SUMMARY
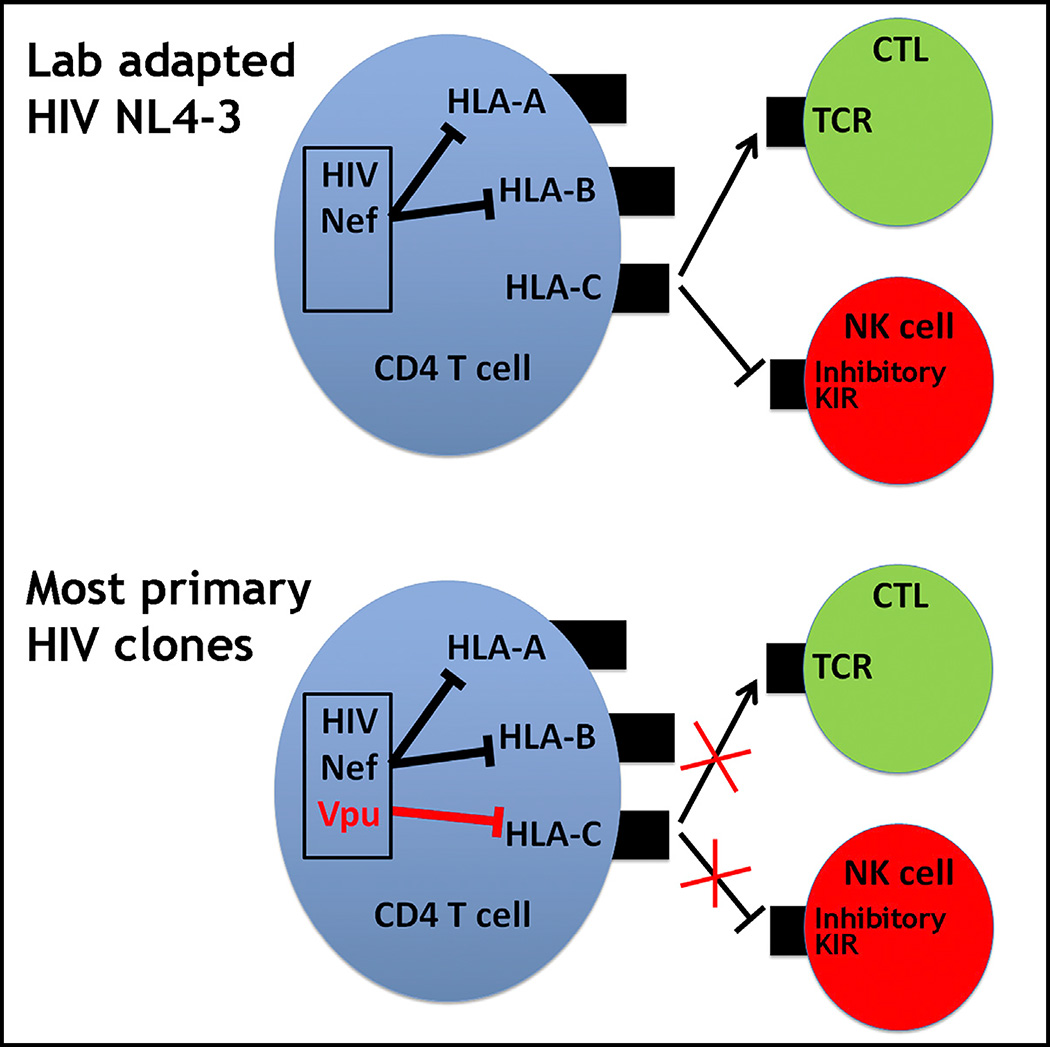
Many pathogens evade cytotoxic T lymphocytes (CTLs) by downregulating HLA molecules on infected cells, but the loss of HLA can trigger NK cell-mediated lysis. HIV-1 is thought to subvert CTLs while preserving NK cell inhibition by Nef-mediated downregulation of HLA-A and -B but not HLA-C molecules. We find that HLA-C is downregulated by most primary HIV-1 clones, including transmitted founder viruses, in contrast to the laboratory-adapted NL4-3 virus. HLA-C reduction is mediated by viral Vpu and reduces the ability of HLA-C restricted CTLs to suppress viral replication in CD4+ cells in vitro. HLA-A/B are unaffected by Vpu, and primary HIV-1 clones vary in their ability to downregulate HLA-C, possibly in response to whether CTLs or NK cells dominate immune pressure through HLA-C. HIV-2 also suppresses HLA-C expression through distinct mechanisms, underscoring the immune pressure HLA-C exerts on HIV. This viral immune evasion casts new light on the roles of CTLs and NK cells in immune responses against HIV.
Graphical Abstract
INTRODUCTION
Human leukocyte antigens (HLA) are highly polymorphic molecules encoded by the HLA-A, -B, and -C loci that present peptides to cytotoxic T lymphocytes (CTLs). Extreme polymorphism of the HLA class I loci enables the presentation of a wide range of peptides, including viral peptides, in the event of HIV-1 infection. HLA-restricted CTL responses to HIV-1 peptides efficiently suppress viral replication in vitro, and extensive evidence in rhesus monkeys and humans, including prevalent HLA-associated viral escape mutations (Moore et al., 2002; Bhattacharya et al., 2007) and HLA allelic associations with viral control (Carrington and O’Brien, 2003), indicate that CTLs also control HIV-1 in vivo. Many pathogens evade CTL immunity by downregulating HLA, and HIV-1 Nef specifically downregulates HLA-A and -B molecules on infected cells, whereas HLA-C is not targeted by Nef (Schwartz et al., 1996; Collins et al., 1998; Cohen et al., 1999; Specht et al., 2010).
The HLA-C locus is distinct relative to HLA-A and HLA-B in that it is less polymorphic, and it encodes molecules that have lower cell surface expression levels (Apps et al., 2015) and more extensive interactions with the killer immunoglobulin-like receptors (KIRs) expressed by natural killer (NK) cells (Parham, 2005). Every HLA-C allotype serves as a ligand for inhibitory KIRs that are present in virtually all individuals, imparting a key role for HLA-C in maintaining NK cell quiescence under healthy conditions. HLA-C alleles vary in expression level under normal conditions (Apps et al., 2013), and evolutionary analyses support selection for this trait (Kulkarni et al., 2011; O’huigin et al., 2011). Opposing disease associations with variable HLA-C expression levels have been observed, where high expression may confer protection in one disease but susceptibility in another (Apps et al., 2013; Petersdorf et al., 2014). Taken together, these data point to the physiological importance of differential HLA-C expression levels. Pathogen-driven downregulation of HLA class I molecules on infected cells can result in strongly diminished CTL recognition but also enhance NK cell-mediated lysis of the infected cell because of the failure of HLA ligand to bind inhibitory KIRs. The specificity of HIV-1 Nef in downregulating HLA-A and -B molecules, but not HLA-C, has been interpreted as an elegant viral mechanism to subvert adaptive HLA-A- and HLA-B-restricted CTL responses (Collins et al., 1998) while simultaneously protecting infected cells against innate NK cell immunity through recognition of unmodulated HLA-C levels by inhibitory NK cell receptors (Cohen et al., 1999). This model is likely to be accurate in some cases, although additional interactions governing innate immune activation can also play a role. Indeed, in vitro data have shown that NK cells are able to lyse HIV-infected cells (Fogli et al., 2008), particularly an NKG2A+ subset that was recently shown to respond to cells infected with viruses that do not downregulate HLA-C (Davis et al., 2016).
Here we measure cell surface protein expression levels of each HLA class I locus on primary CD4+ cells infected in vitro with molecular clones of primary HIV-1 strains, enabled by advances in cloning of primary viruses and characterization of HLA monoclonal antibody (mAb) specificity (Apps et al., 2009). We find that, unlike the widely studied laboratory-adapted HIV-1 isolate NL4-3, most primary clones of HIV-1 do in fact downregulate HLA-C to some extent. Intriguingly, both the viral protein responsible for HLA-C reduction and the range of this modulation between viruses are quite distinct from that of Nef-mediated downregulation of HLA-A and -B. These findings modify models describing the means by which HIV-1 undermines the host immune response.
