Abstract
Purpose
The goals of this study were to evaluate the safety of office-based vitreous sampling, and determine the utility of these samples with multiplex cytokine analysis.
Methods
Vitreous samples were collected from office-based needle aspiration and the rate of adverse events during follow-up was reviewed. The vitreous cytokine concentrations in a subset of patients with diabetic macular edema (DME) were analyzed using a 42 plex-cytokine bead array. These results were compared with vitreous cytokine concentrations in proliferative diabetic retinopathy (PDR) and controls (macular hole, epiretinal membrane, symptomatic vitreous floaters) from pars plana vitrectomy.
Results
An adequate volume of vitreous fluid (100–200 μL) was obtained in 52 (88%) of 59 office-based sampling attempts. The average length of follow-up was 300 days (range, 42–926 days). There were no complications, including cataract, retinal tear or detachment, and endophthalmitis. Two patients (3%) had posterior vitreous detachments within 3 months. Vitreous cytokine concentrations were measured in 44 patients: 14 controls, 13 with DME, and 17 with PDR. The concentration of ADAM11, CXCL-10, IL-8, and PDGF-A were higher in PDR compared with controls and DME. The concentration of IL-6 was higher in PDR compared with controls, but not compared with DME.
Conclusions
Office-based vitreous aspiration is safe and yields high-quality samples for multiplex vitreous cytokine analysis. Significant elevations of vitreous cytokines were found in PDR compared with DME and controls, including the novel finding of elevated ADAM11. As such, office-based aspiration is a safe and effective means to identify vitreous factors associated with vitreoretinal disease.
Keywords: vitreous humor, cytokine, diabetic retinopathy, growth factors
Diabetic retinopathy is a common complication occurring in approximately 35% of patients with diabetes.1 Vision loss from diabetic retinopathy primarily occurs from either diabetic macular edema (DME) or proliferative diabetic retinopathy (PDR). The role of VEGF has been established in the pathogenesis of both DME and PDR.2 The intravitreal use of anti-VEGF drugs, such as bevacizumab, ranibizumab, and aflibercept, is standard practice in the management of patients with diabetic retinopathy, but there are limitations. Many patients are refractory to anti-VEGF pharmacotherapy or demonstrate only a partial response despite multiple, frequent injections.3–5 The reasons for inadequate response are currently poorly understood. It has been suggested that poor responders to anti-VEGF therapy may have additional inflammatory mediators that need to be targeted in addition to VEGF.6
The study of inflammatory mediators in the human vitreous has been gaining increasing attention. Several reports have examined vitreous proteomic changes in diseases such as diabetic retinopathy, AMD, retinal vein occlusion, and retinal detachment.2,7–15 Although human vitreous could serve as an important source of biomarkers for the diagnosis and management of vitreoretinal disease, further clinical application of vitreous cytokine analysis has been limited by two factors: (1) practical approaches to sample intraocular fluid, and (2) methods to analyze very small volume samples. Previous studies have primarily used operating room–based pars plana vitrectomy to obtain vitreous specimen.2,7–15 Attempts to examine cytokine and growth factors levels from more easily accessible anterior chamber samples do not reliably reflect those levels found in the vitreous.16 For vitreous cytokine analysis to be established as a part of the diagnosis and management of patients, a safe and practical approach to obtaining a vitreous sample must be established. One proposed method to more easily sample vitreous fluid is in-office aspiration of vitreous fluid. Giansanti et al.17 reported that microsampling of small volumes (10–15 μL) in rabbit eyes is a useful technique in vitreous pharmacokinetic studies. Pfahler et al.18 found very few complications with a large number of in-office vitreous sampling attempts, but did not report on the ability to analyze samples acquired in this manner.
Although avoiding the need for the operating room, in-office vitreous aspiration is limited by the small volume of a safely obtainable sample. The primary methods of analyzing cytokines in biofluid have been ELISA and Western blot, which are limited to single factor analysis. The recent development of multiplexed cytometric bead assay has allowed for broader vitreous cytokine analysis and can be performed on sample volumes as small as 25 μL. There have been a few reports using cytometric bead assay on vitreous samples from patients undergoing vitrectomy.10,13,19 The practicality of in-office vitreous aspiration paired with multiplexed cytometric bead assay, however, may unlock the true power of vitreous analysis in our understanding of vitreoretinal disease. The clinical potential of proteomic analyses is within reach, but like any clinical tool, must first be validated. The present report examines the safety of office-based vitreous sampling techniques and the feasibility of using this fluid for multiplexed cytokine analysis.
Methods
This study was a prospective case series at the University of Michigan Kellogg Eye Center conducted between November 2011 and May 2015. This study was approved by the University of Michigan Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. Every patient in the study had a vitreoretinal condition (Table 1) that required intraocular injection or surgical intervention. Informed consent was obtained from all subjects undergoing in-office vitreous aspiration after explanation of the nature and possible consequences of the study. Vitreous samples also were collected in the operating room before clinically indicated vitrectomy as part of a larger protocol establishing a vitreous biorepository at the University of Michigan. After vitreous sampling by either method, the patients were observed for follow-up as clinically indicated by their underlying condition. The charts for follow-up office visits were reviewed and all safety and adverse events were recorded.
