movements after parietal cortex damage. Science. 1996;273:1564–1568. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]
- Song S, Sandrini M, Cohen LG. Modifying somatosensory processing with non-invasive brain stimulation. Restor. Neurol. Neurosci. 2011;29:427–437. doi: 10.3233/RNN-2011-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soros P, Knecht S, Imai T, Gurtler S, Lutkenhoner B, Ringelstein EB, Henningsen H. Cortical asymmetries of the human somatosensory hand representation in right- and left-handers. Neurosci. Lett. 1999;271:89–92. doi: 10.1016/s0304-3940(99)00528-5. [DOI] [PubMed] [Google Scholar]
- Sporns O, Chialvo DR, Kaiser M, Hilgetag CC. Organization, development and function of complex brain networks. Trends Cogn. Sci. 2004;8:418–425. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Staines WR, Bolton DA. Transcranial magnetic stimulation techniques to study the somatosensory system: research applications. Handb. Clin. Neurol. 2013;116:671–679. doi: 10.1016/B978-0-444-53497-2.00053-X. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Taskin B, Jungehulsing GJ, Ruben J, Brunecker P, Krause T, Blankenburg F, Villringer A. Preserved responsiveness of secondary somatosensory cortex in patients with thalamic stroke. Cereb. Cortex. 2006;16:1431–1439. doi: 10.1093/cercor/bhj080. [DOI] [PubMed] [Google Scholar]
- Tegenthoff M, Ragert P, Pleger B, Schwenkreis P, Forster AF, Nicolas V, Dinse HR. Improvement of tactile discrimination performance and enlargement of cortical somatosensory maps after 5 Hz rTMS. PLoS Biol. 2005;3:e362. doi: 10.1371/journal.pbio.0030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R. Electromyographic correlates of learning an internal model of reaching movements. J. Neurosci. 1999;19:8573–8588. doi: 10.1523/JNEUROSCI.19-19-08573.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Deschenes M. Single-cell study of motor cortex projections to the barrel field in rats. J. Comp. Neurol. 2003;464:98–103. doi: 10.1002/cne.10769. [DOI] [PubMed] [Google Scholar]
- Vidoni ED, Acerra NE, Dao E, Meehan SK, Boyd LA. Role of the primary somatosensory cortex in motor learning: an rTMS study. Neurobiol. Learn. Mem. 2010;93:532–539. doi: 10.1016/j.nlm.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cortico-cortical connections of somatic sensory cortex (areas 3, 1 and 2) in the rhesus monkey. J. Comp. Neurol. 1978;177:179–191. doi: 10.1002/cne.901770202. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wei K, Kording K. Relevance of error: what drives motor adaptation? J. Neurophysiol. 2009;101:655–664. doi: 10.1152/jn.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werring DJ, Toosy AT, Clark CA, Parker GJ, Barker GJ, Miller DH, Thompson AJ. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J. Neurol. Neurosurg. Psychiatry. 2000;69:269–272. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EL, DeAmicis RA. Afferent and efferent projections of the region in mouse SmL cortex which contains the posteromedial barrel subfield. J. Comp. Neurol. 1977;175:455–482. doi: 10.1002/cne.901750405. [DOI] [PubMed] [Google Scholar]
- Winship IR, Murphy TH. Remapping the somatosensory cortex after stroke: insight from imaging the synapse to network. Neuroscientist. 2009;15:507–524. doi: 10.1177/1073858409333076. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Kawato M. Multiple paired forward and inverse models for motor control. Neural Netw.: Off. J. Int. Neural Netw. Soc. 1998;11:1317–1329. doi: 10.1016/s0893-6080(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat. Neurosci. 2000:1212–1217. doi: 10.1038/81497. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Pearson KG, Ghez CPJ. The organization and planning of movement. In: Kandel ER, Schwartz JH, Jessell TM, Seigelbaum SA, Hudspeth AJ, editors. Principles of Neural Science. 5. New York: McGraw-Hill Companies; 2013. pp. 743–767. [Google Scholar]
- Wu CW, Seo HJ, Cohen LG. Influence of electric somatosensory stimulation on paretic-hand function in chronic stroke. Arch. Phys. Med. Rehabil. 2006;87:351–357. doi: 10.1016/j.apmr.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Xu NL, Harnett MT, Williams SR, Huber D, O'Connor DH, Svoboda K, Magee JC. Nonlinear dendritic integration of sensory and motor input during an active sensing task. Nature. 2012;492:247–251. doi: 10.1038/nature11601. [DOI] [PubMed] [Google Scholar]
- Yamada K, Mori S, Nakamura H, Ito H, Kizu O, Shiga K, Yoshikawa K, Makino M, Yuen S, Kubota T, Tanaka O, Nishimura T. Fiber-tracking method reveals sensorimotor pathway involvement in stroke patients. Stroke. 2003;34:E159–E162. doi: 10.1161/01.STR.0000085827.54986.89. [DOI] [PubMed] [Google Scholar]
- Zapallow CM, Jacobs MF, Lee KG, Asmussen MJ, Tsang P, Nelson AJ. Continuous theta-burst stimulation over the primary somatosensory cortex modulates interhemispheric inhibition. Neurorep. 2013;24:394–398. doi: 10.1097/WNR.0b013e32836131ca. [DOI] [PubMed] [Google Scholar]
- Zarzecki P, Shinoda Y, Asanuma H. Projection from area 3a to the motor cortex by neurons activated from group I muscle afferents. Exp. Brain Res. 1978;33:269–282. doi: 10.1007/BF00238065. [DOI] [PubMed] [Google Scholar]
- Zemke AC, Heagerty PJ, Lee C, Cramer SC. Motor cortex organization after stroke is related to side of stroke and level of recovery. Stroke. 2003;34:e23–e28. doi: 10.1161/01.STR.0000065827.35634.5E. [DOI] [PubMed] [Google Scholar]