RESULTS
HLA-C Is Downregulated by Primary Clones of HIV-1
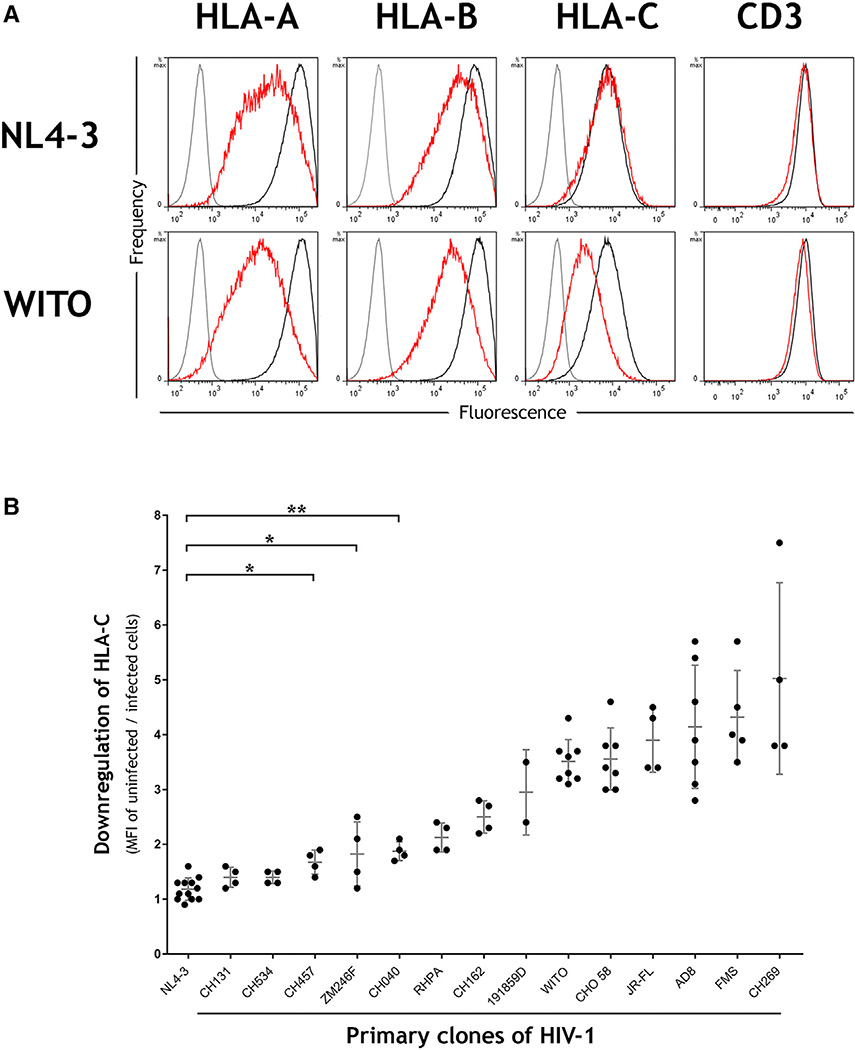
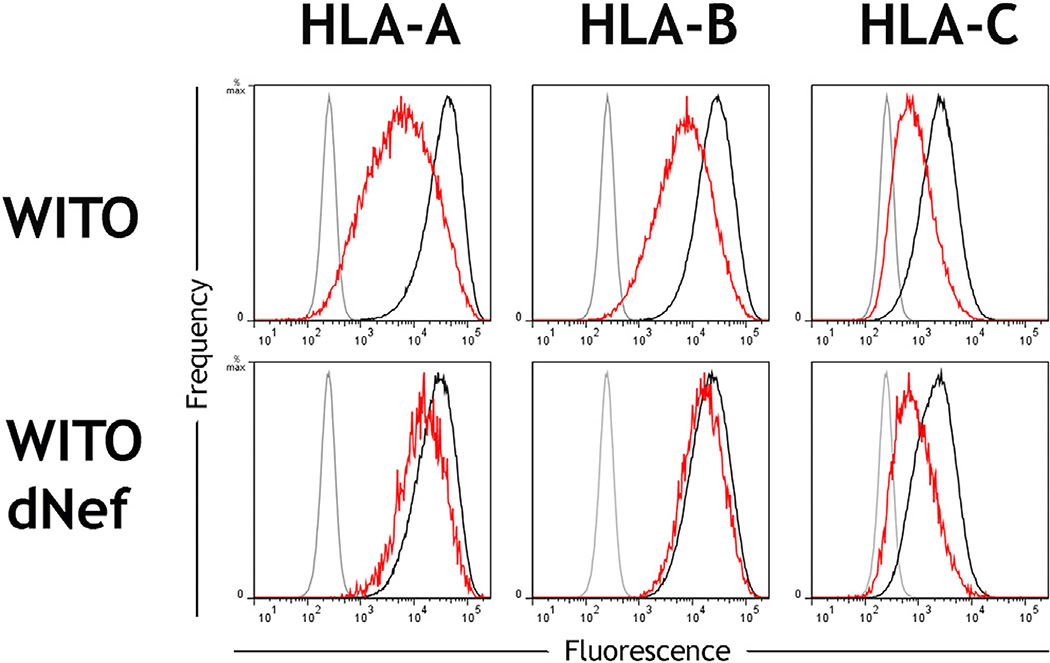
Well characterized mAbs specific to each of the HLA-A, -B, and -C loci (Apps et al., 2015) were used to measure the modulation of HLA expression levels on primary CD4+ cells from healthy donors after in vitro infection with HIV-1 infectious molecular clones. The laboratory-adapted virus NL4-3 downregulated HLA-A and -B molecules but did not affect HLA-C expression levels, consistent with previous observations using HLA-transfected cells infected with NL4-3 or its close relatives (Cohen et al., 1999; Rajapaksa et al., 2012). In contrast, the primary transmitted founder HIV-1 clone WITO robustly downregulated HLA-C in addition to HLA-A/B (Figure 1A; Figure S1). The magnitude of HLA-C reduction was similar to that of HLA-B, whereas other surface antigens, such as CD3, were not affected.
Figure 1. HLA-C Is Downregulated by Most Primary Clones of HIV-1 in Contrast to the Laboratory-Adapted Clone NL4-3.
(A) Primary CD4+ cells were infected in vitro with the HIV-1 molecular clones NL4-3 or WITO. Flow cytometry staining of HLA-A, HLA-B, HLA-C, or CD3 is shown for infected (red) or uninfected cells (black) and an isotype control (gray). Infected cells within cultures were discriminated by co-staining the viral protein Gag, and CD4 cell donors were homozygous for the A*02:01, B*44:02, C*0501 haplotype.
(B) Downregulation of HLA-C is a common feature among primary clones of HIV-1. Primary CD4+ cells were infected in vitro with a panel of HIV-1 infectious molecular clones (x axis), and HLA-C downregulation was determined by flow cytometry (y axis). MFI indicates median fluorescence intensity. Plotted points represent independent infections and include donors with different HLA types. Error bars show ± 1 SD. Statistical comparisons used unpaired t tests where *p < 0.005 and **p < 0.0001, and each virus to the right of CH040 also differs from NL4-3 with p < 0.0001.
Downregulation of HLA-C was confirmed by staining with multiple antibodies that recognize distinct epitopes of HLA-C (Figure S2). The ability of WITO to downregulate HLA-C expression was similar across a broad range of HLA-C alleles (Table S1), suggesting that a common motif across HLA-C alleles is targeted for downregulation. Importantly, the ability to downregulate HLA-C was not restricted to WITO because most viruses among a panel of 14 primary HIV-1 clones were found to downregulate HLA-C to some extent, and several did so more robustly than WITO (Figure 1B). Viruses capable of substantial HLA-C downregulation included transmitted founder viruses, viruses from chronic infection, and viruses representing each of the HIV-1 group M subtypes B, C, and D (Table S2). Thus, the widely held view that HIV-1 downregulates HLA-A/B but not HLA–C molecules on infected cells requires modification, and models of host immune evasion by HIV-1 must be reconsidered.
The HIV-1 Vpu Protein Is Responsible for Downregulation of HLA-C
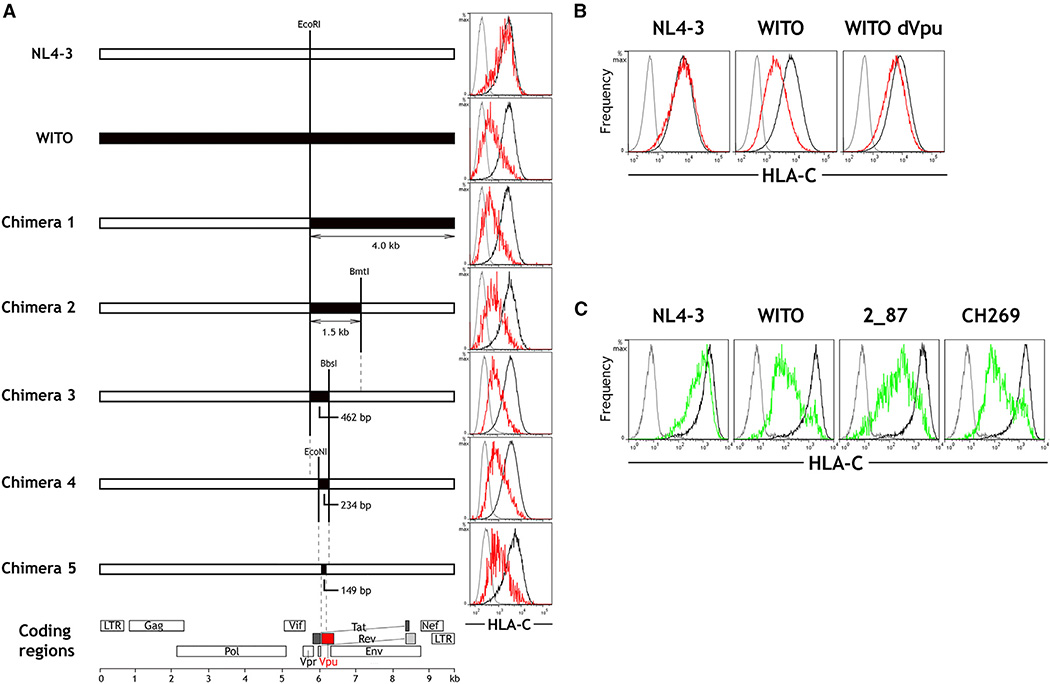
Although other mechanisms were originally proposed (Howcroft et al., 1993; Kerkau et al., 1997), it is now clear that HLA downregulation by NL4-3 is mediated by the viral Nef protein (Schwartz et al., 1996; Collins et al., 1998; Cohen et al., 1999). Nef from NL4-3 does not downregulate HLA-C, but this does not rule out the possibility that Nef from primary strains of HIV-1, with sequences distinct from that of NL4-3, are responsible for downregulation of HLA-C. Nef genes cloned from a battery of primary strains were transfected into cell lines expressing a single HLA allele, and each of them failed to downregulate HLA-C (Figure S3). Furthermore, an HIV-1 molecular clone in which Nef was experimentally deleted retained full ability to downregulate HLA-C upon infection of primary CD4+ cells (Figure S3), definitively ruling out a Nef-mediated effect on HLA-C expression. To identify the gene responsible for HLA-C downregulation, a series of full-length genomic chimeras was constructed, incorporating progressively smaller fragments of the WITO primary HIV-1 clone into an otherwise NL4-3 genome (Figure 2A). The ability of the chimeric constructs to modulate HLA-C was assessed by transfection into HeLa cells. Chimera 5, containing just 149 bp from WITO, demonstrated the full magnitude of HLA-C downregulation conferred by the entire WITO genome. This 149-bp segment encodes the 5′ region of Vpu and no portion of any other gene (Figure 2A; Figure S4).