Table 1.
Patient Characteristics of Office-Based Vitreous Aspiration

Office-Based Vitreous Sampling
Topical anesthesia was initiated with a drop of proparacaine 1% followed by placement of cotton-tip applicators soaked in lidocaine 4% solution on the superotemporal conjunctiva. Subconjunctival lidocaine 1% was infiltrated in the area and the eyelashes and upper and lower eyelids were gently scrubbed with two 10% povidone-iodine sticks. A sterile speculum was placed between the lid fissures with sterile gloves. Another 10% povidone-iodine stick was swabbed over conjunctiva of the superotemporal quadrant and a sterile caliper was used to mark 4.0 mm and 3.5 mm posterior to the corneoscleral limbus for phakic and pseudophakic patients, respectively. A 25-gauge, 5/8-inch needle was introduced through the marked site into the midvitreous cavity. A maximum of 0.2 mL vitreous fluid was gently aspirated into a 1-mL syringe (Supplementary Video S1). If clinically warranted, the patient immediately underwent injection of 0.05 to 0.1 mL therapeutic agent near the same area as vitreous sampling (e.g., an anti-VEGF agent or steroid injection). Optic nerve perfusion was confirmed by counting fingers vision and the patient was discharged without topical antibiotics. Each vitreous sample was stored in a −80°C freezer until cytokine analysis.
Operating Room–Based Vitreous Sampling
The patient was taken to the operating room at the Kellogg Eye Center and either general anesthesia was induced or posterior sub-Tenon's local anesthetic (2% lidocaine mixed 50/50 with 0.5% Marcaine) was injected. Trocars of 23, 25, or 27 gauge were used to place an inferotemporal infusion cannula and another superotemporal cannula 4.0 mm and 3.5 mm posterior to the corneoscleral limbus for phakic and pseudophakic patients, respectively. With the infusion off, a microvitrector was used to cut in the midvitreous cavity while an assistant applied gentle aspiration to an attached 3-mL syringe. Approximately 0.2 mL vitreous fluid was collected, after which the position of the infusion line was checked and the infusion initiated. Any immediate complications from vitreous sampling were recorded. The vitreous fluid was stored in a −80°C freezer until cytokine analysis and the remainder of the vitreoretinal surgical procedure was then performed as planned.
Cytokine Analysis
To examine the feasibility of cytometric bead assay analysis of office-based vitreous aspirates, three groups of patients were identified for comparison. A subset of vitreous samples from both the office-based and operating room–based groups was selected for cytokine analysis. Thirteen patients with DME had vitreous aspirated in the office. Seventeen patients with PDR and 14 age-matched controls without DME or PDR had vitreous sampling in the operating room by vitrectomy. Control patients were defined as those undergoing vitrectomy for a diagnosis of epiretinal membrane, symptomatic vitreous floater, or macular hole. Cytokines and growth factors were assessed from each vitreous sample using 42-plex cytometric bead immunoassay (EMD Millipore, Darmstadt, Germany) according to the manufacturer's instruction manual. Each sample was assessed using 25 μL vitreous in triplicate. The mean concentration of each cytokine was compared among groups with Kruskal-Wallis tests, and P values were adjusted by Holms method for multiple comparisons. Post hoc pairwise group comparisons were performed using 2-sample Wilcoxon tests.