Figure 2. The HIV-1 Protein Vpu Is Responsible for Downregulation of HLA-C.
(A) Full-length HIV-1 genomes were constructed comprising sequences from NL4-3 (white) and WITO (black). Flow cytometry plots show staining of isotype control (gray) and HLA-C on HIV+ (red) or HIV− (black) HeLa cells after transfection with the corresponding HIV-1 constructs and HIV+/− cell discrimination by staining viral Gag. In the final chimera 5, the 149-bp region from WITO encodes part of Vpu.
(B) Primary CD4+ cells were infected in vitro with molecular clones of NL4-3, WITO, or WITO with the Vpu initiation site ablated. Flow cytometry staining for HLA-C on infected (red) and uninfected cells (black) is shown compared with an isotype control (gray), where HIV+ cells were discriminated by staining viral Gag.
(C) Vpu genes cloned from NL4-3 or the primary HIV-1 subtype B viruses WITO and 2_87 and the primary subtype C virus CH269 were expressed in HeLa cells. Flow cytometry staining for HLA-C on Vpu+ (green) or Vpu− (black) cells discriminated by co-transfection with a GFP-expressing plasmid is shown compared with an isotype control (gray) and is representative of three independent experiments.
See also Figures S3 and S4.
Identification of Vpu as the protein responsible for modulating HLA-C expression in the context of infected primary cells was confirmed by disruption of the Vpu start codon in the full molecular clone of WITO. Loss of Vpu expression abrogated the ability of the WITO molecular clone to downregulate HLA-C upon infection of primary CD4+ cells (Figure 2B). Vpu was also shown to be sufficient for HLA-C reduction because the Vpu gene cloned from WITO into a Rev-dependent expression vector downregulated HLA-C strongly when transfected into HeLa cells (Figure 2C). Vpu-mediated influence on HLA-C expression was not unique to WITO because Vpu genes cloned from another B subtype and a C subtype HIV-1 variant were also able to downregulate HLA-C (Figure 2C).
Amino Acid Variants in Vpu that Affect HLA-C Downregulation
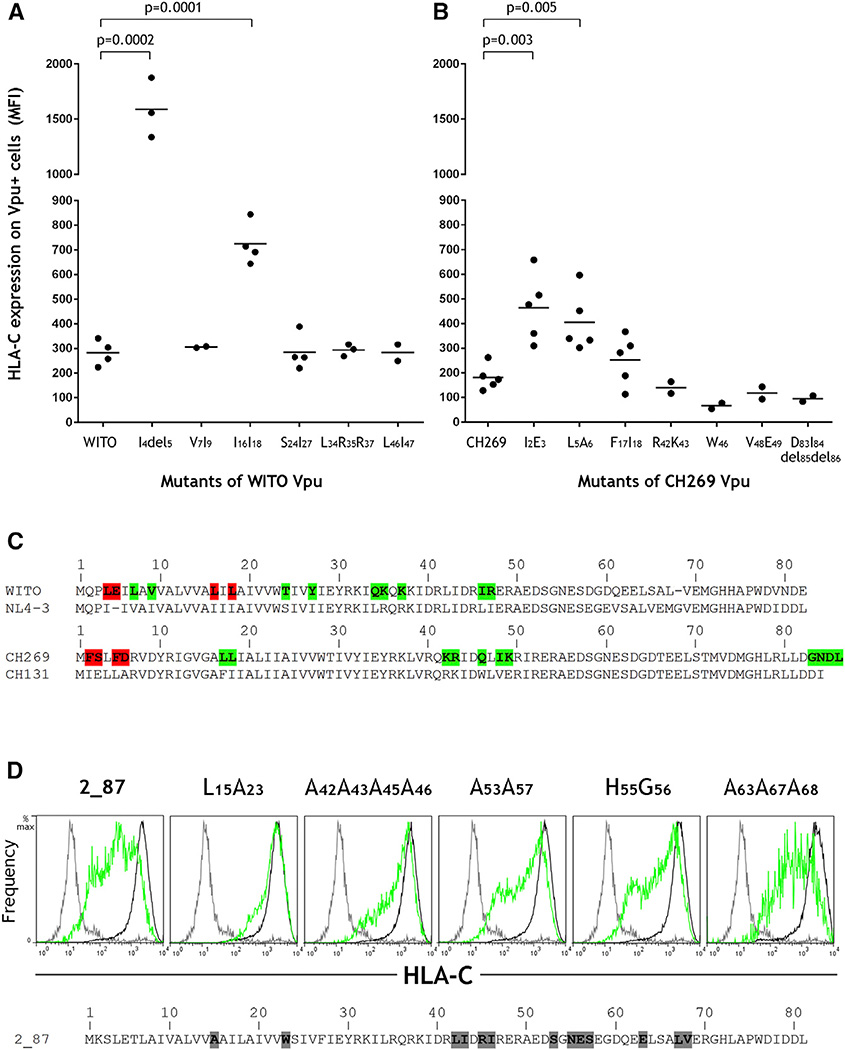
HIV-1-infectious molecular clones varied in magnitude of HLA-C downregulation (Figure 1B), allowing the identification of naturally polymorphic Vpu amino acids that influence HLA-C reduction. Because the Vpu protein sequence varies substantially between HIV-1 subtypes, we considered a pair of subtype B viruses (WITO and NL4-3) as well as a pair of subtype C viruses (CH269 and CH131), where the first virus listed in each of these pairs downregulates HLA-C robustly and the second does not. Specific amino acid sequence differences between the Vpu proteins of a given pair were tested for their contribution to the difference in HLA-C downregulation by incorporating Vpu amino acids present in the virus that does not reduce HLA-C into Vpu cloned from the virus that robustly reduced HLA-C. Mutation of L4E5 to I4 (E deleted) corresponding to the pair of subtype B viruses resulted in abrogation of HLA-C downregulation, and mutation of L16L18 to I16I18 impaired HLA-C downregulation (Figure 3A). Mutation of F2S3 to I2E3 and F5D6 to L5A6 corresponding to the pair of subtype C viruses impaired HLA-C downregulation (Figure 3B). Thus, multiple sites of variation near the N-terminal region of the Vpu protein are implicated in the reduction of HLA-C by HIV-1. Amino acids of Vpu shown to affect HLA-C downregulation are summarized in Figure 3C.
Figure 3. Vpu Amino Acid Residues that Affect HLA-C Downregulation.
(A) Mutants of the Vpu gene cloned from the primary HIV clone WITO were expressed in HeLa cells and analyzed by flow cytometry for their ability to downregulate HLA-C as shown in Figure 2C. Mutants included each of the differences in Vpu protein sequences between WITO and NL4-3 in the region shown to discriminate HLA-C reduction as shown in Figure 2A (i.e., 5′ of the BbsI site).
(B) Mutants of the Vpu gene cloned from the primary HIV clone CH269 were tested in the same way for their ability to downregulate HLA-C. Mutants included each of the differences in Vpu protein sequences between CH269 and CH131.
(C) Vpu sequences from the viruses analyzed are shown, where residues implicated in HLA-C reduction are highlighted red and all others tested but showing little to no effects are highlighted green. Statistical comparisons were determined using an unpaired t test.
(D) The Vpu gene cloned from the primary HIV strain 2_87 and its mutants known to affect CD4 and/or tetherin downregulation were expressed in HeLa cells. Flow cytometry staining for HLA-C on Vpu+ (green) or Vpu− (black) cells discriminated by co-transfection with a GFP-expressing plasmid is shown compared with an isotype control (gray), with data shown representative of three independent experiments. The wild-type 2_87 Vpu sequence is shown, with mutants detailed above each cytometry plot.
Vpu is known to downregulate host CD4 and tetherin molecules by distinct mechanisms, involving β-TrCP-dependent misdirection from the endoplasmic reticulum or AP/clathrin-dependent endocytic sorting, respectively (Magadán et al., 2010; Jia et al., 2014; Kueck et al., 2015). To evaluate the effect on HLA-C downregulation of specific mutations within the Vpu gene known to affect CD4 or tetherin modulation, we tested mutants identified previously in the context of Vpu from the primary HIV-1 virus 2_87. Substitution of conserved serines at positions 53 and 57 of Vpu is known to abrogate reduction of CD4, whereas tetherin antagonism is only partially reduced (Kueck et al., 2015; Schubert and Strebel, 1994). Mirroring the effect on tetherin antagonism, HLA-C downregulation was impaired but not abrogated by substitution of serine to alanine residues at positions 53 and 57 of Vpu from 2_87 (Figure 3D). Additional mutations of Vpu from 2_87 that reduce tetherin antagonism include substitutions of A15W23 to L15A23, L42I43R45I46 to alanines, N55E56 to H55G56, and E63L67V68 to alanines (Kueck et al., 2015; Pickering et al., 2014), of which all except the last impair reduction of HLA-C (Figure 3D). These data suggest that HLA-C is downregulated by a mechanism more similar to that of the tetherin than CD4 antagonism.