Results
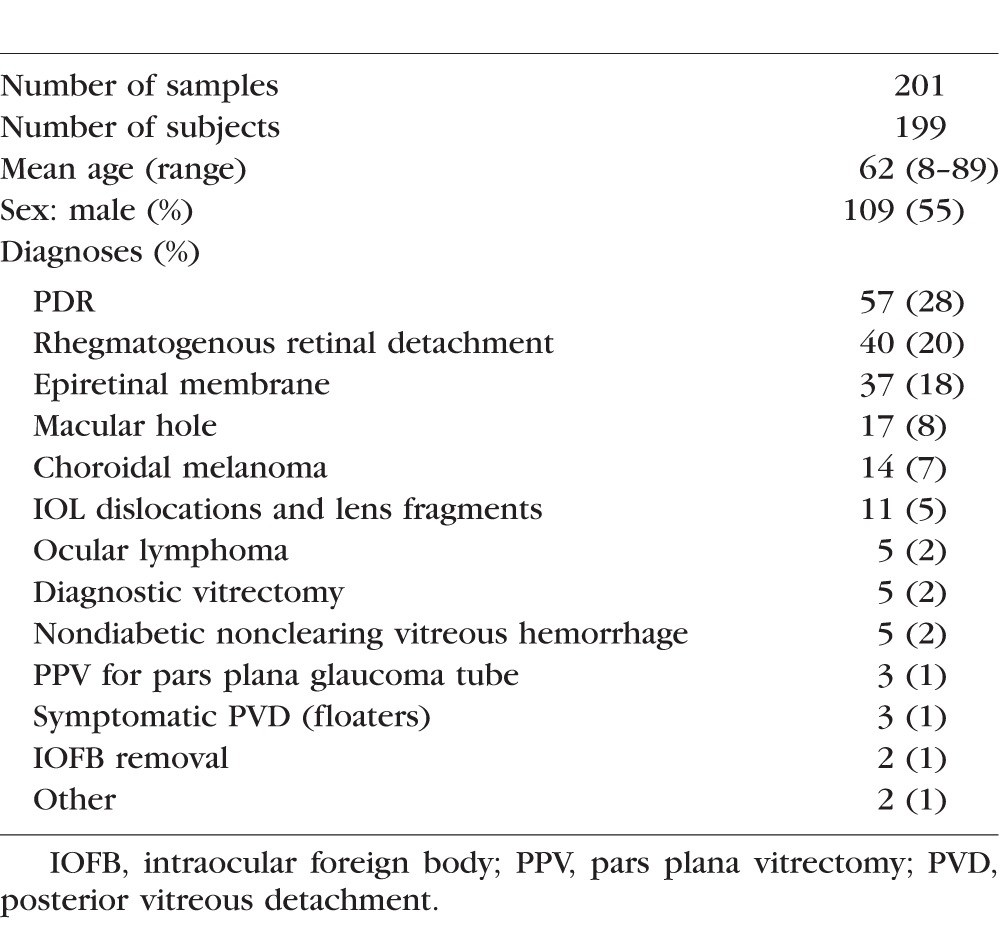
A total of 264 vitreous samplings were attempted: 63 from office-based sampling and 201 from operating room–based sampling. Of the office-based group, four patients with choroidal melanoma were excluded due to not having follow-up. Three patients had the eye enucleated and one patient did not return for follow-up. Fifty-nine office-based vitreous aspirations from 48 patients were included for safety and feasibility analysis. Of these 48 patients, 38 patients had one office-based vitreous sample, 9 patients had two samples at different visits, and 1 patient had three samples at different visits. Twenty-eight (58%) were male and the average age was 61 (range, 24–90). Thirty-three eyes (69%) were phakic and 15 (31%) were pseudophakic. Patient demographics and underlying vitreoretinal diagnoses of subjects in the office-based group are listed in Table 1.
A total of 201 operating room–based vitreous samples were performed in 199 patients. Two patients with PDR had both eyes included. Of this group, 109 (55%) were male and the average age was 62 (range, 8–89). The underlying vitreoretinal diagnoses for which study subjects were being treated are presented in Table 2.
Table 2.
Patient Characteristics of Operating Room–Based Vitreous Sampling
Of the 59 office-based vitreous samples included with follow-up, 52 (88%) had adequate volume of aspirated vitreous. There were four “dry” samples (7%) in which vitreous fluid could not be aspirated, and three samples (5%) in which only a very small volume could be obtained. Of the successful 52 samples, 9 (17%) had a prior posterior vitreous detachment documented. Of the seven inadequate samples, four (57%) had a prior posterior vitreous detachment documented. This was a statistically significant association by Fischer's exact test (P = 0.0360). For all office-based vitreous samples, the average length of follow-up was 300 days (range, 42–926 days; SD, 176 days).
There were no documented instances of cataract, retinal tear, retinal detachment, or endophthalmitis in patients undergoing vitreous aspiration. No patients with “dry” or inadequate vitreous sampling attempt had any complications. There were two (3%) patients with posterior vitreous detachments documented within 3 months after vitreous aspiration. One patient was found to have asymptomatic posterior vitreous detachment 2 months after vitreous aspiration. The other patient presented 14 days after vitreous aspiration with complaints of flashing photopsias and a new floater in the study eye. Both patients had no retinal tears or detachments on complete dilated fundus examination with scleral depression, and continued to do well without complications after 3 and 13 months of follow-up, respectively. Four patients (7%) had new posterior vitreous detachment documented between 3 and 6 months. Five patients (8%) had new posterior vitreous detachment documented between 6 and 12 months. All patients with new posterior vitreous detachment after 3 months had additional intravitreal injections after vitreous aspiration. No retinal tears or detachments were noted in any patients with new documented posterior vitreous detachment. Of the 11 patients with new posterior vitreous detachment within 12 months of vitreous aspiration, 9 (82%) were asymptomatic.
There were no intraoperative complications from vitreous sampling during operating room–based procedures. There were no additional retinal breaks or signs of hypotony, such as corneal striae, choroidal folds, or choroidal detachment.
Vitreous cytokines were measured from 44 patients in three groups: DME (n = 13), PDR (n = 17), and controls (n = 14). All 13 DME samples were obtained by office-based vitreous aspiration, and the 17 PDR samples and 14 controls (epiretinal membrane, symptomatic vitreous floaters, and macular holes) were obtained by office-based vitrectomy. Subjects were selected chronologically by date of vitreous sampling from each group for inclusion in the subset of patients undergoing cytokine analysis. The cytokines included in the multiplex bead assay were the following: chemokine ligand 2 (CCL2/monocyte chemotactic protein 1), CCL3, CCL4, CCL5, CCL7 (monocyte chemoattractant protein 3), C-X-C motif chemokine 1 (CXCL1/growth-regulated alpha protein), C-X-C motif chemokine 10 (CXCL10/IP-10), disintegrin and metalloproteinase domain-containing protein 11 (ADAM11), fractalkine, eotaxin, epidermal growth factor (EGF), FGF, fms-related tyrosine kinase 3 ligand, granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony stimulating factor (GM-CSF), IFN-α-2, INF-gamma, IL-1 receptor antagonist protein, IL-1-α, IL-1-β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17A, platelet-derived growth factor (PDGF)-A, PDGF-B, soluble CD 40 ligand, TGF-α, TNF-α, TNF-β (lymphotoxin-α), and VEGF. The mean concentrations of each cytokine stratified by group (DME, PDR, and control) are shown in Table 3.
Table 3.
Mean Concentrations (pg/mL) and SDs of Vitreous Cytokines Compared Among Control (Macular Holes, Symptomatic Vitreous Floaters, and Epiretinal Membranes), DME, and PDR Patients
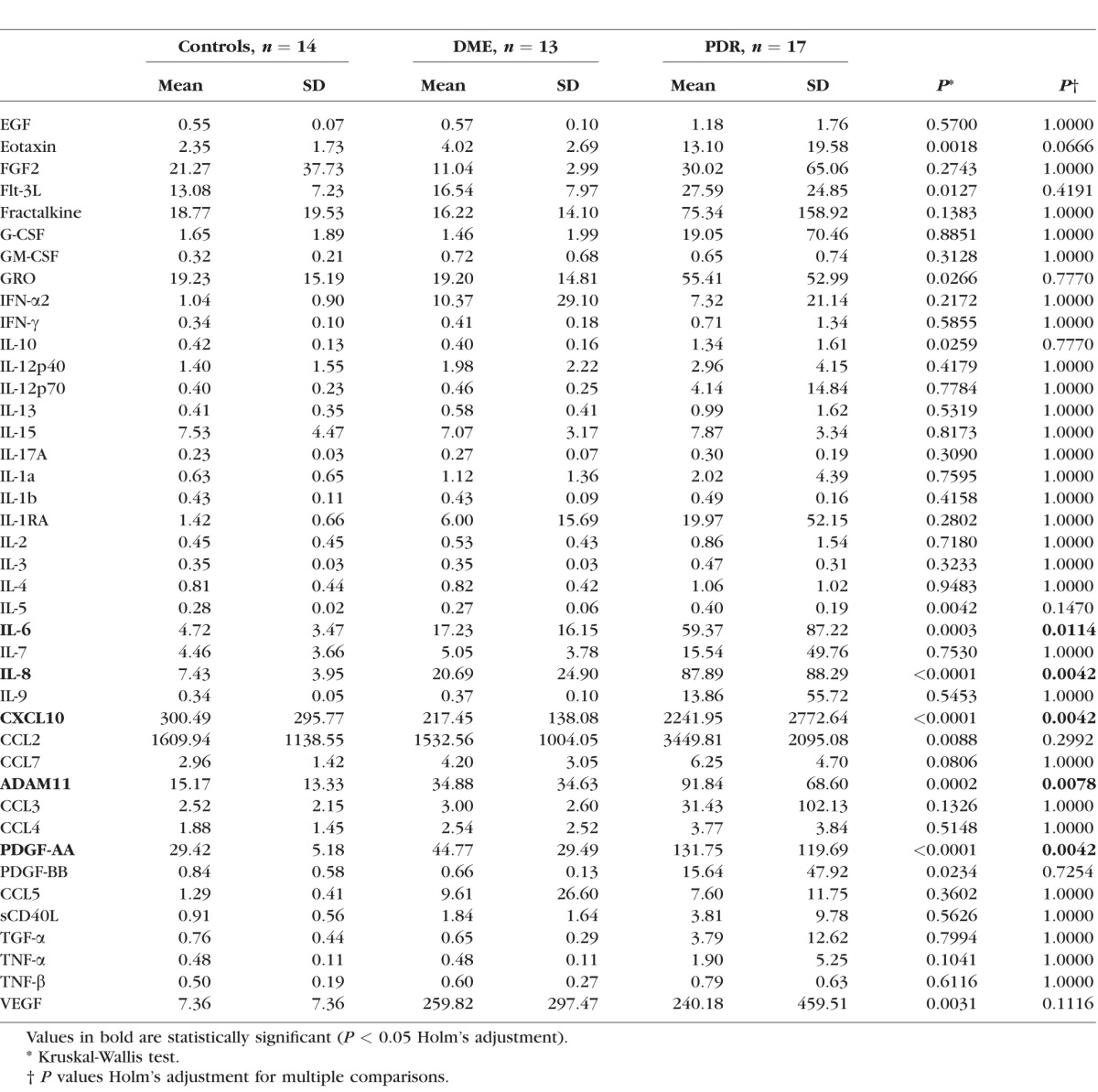
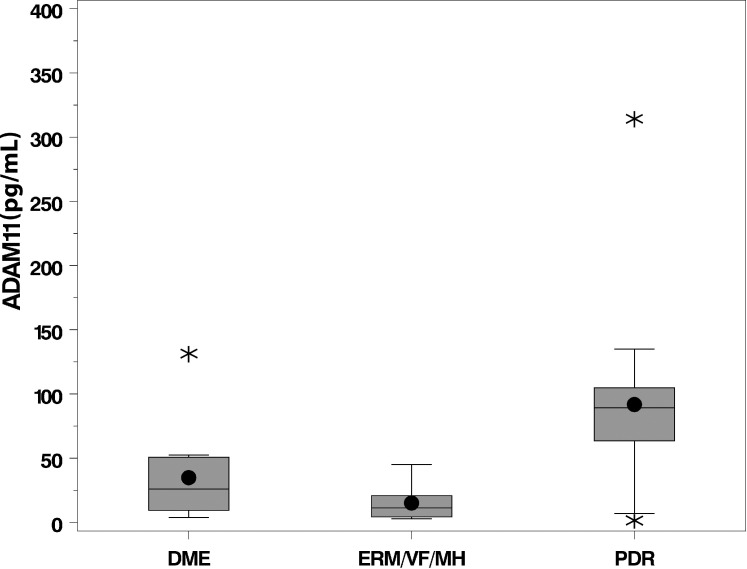
After Holm's adjustment for multiple comparisons, there were statistically significant differences between groups (P < 0.05) observed for five vitreous cytokines: ADAM11, CXCL2, IL-6, IL-8, and PDGF-A. The pairwise comparisons between groups for significant cytokines are shown in Table 4. The vitreous concentrations of each of the five cytokines were significantly higher in patients with PDR compared with controls. In addition, the average concentration of each cytokine except for IL-6 was significantly higher in the PDR group compared with the DME group. The Figure shows a boxplot comparing the distribution of vitreous concentrations of the novel cytokine ADAM11 for each group.
Table 4.
P Values for Pairwise Comparisons Using Wilcoxon 2-Sample Test of Control, DME, and PDR Patients
Figure.