Distinctions in Viral Targeting of HLA-C Compared with HLA-A/B
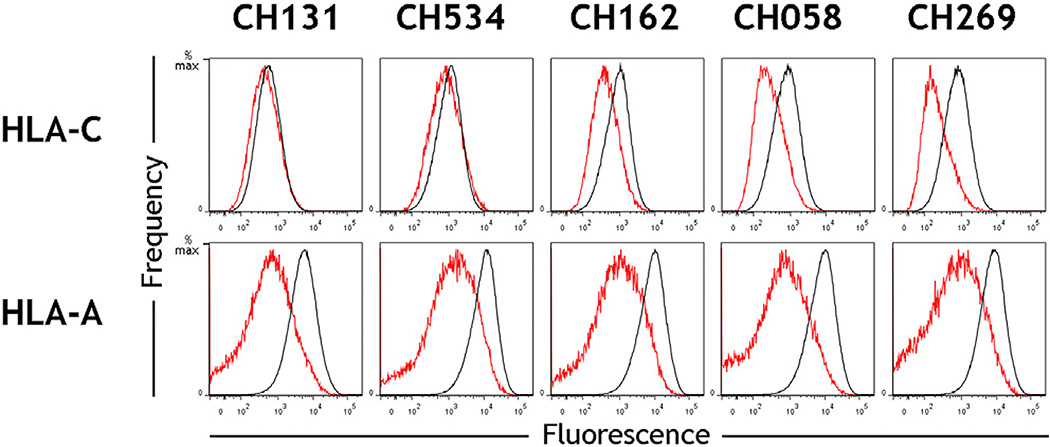
HIV-1 has evolved to subvert host resistance by degenerate and flexible mechanisms, as exemplified by the ability of both Nef and Vpu to downregulate CD4. The possibility that Vpu in addition to Nef in primary viruses can downregulate HLA-A and -B, was tested by infection of primary CD4+ cells with a mutant of the WITO molecular clone lacking Nef expression. This clone demonstrated negligible downregulation of HLA-A/B, whereas HLA-C was reduced to the same extent as the primary WITO clone (Figure 4), indicating that Vpu is specific in downregulating HLA-C and that it does not target HLA-A/B. Thus, Nef downregulation of HLA-A/B and Vpu downregulation of HLA-C represent non-overlapping viral immune evasion mechanisms that are further differentiated by the observation that primary HIV-1 viruses differ substantially in the magnitude by which they reduce HLA-C (Figure 1B), with a range far more pronounced than is observed for the reduction of HLA-A/B by common variants of Nef (Figure 5; Mann et al., 2013).
Figure 4. Vpu Downregulates HLA-C Specifically and Not HLA-B or HLA-A.
Primary CD4+ cells were infected in vitro with the HIV molecular clone WITO (top) or a mutant of WITO that does not express Nef (bottom). Cytometry staining of HLA-A, HLA-B, or HLA-C is shown for infected (red) and uninfected cells (black) and for an isotype control (gray). Infected cells within cultures were discriminated by co-staining for the viral protein Gag. CD4 cells were derived from donors homozygous for the A*02:01, B*44:02, C*0501 haplotype. See also Figure S5.
Figure 5. Primary HIV-1 Clones Show Greater Variation in Downregulation of HLA-C Than HLA-A.
Primary CD4+ cells were infected in vitro with the HIV-1 primary molecular clones CH131, CH534, CH162, CH058, and CH269. Flow cytometry staining of HLA-C (top) or HLA-A (bottom) is shown for infected cells (red) and uninfected cells (black), which were discriminated within a culture by co-staining of the viral protein Gag. Data are representative of experiments using CD4 cells from donors with multiple HLA-A and -C genotypes.
Viral Downregulation of HLA-C Impairs T Cell Inhibition of Viral Replication
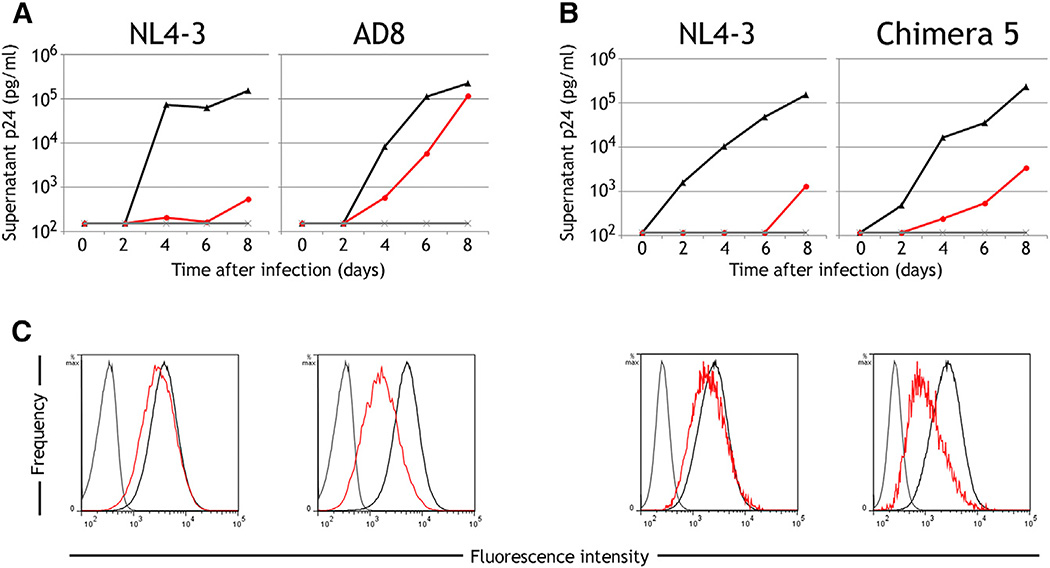
Vpu-mediated downregulation of HLA-C may represent a mechanism of escape from CTL analogous to that of Nef in response to HLA-A/-B-restricted CTL activity. This possibility was tested by determining the effect of HLA-C reduction on the ability of CTL clones derived from HIV-infected patients to suppress viral replication in vitro. An HIV-specific HLA-C restricted CTL clone poorly inhibited replication of AD8, an HIV clone that efficiently downregulates HLA-C after infection of primary CD4+ T cells, whereas this same CTL clone strongly inhibited replication of NL4-3, the laboratory-adapted virus that does not downregulate HLA-C (Figures 6A and 6C). Similar results were seen when comparing NL4-3 to chimera 5 (characterized in Figure 2A and Figure S4), a pair of viruses that differ by only 20 single nucleotide polymorphisms, where the CTL clone inhibited NL4-3 more effectively than it did the chimera that is able to downregulate HLA-C (Figures 6B and 6C).
Figure 6. Viral Downregulation of HLA-C Impairs T Cell Inhibition of Viral Replication In Vitro.
(A and B) Primary CD4+ cells expressing HLA-C* 03:04 were infected in vitro with the HIV-1 molecular clones NL4-3 and AD8 (A) or NL4-3 and chimera 5, the mutant of NL4-3 containing a 149-bp segment substituted from WITO (B). Viral replication quantified over the course of 8 days is shown for CD4 cells cultured alone (black), cocultured with an Env-specific HLA-C*03-restricted CTL clone at a CTL:CD4 ratio of 1:4 (red) and uninfected cultures (gray). Results are representative of CTL:CD4 ratios from 1:1 to 1:20 and four replicate experiments using multiple CD4 donors.
(C) Flow cytometry staining of HLA-C on infected (red) or uninfected cells (black) and an isotype control (gray) is shown for each of the above experiments at day 6 post-infection, with infected cells in a culture discriminated by co-staining the viral protein Gag.
See also Figure S4.
HIV-2 Clones Can Also Downregulate HLA-C Although HIV-2 Lacks a Vpu Gene
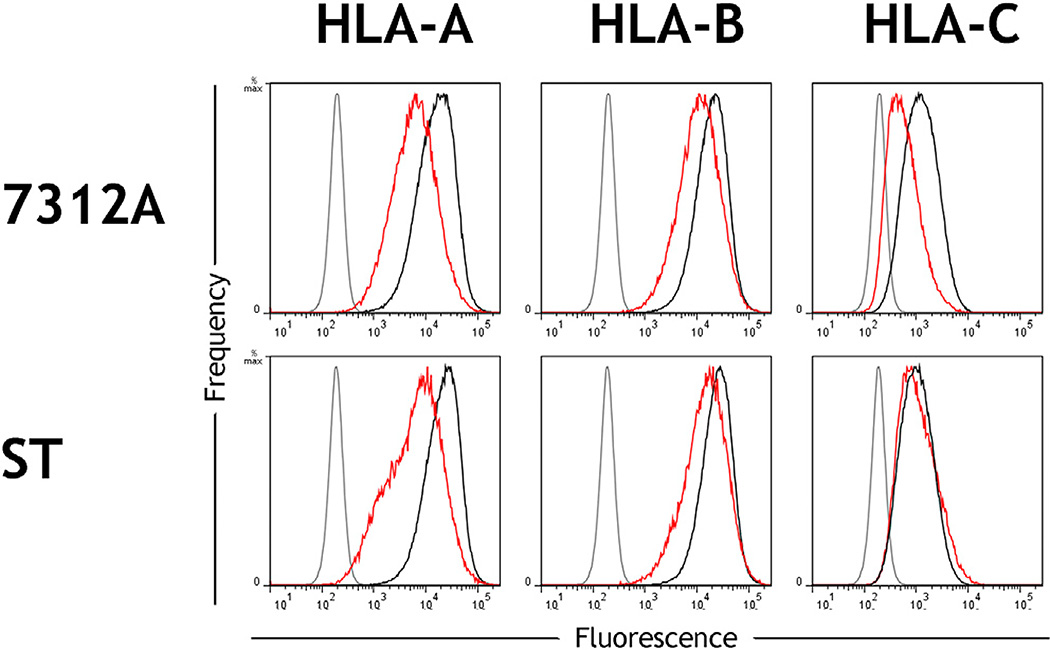
As with HIV-1, Nef alleles from HIV-2 downregulate HLA-A/B but not HLA-C (DeGottardi et al., 2008). Having shown that Vpu from multiple primary HIV-1 clones downregulates HLA-C (Figure 2), we tested whether HIV-2 was capable of modulating HLA-C. The HIV-2 genome does not encode Vpu, but other HIV-2 proteins are adapted to perform certain functions of HIV-1 Vpu, such as tetherin antagonism and, likely, also CD4 downregulation (Le Tortorec and Neil, 2009). Infectious molecular clones of two HIV-2 isolates, 7312A and ST, were tested for their ability to downregulate HLA-C. In vitro infection of primary CD4+ cells showed that 7312A but not ST downregulated HLA-C, whereas both viruses demonstrated moderate downregulation of HLA-A and -B (Figure 7). Downregulation of HLA-C by 7312A indicates a Vpu-independent mechanism by which HIV can downregulate HLA-C. The distinction in ability of 7312A but not ST to downregulate HLA-C may reflect the variation observed in modulation of HLA-C between primary HIV-1 clones.