Boxplots comparing the vitreous concentration (pg/mL) of ADAM11 among controls (epiretinal membrane, vitreous floaters, macular hole), DME, and PDR. The interquartile range (IQR), including the lower quartile/25th percentile (bottom of the box) and the upper quartile/75th percentile (top of the box), the median/50th percentile (line within the box), the mean (dot within the box), the lower fence/whisker (smallest observation within 1.5*IQR), the upper fence/whisker (largest observation within 1.5*IQR), and outliers (stars; observations located outside 1.5*IQR).
Discussion
The identification of protein biomarkers in the vitreous could elucidate pathophysiologic mechanisms of vitreoretinal disease and potentially serve as targets for future therapies. The proximity of vitreous body to the retina and its interaction in various disease states provides a unique means of studying the retina itself. Several reports have characterized vitreous cytokines and have shown the concentrations of various inflammatory and angiogenic factors to be altered in a number of disease states.10,11,16,20–23
Beyond its use a research tool, clinical application of proteomic analysis has been limited by a safe method to sample vitreous and a reliable means to analyze small samples. Vitreous can be sampled by two methods: vitreous aspiration of a small volume of liquid vitreous through the pars plana with a small-gauge needle, and acquisition of a larger volume of vitreous liquid and gel with a vitreous cutter at the beginning of pars plana vitrectomy. The former can be conveniently performed in an office setting, but is limited by small volumes of sample and possible increased risk of retinal break or detachment from vitreoretinal traction during aspiration. The latter has the benefit of increased sample volume and lower risk of vitreoretinal traction, but is limited to an operating room setting and is associated with side effects typical of vitrectomy, such as cataract progression. A few reports have examined office-based vitrectomy systems (Intrector [Insight Instruments, Stuart, FL, USA] and VersVIT [Synergetics, O'Fallon, MO, USA]) for various retinal procedures, but these systems have not been widely adopted.24–26 Current reports examining vitreous proteins have mostly used typical techniques of diagnostic pars plana vitrectomy in the operating room to acquire vitreous sample.2,7,10,20,21 Office-based aspiration of vitreous sample for proteomic analysis has less commonly been used.27 Despite theoretical risks of vitreoretinal traction, a large review of in-office vitreous sampling by Pfahler et al.18 showed that aspiration of small volumes was a safe and reproducible procedure. Lobo and Lightman28 reported a similar rate, 92%, of obtaining adequate sample from vitreous aspiration and few complications in diagnosing patients with infectious, malignant, and inflammatory causes of uveitis. Feasibility of proteomic analysis from their vitreous aspiration samples, however, was not discussed.
The second major challenge of cytokine analysis of the vitreous lies in the limitations imposed by small sample volumes. Most current reports have used ELISA or Western blot to quantitatively analyze vitreous proteins. These techniques, however, are limited to single factor analysis and require larger volumes of fluid for each measurement. Polymerase chain reaction can be performed on very small volumes, but is limited to DNA markers. The development of cytometric bead array has allowed the quantification of multiple proteins simultaneously from small sample volumes.29 This technique uses antibody-coated beads and flow-cytometry to capture a broad dynamic range of fluorescence in the measurement of a variety of soluble and intracellular proteins, including cytokines, chemokines, growth factors, and phosphorylated cell-signaling proteins. A few studies have used such methods for proteomic analysis of the vitreous.11,13,14,30–34 Although currently an interesting research tool, the full utility of cytometric bead analysis of the vitreous could be unlocked when paired with more practical office-based means of vitreous sampling. Before adopting a new technique into clinical practice, the safety and feasibility must be examined.
Despite our relatively small study cohort, we found statistically significant differences in certain cytokine concentrations among PDR, DME, and controls. Similar to previous reports, the present study found that patients with PDR have significantly higher levels of IL-6,7,13,33,35–37 IL-8,30,37–41 CXCL10,12,14,33,42,43 and PDGF-AA36,44–49 compared with controls. A novel finding not previously reported in the literature is the significantly higher concentration of ADAM11 in PDR compared with DME and controls; ADAM11 was originally identified as a candidate tumor suppressor gene for breast cancer.50 It is thought to be an adhesion molecule that plays a role in cell-cell or cell–extracellular matrix interactions. It is mainly expressed in the nervous system but has been implicated in a variety of biological processes including fertilization, muscle development, and neurogenesis. Takahashi and colleagues51 described learning and motor coordination deficiencies in ADAM11-deficient mice. They also suggested that ADAM11 plays a role in pain transmission and in inflammatory regulation mechanisms underlying changes in the threshold for pain perception.52 At present, there have been no reports associating ADAM11 to diabetes or diabetic retinopathy, but one could reasonably speculate that ADAM11 is involved in the neuropathic changes frequently associated with diabetes.
There are several limitations to this study. This is a small pilot study to assess safety and efficacy of vitreous cytokine analysis through office- versus operating room–based vitreous sampling. Larger randomized studies could help transition office-based vitreous aspiration from a research tool into a standard procedure in the diagnosis and management of vitreoretinal disease, following validation of specific vitreous biomarkers. Second, we did not implement a standardized follow-up protocol in determining complications associated with office-based vitreous sampling. Follow-up intervals were determined by the patient's underlying clinical diagnosis. Despite this, there was an adequate overall length of follow-up with the average patient followed 300 days after office-based vitreous sampling. Third, it is possible that office-based vitreous aspiration may capture only soluble or nonbound proteins, whereas vitreous cutter–based biopsies may gather additional insoluble or bound factors. However, Skeie and colleagues53 found that proteins collected from sequential vitreous needle and vitreous cutter biopsies were nearly equivalent.
In conclusion, this study supports the safety and feasibility of obtaining vitreous by office-based aspiration for analysis with cytometric bead array. Our results show a very low rate of complications from in-office vitreous sampling of a small volume of fluid with the method described. A single patient experienced an uncomplicated acute posterior vitreous detachment. The procedure was well tolerated and there was sufficient length of follow-up to rule out complications, such as endophthalmitis, retina tear or detachment, and cataract. At present, we believe that office-based vitreous aspiration can be a safe, effective way to acquire vitreous fluid for research. With increasing insight into the role of vitreous biomarkers, office-based vitreous sampling paired with multiplexed cytokine analysis could one day be clinically useful in diagnosing, monitoring, and treating vitreoretinal disease, such as diabetic retinopathy.
Supplementary Material
Acknowledgments
The authors acknowledge Grant Comer, MD, for collection of operating room–based vitrectomy samples.
Supported by Michigan Eye Bank, EY 20582, Physician Scientist Award from Research to Prevent Blindness, and the Taubman Institute.