Figure 7. HLA-C Can Be Downregulated by Clones of HIV-2.
Primary CD4+ cells were infected in vitro with the HIV-2 molecular clones 7312A or ST. Flow cytometry staining of HLA-A, HLA-B, or HLA-C is shown for infected (red) or uninfected cells (black) and an isotype control (gray). Infected cells within cultures were discriminated by CD4 downregulation among CD3+ CD8− cells. CD4 cell donors were homozygous for the A*02:01, B*44:02, C*0501 haplotype.
DISCUSSION
The characterization of HLA modulation by primary HIV clones described here refines the widely held view that HIV specifically downregulates HLA-A/B to diminish CTL recognition of virally infected cells, while leaving HLA-C expression intact as a means of inhibiting NK cells by interaction with inhibitory receptors for self HLA-C (Paul, 2012). In this respect, study of the laboratory-adapted HIV-1 virus NL4-3, which has long been propagated in vitro in the absence of immune-selective pressure and does not downregulate HLA-C, has been misleading to some extent. Most primary HIV-1 clones from a panel including transmitted founder viruses and viruses of multiple HIV-1 subtypes demonstrated the ability to downregulate HLA-C in infected primary CD4+ cells. We determined, using several primary HIV-1 strains, that the viral Vpu protein is responsible for HLA-C downregulation.
Mutations of naturally polymorphic residues implicate a role for the N-terminal region of Vpu in HLA-C reduction. Despite substantial sequence divergence between subtype B and C viruses in this region, Vpu-mediated HLA-C reduction can occur across primary strains of these subtypes. Notably, specific naturally occurring amino acid variants in the N-terminal region of Vpu that affect the differential ability to downregulate HLA-C (Figure 3) are located within peptides known to bind HLA alleles (Ternette et al., 2016), suggesting that the ability of HIV-1 to modulate HLA-C could be altered over the course of viral adaptation to certain CTL responses. Vpu is an accessory molecule known to manipulate the host immune defense by downregulation of multiple cellular proteins such as tetherin, the HLA-related molecule CD1d, and CD4 (Willey et al., 1992; Neil et al., 2008; Moll et al., 2010). Analyses using loss-of-function Vpu mutants suggest that HLA-C is downregulated by a mechanism more similar to that of tetherin than CD4 antagonism. Tetherin antagonism by Vpu is AP/clathrin-dependent, showing parallels to the downregulation of HLA-A/B by Nef (Jia et al., 2012). Whereas Nef recruits AP-1 and clathrin to HLA-A/B for endocytic degradation, Vpu may induce a similar process for HLA-C.
Downregulation of HLA-C by HIV is likely to be physiologically significant and of benefit to the virus, as supported by convergent evolution in the ability of HIV-1 and HIV-2 to downregulate HLA-C using distinct viral proteins. Differences in HLA-C expression levels have been shown to influence the outcome of HIV-1 infection at a population level where natural variation in HLA-C expression levels across individuals associates inversely with viral load in the absence of anti-retroviral therapy (Apps et al., 2013). Higher HLA-C expression levels also correlate with greater frequencies of HLA-C-associated CTL responses and a higher degree of viral mutation (Apps et al., 2013; Blais et al., 2012), illustrating the immune pressure that higher HLA-C expression exerts on the virus. These findings likely explain the observation that a variant marking HLA-C expression level (Fellay et al., 2007; Thomas et al., 2009) had the strongest (Pereyra et al., 2010) and second strongest (Fellay et al., 2007) host genome-wide effects on viral control in two large studies. That higher HLA-C expression levels associate with viral control is consistent with HIV targeting HLA-C for downregulation. Increasing numbers of HLA-C-restricted CTL epitopes are being defined for HIV-1 (Makadzange et al., 2010; Honda et al., 2011; Stoll et al., 2015), some of which could drive the selection for HLA-C downregulation. We show here that Vpu-mediated downregulation of HLA-C in HIV-1-infected cells inhibited CTL control of viral replication in vitro in a manner that mirrors the effects observed for Nef-mediated downregulation of HLA-A/B on CTL killing of infected cells (Collins et al., 1998; Adnan et al., 2006). The impact of MHC class I downregulation by Nef in vivo has been confirmed by individuals who are infected with rare forms of HIV-1 that lack Nef and spontaneously control the virus in the absence of anti-retroviral therapy (Deacon et al., 1995; Kirchhoff et al., 1995). Rhesus monkeys infected with simian immunodeficiency virus (SIV) containing a Nef mutation that specifically abrogates MHC downregulation without disrupting Nef-mediated modulation of other host proteins initially demonstrated increased CTL responses, followed by complex evolution of Nef to regain the ability to downregulate MHC class I (Swigut et al., 2004).
We find that Vpu from the primary HIV-1 clone WITO is specific in downregulating HLA-C and does not downregulate HLA-A/B. Vpu could interact with several transmembrane and cytoplasmic residues that discriminate HLA-C alleles from HLA-A and -B (Figure S5). A distinct viral mechanism for targeting HLA-C relative to HLA-A/B may have evolved as a result of immune pressure conferred by unique properties of HLA-C, such as its function as a ligand for both T cell receptors and NK cell receptors in virtually all individuals. Although viruses unable to downregulate HLA-A/B have been identified (Bonaparte and Barker, 2004), it is notable that primary HIV-1 clones showed more common variation in downregulation of HLA-C than what was observed for the downregulation of HLA-A in a comprehensive study of 360 primary Nef variants (Mann et al., 2013). HLA-A/B are dominant CTL ligands throughout infection (Llano et al., 2009), consistently showing stronger allelic effects in HIV-1 disease compared with HLA-C (Carrington and O’Brien, 2003), so that fixed downregulation of HLA-A/B molecules by Nef is likely advantageous to the virus. In contrast, the virus may benefit from a flexible, dynamic mechanism for HLA-C regulation that can be varied depending on the prevailing immune pressure through HLA-C at any given time (i.e., inhibition of NK cells by preservation of HLA-C versus evasion of CTL by downregulation of HLA-C). The unique characteristics between HLA-A/B versus HLA-C downregulation by HIV-1 may thus reflect the distinct biological functions of these class I molecules. Evolution of the Vpu gene over the course of infection may occur in response to a fluctuating host immune response mediated by HLA-C. Alternatively, modulation of HLA-C may be fixed in a given individual if host factors such as genetic background determine whether the virus benefits more by decreasing HLA-C expression or maintaining normal physiological levels of HLA-C. In this regard, it will be of interest to determine whether the host KIR genotype associates with the downregulation of HLA-C by HIV-1. Notably, associations between Vpu sequence variants and the host KIR2DL2/3 genotype have already been identified (Alter et al., 2011).
Revision of the prevailing model in which HIV evades both CTL and NK cell immune responses through selective downregulation of HLA loci is required and must now take into account mechanisms by which HIV-infected cells evade innate immune cells when HLA-C expression is downregulated (Shah et al., 2010; Matusali et al., 2012). The findings also pose broader biological questions regarding our understanding of NK function, such as differences in the magnitude or breadth of their responses as a consequence of the continuum of differential HLA-C expression levels.
EXPERIMENTAL PROCEDURES
Ethics Statement
This study was approved by the local institutional review boards, and all donors gave informed consent.
HLA Class I Antibodies
Four mAbs that recognize HLA-C were used: mAbs DT9 and WK 4C11, which have been described previously (Thomas et al., 2009; Hiby et al., 2010), and mAbs TRA 4G9 and VP 6G3, provided by Dr. A. Mulder (University of Leiden), which were generated from hybridomas of B cells isolated from the peripheral blood of multiparous women (Mulder et al., 2010). Where not otherwise specified, the antibody used for HLA-C staining was mAb DT9. mAbs PA2.1 (Parham and Bodmer, 1978) and 22E-1 (Tahara et al., 1990) bind specifically to HLA-A and -B, respectively, in subjects homozygous for the A*02:01, B*44:02, C*0501 haplotype (Apps et al., 2015) and were supplied by Drs. N. Holmes and I. Smith (University of Cambridge). mAb W6/32 (Barnstable et al., 1978) reacts with all HLA class I alleles, mAb BBM.1 (Brodsky et al., 1979) binds β-2-microglobulin, and mAb MEM-E/08 (Menier et al., 2003) recognizes HLA-E. These mAbs were purchased from Serotec, Santa Cruz Biotechnology, and Abcam, respectively. All HLA mAbs were characterized for reactivity against 97 common classical HLA class I alleles using commercially available beads coated with individual HLA allotypes as described previously (Apps et al., 2009). mAbs binding HLA-C were further characterized for reactivity with HLA-E as described in the Supplemental Experimental Procedures.