Disclosure: D.H. Ghodasra, None; R. Fante, None; T.W. Gardner, None; M. Langue, None; L.M. Niziol, None; C. Besirli, None; S.R. Cohen, None; V.S. Dedania, None; H. Demirci, None; N. Jain, None; K.T. Jayasundera, None; M.W. Johnson, None; P.S. Kalyani, None; R.C. Rao, None; D.N. Zacks, None, J.M. Sundstrom, None
References
- 1. Yau JWY,, Rogers SL,, Kawasaki R,, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012; 35: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aiello L,, Avery R,, Arrigg P. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331: 1480–1487. [DOI] [PubMed] [Google Scholar]
- 3. Dobrogowska DH,, Lossinsky AS,, Tarnawski M,, Vorbrodt AW. Increased blood–brain barrier permeability and endothelial abnormalities induced by vascular endothelial growth factor. J Neurocytol. 1998; 27: 163–173. [DOI] [PubMed] [Google Scholar]
- 4. Nicholson BP,, Schachat AP. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010; 248: 915–930. [DOI] [PubMed] [Google Scholar]
- 5. Wu L,, Martínez-Castellanos MA,, Quiroz-Mercado H,, et al. Twelve-month safety of intravitreal injections of bevacizumab (Avastin): results of the Pan-American Collaborative Retina Study Group (PACORES). Graefes Arch Clin Exp Ophthalmol. 2008; 246: 81–87. [DOI] [PubMed] [Google Scholar]
- 6. Das A,, McGuire PG,, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015; 122: 1375–1394. [DOI] [PubMed] [Google Scholar]
- 7. Funatsu H,, Yamashita H,, Ikeda T,, Mimura T,, Eguchi S,, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003; 110: 1690–1696. [DOI] [PubMed] [Google Scholar]
- 8. Funatsu H,, Yamashita H,, Nakamura S,, et al. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2006; 113: 294–301. [DOI] [PubMed] [Google Scholar]
- 9. Noma H,, Funatsu H,, Mimura T,, Eguchi S,, Shimada K,, Hori S. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Curr Eye Res. 2011; 36: 256–263. [DOI] [PubMed] [Google Scholar]
- 10. Bromberg-White JL,, Glazer L,, Downer R,, Furge K,, Boguslawski E,, Duesbery NS. Identification of VEGF-independent cytokines in proliferative diabetic retinopathy vitreous. Invest Ophthalmol Vis Sci. 2013; 54: 6472–6480. [DOI] [PubMed] [Google Scholar]
- 11. Yoshimura T,, Sonoda K,, Sugahara M,, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009; 4: e8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elner SG,, Strieter R,, Bian ZM,, et al. Interferon-induced protein 10 and interleukin 8. C-X-C chemokines present in proliferative diabetic retinopathy. Arch Ophthalmol. 1998; 116: 1597–1601. [DOI] [PubMed] [Google Scholar]
- 13. Banerjee S,, Savant V,, Scott RAH,, Curnow SJ,, Wallace GR,, Murray PL. Multiplex bead analysis of vitreous humor of patients with vitreoretinal disorders. Invest Ophthalmol Vis Sci. 2007; 48: 2203–2207. [DOI] [PubMed] [Google Scholar]
- 14. Maier R,, Weger M,, Haller-Schober E-M,, et al. Multiplex bead analysis of vitreous and serum concentrations of inflammatory and proangiogenic factors in diabetic patients. Mol Vis. 2008; 14: 637–643. [PMC free article] [PubMed] [Google Scholar]
- 15. Asato R,, Kita T,, Kawahara S,, et al. Vitreous levels of soluble vascular endothelial growth factor receptor (VEGFR)-1 in eyes with vitreoretinal diseases. Br J Ophthalmol. 2011; 95: 1745–1748. [DOI] [PubMed] [Google Scholar]
- 16. Ecker S,, Hines J,, Pfahler S,, Glaser B. Aqueous cytokine and growth factor levels do not reliably reflect those levels found in the vitreous. Mol Vis. 2011; 17: 2856–2863. [PMC free article] [PubMed] [Google Scholar]
- 17. Giansanti F,, Ramazzotti M,, Giuntoli M,, et al. Intravitreal infliximab clearance in a rabbit model: different sampling methods and assay techniques. Invest Ophthalmol Vis Sci. 2009; 50: 5328–5335. [DOI] [PubMed] [Google Scholar]
- 18. Pfahler S,, Brandford A,, Glaser B. A prospective study of in-office diagnostic vitreous sampling in patients with vitreoretinal pathology. Retina. 2009; 29: 2007–2010. [DOI] [PubMed] [Google Scholar]
- 19. Koss MJ,, Pfister M,, Koch FH. Inflammatory and angiogenic protein detection in the human vitreous: cytometric bead assay. J Ophthalmol. 2011; 2011: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davuluri G,, Espina V,, Petricoin EF,, III, et al. Activated VEGF receptor shed into the vitreous in eyes with wet AMD: a new class of biomarkers in the vitreous with potential for predicting the treatment timing and monitoring response. Arch Ophthalmol. 2009; 127: 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noma H,, Funatsu H,, Mimura T,, Eguchi S,, Shimada K,, Hori S. Vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Curr Eye Res. 2011; 36: 256–263. [DOI] [PubMed] [Google Scholar]