Primary Infectious Molecular Clones of HIV
The HIV-1 clone NL4-3, generated as a chimera from two viral isolates after both were subjected to prolonged in vitro culture (Adachi et al., 1986), was obtained from Dr. R. Gorelick (Frederick National Laboratory). HIV-1 isolate clones AD8 (Theodore et al., 1996) and JRFL (O’Brien et al., 1990), generated after brief in vitro culture in primary CD4+ cells, were obtained from Drs. M. Martin (National Institute of Allergy and Infectious Disease [NIAID]) and Y. Koyanagi (Kyoto University) respectively. The following primary transmitted founder and chronic HIV-1 clones were inferred from plasma viral sequences as reported previously: AD17 (Li et al., 2010); CH040, CH058, WITO, and RHPA (Ochsenbauer et al., 2012); ZM246F (Salazar-Gonzalez et al., 2009); CH131, CH162, and CH269 (Parrish et al., 2013); CH457 and CH534 (Parrish et al., 2012); and 191859D (Baalwa et al., 2013). Clones of the primary HIV-2 isolates 7312A (Gao et al., 1994) and ST (Kong et al., 1988) have been reported previously.
Mutant Infectious Molecular Clones of HIV-1
A mutant of the AD8 clone in which Nef was experimentally deleted by a 4-bp insertion, resulting in missense after 74 residues and premature truncation (Theodore et al., 1996), was supplied by Dr. A. Mergia (University of Florida). Mutants of WITO were generated that were either unable to express Vpu (because of substitution of A to C in the Vpu initiation codon, which is known to abrogate Vpu expression in the primary HIV-1 isolate YU-2 (Doehle et al., 2012)), or unable to express Nef (because of substitution of AT to TA in the Nef initiation codon). EcoRI-BamHI or BamHI-BlpI fragments of WITO containing Vpu or Nef initiation codons, respectively, were cloned into pBR322, and mutations were made using the Q5 site-directed mutagenesis kit (New England Biolabs) before transfer of the cloned fragment back into the full-length WITO genome. Construction of chimeras of the NL4-3 and WITO clones is described in the Supplemental Experimental Procedures. All PCR amplifications used Platinum Pfx DNA polymerase (Invitrogen), and all restriction enzymes were from New England Biolabs. Plasmids were amplified in the E. coli strain Stbl2 (Invitrogen), and the sequences of the full regions manipulated were confirmed by Sanger sequencing (Applied Biosystems 3730) using dedicated primers for each construct.
Infection of Primary Cells In Vitro with HIV
Leukocytes were isolated by density gradient separation from peripheral blood freshly drawn from healthy donors and positively selected using anti-CD4 mAb-based magnetic selection (EasySep) to attain a purity exceeding 95% CD4+ with less than 1% CD8+ cells, as assessed by cytometry staining with L120-phycoerythrin (PE) and SK1-FITC (both Becton Dickinson). CD4+ cell preparations were expanded for 3–5 days in RPMI medium (Quality Biological) supplemented with 100 U/ml IL-2 (PeproTech), anti-CD3/28 beads (Invitrogen), fetal bovine serum (FBS) (Lonza) and penicillin, streptomycin, and glutamine (PSG) (Invitrogen) before infection. HEK293T cells (ATCC, CRL-11268) were transfected with plasmids containing primary or mutated infectious molecular clones using TransIT-293 transfection reagent (Mirus) according to the manufacturer’s instructions. 48 hr post-transfection, virus-containing supernatants were clarified by centrifugation and stored at −80°C, with the infectious titer of thawed supernatants determined using TZM-Bl cells (Morcock et al., 2008). Infection was performed by incubation of cells with virus at a nominal MOI of 0.1 for 4–6 hr at 37°C. Cells were then washed and cultured in RPMI medium with IL-2 for a further 6 or 7 days before analysis by cytometry. Cells were incubated with unlabeled primary antibody to HLA or isotype controls, followed by PE-conjugated anti-mouse immunoglobulin G (IgG) (Sigma-Aldrich). Free secondary antibody-binding sites were blocked with murine immunoglobulin before further staining with CD4-PB (BioLegend), CD8-APC and CD3-APC.Cy7 (both from Becton Dickinson), and yellow fluorescent reactive viability dye (Invitrogen). Cells were then fixed and permeabilized by incubation with paraformaldehyde and saponin (Becton Dickinson) before staining of HIV-1 intracellular Gag with KC57-fluorescein isothiocyanate (FITC) (Beckman Coulter). Cells infected with HIV-2 were identified by CD4 downregulation as described previously (Apps et al., 2015), with CD3+ CD8− CD4− populations >20% of infected cultures compared with <2% of uninfected cultures. Staining results were acquired using an LSRII flow cytometer (Becton Dickinson), with analysis performed using FlowJo (Tree Star).
Transfection of HeLa Cells with Full-Length HIV Genomes
HeLa cells (ATCC, CCL-2) cultured in DMEM (Quality Biological) supplemented with FBS (Lonza) and PSG (Invitrogen) were transfected with plasmids containing full-length genomes of primary or mutated HIV using the TransIT-HeLaMONSTER transfection kit (Mirus) according to the manufacturer’s instructions and analyzed by cytometry after 48 hr of culture. Trypsinized cells were incubated with mAb DT9 or isotype control, followed by PE-conjugated anti-mouse IgG (Sigma-Aldrich) before fixation and permeabilization by incubation with paraformaldehyde and saponin (Becton Dickinson) and then staining of intracellular Gag with KC57-FITC (Beckman Coulter). Staining results were acquired using an LSRII flow cytometer (Becton Dickinson), with analysis performed using FlowJo (Tree Star).
Transfections with Vpu or Nef Clones
A Rev-dependent vector has been described previously to express Vpu genes from the HIV-1 clones NL4-3 and WITO and the primary HIV-1 isolate RP2v16_2_87 (abbreviated 2_87) (Pickering et al., 2014; Zennou et al., 2004). Cloning of additional Vpu genes and their mutation in this vector is described in the Supplemental Experimental Procedures. HeLa cells cultured as described above were co-transfected with a Vpu clone and EGFP-expressing plasmid (Pickering et al., 2014) using the TransIT-HeLaMONSTER transfection kit (Mirus) according to the manufacturer’s instructions and analyzed by cytometry after 24 hr of culture. Trypsinized cells were incubated with mAb DT9 or isotype control followed by APC-conjugated anti-mouse IgG (BioLegend), and staining results were acquired using a FACSCalibur flow cytometer (Becton Dickinson), with analysis performed using FlowJo (Tree Star). Transfection of 721.221 cells with Nef clones is described in the Supplemental Experimental Procedures.
Inhibition of In Vitro Infection with CTL Clones
HIV-1-specific CTL clones were obtained by limiting dilution cloning from the peripheral blood of HIV-1-infected individuals and characterized for epitope specificity and HLA restriction as described previously (Yang et al., 1997; Walker et al., 1989). The specific clone used, 161jC64, recognizes an HLA-C*03-restricted Env epitope, RAIEAQQHL, that is conserved in the AD8, NL4-3, and chimera 5 viruses. CTL clones were cultured with 50 U/ml IL-2 and maintained by restimulation every 2 weeks with anti-CD3 monoclonal antibody (40 ng/ml) and a feeder population of human peripheral blood mononuclear cells and human Epstein-Barr virus-transformed B cells, both irradiated at 12,500 rad, with other details as described previously (Minang et al., 2008). Suppression of acute in vitro infection by HIV-1-specific clones was assayed as described previously (Adnan et al., 2006). Briefly, primary CD4+ cells isolated from normal donors carrying HLA-C*03:04 and activated as described above were infected at a nominal MOI of 0.01 for 4–6 hr at 37°C. Cells were then washed and cultured with CTLs at an effector-target ratio of 1:20, 1:4, or 1:1 in 24-well plates (5 × 105 target cells in 2 ml medium). Supernatant samples taken at 2-day intervals were stored frozen until analysis by quantitative p24 ELISA was performed (Advanced Bioscience Laboratories) to determine viral replication. The specificity of the viral suppression observed is indicated by viral inhibition increasing with the number of effector cells used, absence of inhibition by CTL clone 161jC64 against the WITO virus lacking an RAIEAQQHL sequence, and absence of inhibition by this CTL when using CD4 cell donors lacking HLA-C*03.
Supplementary Material
In Brief.
HIV-1 Nef downregulates HLA-A and -B but not HLA-C molecules on infected cells, presumably allowing viral subversion of CTL responses while preserving NK cell inhibition. Apps et al. determine that Vpu in primary HIV clones downregulates HLA-C, collectively resulting in decreased expression of all classical HLA class I molecules by HIV.
Highlights.
Most primary HIV-1 clones downregulate HLA-C, in contrast to lab-adapted strains
Vpu is responsible for HLA-C downregulation in viruses of multiple HIV-1 subtypes
Primary HIV-1 clones frequently vary in their magnitude of HLA-C reduction
HIV-2 shows a Vpu-independent mechanism of HLA-C downregulation
Acknowledgments
We thank Dr. K. Strebel (NIAID) for providing informative viral constructs. This project has been funded in part with federal funds from the Frederick National Laboratory for Cancer Research under contract no. HHSN261200800001E and the Center for HIV/AIDS Vaccine Immunology (U19 AI067854). This Research was supported in part by the Intramural Research Program of the NIH, Frederick National Laboratory, Center for Cancer Research. Z.L.B. was supported by a Scholar Award from the Michael Smith Foundation for Health Research. M.A.B. holds a Canada Research Chair in Viral Pathogenesis and Immunity.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, five figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.chom.2016.04.005.
AUTHOR CONTRIBUTIONS
R.A., G.D.P., R.T., J.D.L., and M.C. designed the study and prepared the manuscript. Z.L.B., M.A.B., S.N., S.P., D.K.S., A.P.T., B.D.W., G.M.S., B.H.H., and B.F.K. generated primary samples. R.A., G.D.P., P.C., and A.L. performed all other experiments.
REFERENCES
- Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adnan S, Balamurugan A, Trocha A, Bennett MS, Ng HL, Ali A, Brander C, Yang OO. Nef interference with HIV-1-specific CTL antiviral activity is epitope specific. Blood. 2006;108:3414–3419. doi: 10.1182/blood-2006-06-030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter G, Heckerman D, Schneidewind A, Fadda L, Kadie CM, Carlson JM, Oniangue-Ndza C, Martin M, Li B, Khakoo SI, et al. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature. 2011;476:96–100. doi: 10.1038/nature10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. Human leukocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009;127:26–39. doi: 10.1111/j.1365-2567.2008.03019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Qi Y, Carlson JM, Chen H, Gao X, Thomas R, Yuki Y, Del Prete GQ, Goulder P, Brumme ZL, et al. Influence of HLA-C expression level on HIV control. Science. 2013;340:87–91. doi: 10.1126/science.1232685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Meng Z, Del Prete GQ, Lifson JD, Zhou M, Carrington M. Relative expression levels of the HLA class-I proteins in normal and HIV-infected cells. J. Immunol. 2015;194:3594–3600. doi: 10.4049/jimmunol.1403234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baalwa J, Wang S, Parrish NF, Decker JM, Keele BF, Learn GH, Yue L, Ruzagira E, Ssemwanga D, Kamali A, et al. Molecular identification, cloning and characterization of transmitted/founder HIV-1 subtype A, D and A/D infectious molecular clones. Virology. 2013;436:33–48. doi: 10.1016/j.virol.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Bhattacharya T, Daniels M, Heckerman D, Foley B, Frahm N, Kadie C, Carlson J, Yusim K, McMahon B, Gaschen B, et al. Founder effects in the assessment of HIV polymorphisms and HLA allele associations. Science. 2007;315:1583–1586. doi: 10.1126/science.1131528. [DOI] [PubMed] [Google Scholar]
- Blais ME, Zhang Y, Rostron T, Griffin H, Taylor S, Xu K, Yan H, Wu H, James I, John M, et al. High frequency of HIV mutations associated with HLA-C suggests enhanced HLA-C-restricted CTL selective pressure associated with an AIDS-protective polymorphism. J. Immunol. 2012;188:4663–4670. doi: 10.4049/jimmunol.1103472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaparte MI, Barker E. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood. 2004;104:2087–2094. doi: 10.1182/blood-2004-02-0696. [DOI] [PubMed] [Google Scholar]
- Brodsky FM, Bodmer WF, Parham P. Characterization of a monoclonal anti-beta 2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur. J. Immunol. 1979;9:536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- Carrington M, O’Brien SJ. The influence of HLA genotype on AIDS. Annu. Rev. Med. 2003;54:535–551. doi: 10.1146/annurev.med.54.101601.152346. [DOI] [PubMed] [Google Scholar]
- Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- Davis ZB, Cogswell A, Scott H, Mertsching A, Boucau J, Wambua D, Le Gall S, Planelles V, Campbell KS, Barker E. A Conserved HIV-1-Derived Peptide Presented by HLA-E Renders Infected T-cells Highly Susceptible to Attack by NKG2A/CD94-Bearing Natural Killer Cells. PLoS Pathog. 2016;12:e1005421. doi: 10.1371/journal.ppat.1005421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker DJ, McPhee DA, Greenway AL, Ellett A, Chatfield C, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- DeGottardi MQ, Specht A, Metcalf B, Kaur A, Kirchhoff F, Evans DT. Selective downregulation of rhesus macaque and sooty mangabey major histocompatibility complex class I molecules by Nef alleles of simian immunodeficiency virus and human immunodeficiency virus type 2. J. Virol. 2008;82:3139–3146. doi: 10.1128/JVI.02102-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehle BP, Chang K, Rustagi A, McNevin J, McElrath MJ, Gale M., Jr Vpu mediates depletion of interferon regulatory factor 3 during HIV infection by a lysosome-dependent mechanism. J. Virol. 2012;86:8367–8374. doi: 10.1128/JVI.00423-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, Weale M, Zhang K, Gumbs C, Castagna A, Cossarizza A, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogli M, Mavilio D, Brunetta E, Varchetta S, Ata K, Roby G, Kovacs C, Follmann D, Pende D, Ward J, et al. Lysis of endogenously infected CD4+ T cell blasts by rIL-2 activated autologous natural killer cells from HIV-infected viremic individuals. PLoS Pathog. 2008;4:e1000101. doi: 10.1371/journal.ppat.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Yue L, Robertson DL, Hill SC, Hui H, Biggar RJ, Neequaye AE, Whelan TM, Ho DD, Shaw GM, et al. Genetic diversity of human immunodeficiency virus type 2: evidence for distinct sequence subtypes with differences in virus biology. J. Virol. 1994;68:7433–7447. doi: 10.1128/jvi.68.11.7433-7447.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, Claas FH, Walker JJ, Redman CW, Morgan L, et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J. Clin. Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Zheng N, Murakoshi H, Hashimoto M, Sakai K, Borghan MA, Chikata T, Koyanagi M, Tamura Y, Gatanaga H, et al. Selection of escape mutant by HLA-C-restricted HIV-1 Pol-specific cytotoxic T lymphocytes carrying strong ability to suppress HIV-1 replication. Eur. J. Immunol. 2011;41:97–106. doi: 10.1002/eji.201040841. [DOI] [PubMed] [Google Scholar]
- Howcroft TK, Strebel K, Martin MA, Singer DS. Repression of MHC class I gene promoter activity by two-exon Tat of HIV. Science. 1993;260:1320–1322. doi: 10.1126/science.8493575. [DOI] [PubMed] [Google Scholar]
- Jia X, Singh R, Homann S, Yang H, Guatelli J, Xiong Y. Structural basis of evasion of cellular adaptive immunity by HIV-1 Nef. Nat. Struct. Mol. Biol. 2012;19:701–706. doi: 10.1038/nsmb.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Weber E, Tokarev A, Lewinski M, Rizk M, Suarez M, Guatelli J, Xiong Y. Structural basis of HIV-1 Vpu-mediated BST2 antagonism via hijacking of the clathrin adaptor protein complex 1. eLife. 2014;3:e02362. doi: 10.7554/eLife.02362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkau T, Bacik I, Bennink JR, Yewdell JW, Húnig T, Schimpl A, Schubert U. The human immunodeficiency virus type 1 (HIV-1) Vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J. Exp. Med. 1997;185:1295–1305. doi: 10.1084/jem.185.7.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- Kong LI, Lee SW, Kappes JC, Parkin JS, Decker D, Hoxie JA, Hahn BH, Shaw GM. West African HIV-2-related human retrovirus with attenuated cytopathicity. Science. 1988;240:1525–1529. doi: 10.1126/science.3375832. [DOI] [PubMed] [Google Scholar]
- Kueck T, Foster TL, Weinelt J, Sumner JC, Pickering S, Neil SJ. Serine Phosphorylation of HIV-1 Vpu and Its Binding to Tetherin Regulates Interaction with Clathrin Adaptors. PLoS Pathog. 2015;11:e1005141. doi: 10.1371/journal.ppat.1005141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S, Savan R, Qi Y, Gao X, Yuki Y, Bass SE, Martin MP, Hunt P, Deeks SG, Telenti A, et al. Differential microRNA regulation of HLA-C expression and its association with HIV control. Nature. 2011;472:495–498. doi: 10.1038/nature09914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tortorec A, Neil SJ. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 2009;83:11966–11978. doi: 10.1128/JVI.01515-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Bar KJ, Wang S, Decker JM, Chen Y, Sun C, Salazar-Gonzalez JF, Salazar MG, Learn GH, Morgan CJ, et al. High Multiplicity Infection by HIV-1 in Men Who Have Sex with Men. PLoS Pathog. 2010;6:e1000890. doi: 10.1371/journal.ppat.1000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano A, Frahm N, Brander C. How to Optimally Define Optimal Cytotoxic T Lymphocyte Epitopes in HIV Infection? In: Yusim K, Brander H, Koup M, Walker W, editors. HIV Molecular Immunology. New Mexico: Los Alamos National Laboratory; 2009. pp. 3–24. [Google Scholar]
- Magadán JG, Pérez-Victoria FJ, Sougrat R, Ye Y, Strebel K, Bonifacino JS. Multilayered mechanism of CD4 downregulation by HIV-1 Vpu involving distinct ER retention and ERAD targeting steps. PLoS Pathog. 2010;6:e1000869. doi: 10.1371/journal.ppat.1000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makadzange AT, Gillespie G, Dong T, Kiama P, Bwayo J, Kimani J, Plummer F, Easterbrook P, Rowland-Jones SL. Characterization of an HLA-C-restricted CTL response in chronic HIV infection. Eur. J. Immunol. 2010;40:1036–1041. doi: 10.1002/eji.200939634. [DOI] [PubMed] [Google Scholar]
- Mann JK, Byakwaga H, Kuang XT, Le AQ, Brumme CJ, Mwimanzi P, Omarjee S, Martin E, Lee GQ, Baraki B, et al. Ability of HIV-1 Nef to downregulate CD4 and HLA class I differs among viral subtypes. Retrovirology. 2013;10:100. doi: 10.1186/1742-4690-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusali G, Potestà M, Santoni A, Cerboni C, Doria M. The human immunodeficiency virus type 1 Nef and Vpu proteins downregulate the natural killer cell-activating ligand PVR. J. Virol. 2012;86:4496–4504. doi: 10.1128/JVI.05788-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menier C, Saez B, Horejsi V, Martinozzi S, Krawice-Radanne I, Bruel S, Le Danff C, Reboul M, Hilgert I, Rabreau M, et al. Characterization of monoclonal antibodies recognizing HLA-G or HLA-E: new tools to analyze the expression of nonclassical HLA class I molecules. Hum. Immunol. 2003;64:315–326. doi: 10.1016/s0198-8859(02)00821-2. [DOI] [PubMed] [Google Scholar]
- Minang JT, Barsov EV, Yuan F, Trivett MT, Piatak M, Jr, Lifson JD, Ott DE, Ohlen C. Efficient inhibition of SIV replication in rhesus CD4+ T-cell clones by autologous immortalized SIV-specific CD8+ T-cell clones. Virology. 2008;372:430–441. doi: 10.1016/j.virol.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Moll M, Andersson SK, Smed-Sörensen A, Sandberg JK. Inhibition of lipid antigen presentation in dendritic cells by HIV-1 Vpu interference with CD1d recycling from endosomal compartments. Blood. 2010;116:1876–1884. doi: 10.1182/blood-2009-09-243667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CB, John M, James IR, Christiansen FT, Witt CS, Mallal SA. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science. 2002;296:1439–1443. doi: 10.1126/science.1069660. [DOI] [PubMed] [Google Scholar]
- Morcock DR, Thomas JA, Sowder RC, 2nd, Henderson LE, Crise BJ, Gorelick RJ. HIV-1 inactivation by 4-vinylpyridine is enhanced by dissociating Zn(2+) from nucleocapsid protein. Virology. 2008;375:148–158. doi: 10.1016/j.virol.2008.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder A, Kardol MJ, Arn JS, Eijsink C, Franke ME, Schreuder GM, Haasnoot GW, Doxiadis II, Sachs DH, Smith DM, Claas FH. Human monoclonal HLA antibodies reveal interspecies crossreactive swine MHC class I epitopes relevant for xenotransplantation. Mol. Immunol. 2010;47:809–815. doi: 10.1016/j.molimm.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- O’Brien WA, Koyanagi Y, Namazie A, Zhao JQ, Diagne A, Idler K, Zack JA, Chen IS. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- O’huigin C, Kulkarni S, Xu Y, Deng Z, Kidd J, Kidd K, Gao X, Carrington M. The molecular origin and consequences of escape from miRNA regulation by HLA-C alleles. Am. J. Hum. Genet. 2011;89:424–431. doi: 10.1016/j.ajhg.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsenbauer C, Edmonds TG, Ding H, Keele BF, Decker J, Salazar MG, Salazar-Gonzalez JF, Shattock R, Haynes BF, Shaw GM, et al. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J. Virol. 2012;86:2715–2728. doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat. Rev. Immunol. 2005;5:201–214. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- Parham P, Bodmer WF. Monoclonal antibody to a human histocompatibility alloantigen, HLA-A2. Nature. 1978;276:397–399. doi: 10.1038/276397a0. [DOI] [PubMed] [Google Scholar]
- Parrish NF, Wilen CB, Banks LB, Iyer SS, Pfaff JM, Salazar-Gonzalez JF, Salazar MG, Decker JM, Parrish EH, Berg A, et al. Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin α4β7. PLoS Pathog. 2012;8:e1002686. doi: 10.1371/journal.ppat.1002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish NF, Gao F, Li H, Giorgi EE, Barbian HJ, Parrish EH, Zajic L, Iyer SS, Decker JM, Kumar A, et al. Phenotypic properties of transmitted founder HIV-1. Proc. Natl. Acad. Sci. USA. 2013;110:6626–6633. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. Fundamental Immunology. Seventh. Lippincott Williams & Wilkins; 2012. [Google Scholar]
- Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI, Walker BD, Ripke S, Brumme CJ, Pulit SL, Carrington M, et al. International HIV Controllers Study. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330:1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersdorf EW, Gooley TA, Malkki M, Bacigalupo AP, Cesbron A, Du Toit E, Ehninger G, Egeland T, Fischer GF, Gervais T, et al. International Histocompatibility Working Group in Hematopoietic Cell Transplantation. HLA-C expression levels define permissible mismatches in hematopoietic cell transplantation. Blood. 2014;124:3996–4003. doi: 10.1182/blood-2014-09-599969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering S, Hué S, Kim EY, Reddy S, Wolinsky SM, Neil SJ. Preservation of tetherin and CD4 counter-activities in circulating Vpu alleles despite extensive sequence variation within HIV-1 infected individuals. PLoS Pathog. 2014;10:e1003895. doi: 10.1371/journal.ppat.1003895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapaksa US, Li D, Peng YC, McMichael AJ, Dong T, Xu XN. HLA-B may be more protective against HIV-1 than HLA-A because it resists negative regulatory factor (Nef) mediated down-regulation. Proc. Natl. Acad. Sci. USA. 2012;109:13353–13358. doi: 10.1073/pnas.1204199109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H, Decker JM, Wang S, Baalwa J, Kraus MH, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 2009;206:1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert U, Strebel K. Differential activities of the human immunodeficiency virus type 1-encoded Vpu protein are regulated by phosphorylation and occur in different cellular compartments. J. Virol. 1994;68:2260–2271. doi: 10.1128/jvi.68.4.2260-2271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O, Maréchal V, Le Gall S, Lemonnier F, Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- Shah AH, Sowrirajan B, Davis ZB, Ward JP, Campbell EM, Planelles V, Barker E. Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell Host Microbe. 2010;8:397–409. doi: 10.1016/j.chom.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht A, Telenti A, Martinez R, Fellay J, Bailes E, Evans DT, Carrington M, Hahn BH, Goldstein DB, Kirchhoff F. Counteraction of HLA-C-mediated immune control of HIV-1 by Nef. J. Virol. 2010;84:7300–7311. doi: 10.1128/JVI.00619-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll A, Bergmann S, Mummert C, Mueller-Schmucker SM, Spriewald BM, Harrer EG, Harrer T. Identification of HLA-C restricted, HIV-1-specific CTL epitopes by peptide induced upregulation of HLA-C expression. J. Immunol. Methods. 2015;418:9–18. doi: 10.1016/j.jim.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Swigut T, Alexander L, Morgan J, Lifson J, Mansfield KG, Lang S, Johnson RP, Skowronski J, Desrosiers R. Impact of Nef-mediated downregulation of major histocompatibility complex class I on immune response to simian immunodeficiency virus. J. Virol. 2004;78:13335–13344. doi: 10.1128/JVI.78.23.13335-13344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara T, Yang SY, Khan R, Abish S, Hämmerling GJ, Hämmerling U. HLA antibody responses in HLA class I transgenic mice. Immunogenetics. 1990;32:351–360. doi: 10.1007/BF00211650. [DOI] [PubMed] [Google Scholar]
- Ternette N, Yang H, Partridge T, Llano A, Cedeño S, Fischer R, Charles PD, Dudek NL, Mothe B, Crespo M, et al. Defining the HLA class I-associated viral antigen repertoire from HIV-1-infected human cells. Eur. J. Immunol. 2016;46:60–69. doi: 10.1002/eji.201545890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore TS, Englund G, Buckler-White A, Buckler CE, Martin MA, Peden KWC. Construction and characterization of a stable full-length macrophage-tropic HIV type 1 molecular clone that directs the production of high titers of progeny virions. AIDS Res. Hum. Retroviruses. 1996;12:191–194. doi: 10.1089/aid.1996.12.191. [DOI] [PubMed] [Google Scholar]
- Thomas R, Apps R, Qi Y, Gao X, Male V, O’huigin C, O’Connor G, Ge D, Fellay J, Martin JN, et al. HLA-C cell surface expression and control of HIV/AIDS correlate with a variant upstream of HLA-C. Nat. Genet. 2009;41:1290–1294. doi: 10.1038/ng.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BD, Flexner C, Birch-Limberger K, Fisher L, Paradis TJ, Aldovini A, Young R, Moss B, Schooley RT. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA. 1989;86:9514–9518. doi: 10.1073/pnas.86.23.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey RL, Maldarelli F, Martin MA, Strebel K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 1992;66:7193–7200. doi: 10.1128/jvi.66.12.7193-7200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang OO, Kalams SA, Trocha A, Cao H, Luster A, Johnson RP, Walker BD. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 1997;71:3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zennou V, Perez-Caballero D, Göttlinger H, Bieniasz PD. APOBEC3G incorporation into human immunodeficiency virus type 1 particles. J. Virol. 2004;78:12058–12061. doi: 10.1128/JVI.78.21.12058-12061.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.