- 22. Gariano RF,, Nath AK,, D'Amico DJ,, Lee T,, Sierra-Honigmann MR. Elevation of vitreous leptin in diabetic retinopathy and retinal detachment. Invest Ophthalmol Vis Sci. 2000; 41: 3576–3581. [PubMed] [Google Scholar]
- 23. Koss MJ,, Hoffmann J,, Nguyen N,, et al. Proteomics of vitreous humor of patients with exudative age-related macular degeneration. PLoS One. 2014; 9: e96895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morales-Canton V,, Kawakami-Campos PA. Machines and cutters: VersaVIT—potential and perspectives of office-based vitrectomy. Dev Ophthalmol. 2014; 54: 17–22. [DOI] [PubMed] [Google Scholar]
- 25. Hilton GF,, Josephberg RG,, Halperin LS,, et al. Office-based sutureless transconjunctival pars plana vitrectomy. Retina. 2002; 22: 725–732. [DOI] [PubMed] [Google Scholar]
- 26. Koch FH,, Koss MJ. Microincision vitrectomy procedure using Intrector technology. Arch Ophthalmol. 2011; 129: 1599–1604. [DOI] [PubMed] [Google Scholar]
- 27. Ecker S,, Pfahler S,, Hines J. Sequential in-office vitreous aspirates demonstrate vitreous matrix metalloproteinase 9 levels correlate with the amount of subretinal fluid in eyes with wet age. Mol Vis. 2012; 18: 1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 28. Lobo A,, Lightman S. Vitreous aspiration needle tap in the diagnosis of intraocular inflammation. Ophthalmology. 2003; 110: 595–599. [DOI] [PubMed] [Google Scholar]
- 29. Morgan E,, Varro R,, Sepulveda H,, et al. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol. 2004; 110: 252–266. [DOI] [PubMed] [Google Scholar]
- 30. Yoshida S,, Kubo Y,, Kobayashi Y,, et al. Increased vitreous concentrations of MCP-1 and IL-6 after vitrectomy in patients with proliferative diabetic retinopathy: possible association with postoperative macular oedema. Br J Ophthalmol. 2015; 99: 960–966. [DOI] [PubMed] [Google Scholar]
- 31. Weiss K,, Steinbrugger I,, Weger M,, et al. Intravitreal VEGF levels in uveitis patients and treatment of uveitic macular oedema with intravitreal bevacizumab. Eye (Lond). 2009; 23: 1812–1818. [DOI] [PubMed] [Google Scholar]
- 32. Maier R,, Weger M,, Haller-Schober E-M,, et al. Application of multiplex cytometric bead array technology for the measurement of angiogenic factors in the vitreous. Mol Vis. 2006; 12: 1143–1147. [PubMed] [Google Scholar]
- 33. Suzuki Y,, Nakazawa M,, Suzuki K,, Yamazaki H,, Miyagawa Y. Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn J Ophthalmol. 2011; 55: 256–263. [DOI] [PubMed] [Google Scholar]
- 34. DjobaSiawaya JF,, Roberts T,, Babb C, et al. An evaluation of commercial fluorescent bead-based luminex cytokine assays. PLoS One. 2008; 3: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koskela UE,, Kuusisto SM,, Nissinen AE,, Savolainen MJ,, Liinamaa MJ. High vitreous concentration of IL-6 and IL-8, but not of adhesion molecules in relation to plasma concentrations in proliferative diabetic retinopathy. Ophthalmic Res. 2013; 49: 108–114. [DOI] [PubMed] [Google Scholar]
- 36. Zhou J,, Wang S,, Xia X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res. 2012; 37: 416–420. [DOI] [PubMed] [Google Scholar]
- 37. Chernykh VV,, Varvarinsky EV,, Smirnov EV,, Chernykh DV,, Trunov AN. Proliferative and inflammatory factors in the vitreous of patients with proliferative diabetic retinopathy. Indian J Ophthalmol. 2015; 63: 33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murugeswari P,, Shukla D,, Kim R,, Namperumalsamy P,, Stitt AW,, Muthukkaruppan V. Angiogenic potential of vitreous from proliferative diabetic retinopathy and Eales' disease patients. PLoS One. 2014; 9: e107551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Canataroglu H,, Varinli I,, Ozcan AA,, Canataroglu A,, Doran F,, Varinli S. Interleukin (IL)-6, interleukin (IL)-8 levels and cellular composition of the vitreous humor in proliferative diabetic retinopathy, proliferative vitreoretinopathy, and traumatic proliferative vitreoretinopathy. Ocul Immunol Inflamm. 2005; 13: 375–381. [DOI] [PubMed] [Google Scholar]
- 40. Yuuki T,, Kanda T,, Kimura Y,, et al. Inflammatory cytokines in vitreous fluid and serum of patients with diabetic vitreoretinopathy. J Diabetes Complications. 2001; 15: 257–259. [DOI] [PubMed] [Google Scholar]
- 41. Schwartzman ML,, Iserovich P,, Gotlinger K,, et al. Profile of lipid and protein autacoids in diabetic vitreous correlates with the progression of diabetic retinopathy. Diabetes. 2010; 59: 1780–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wakabayashi Y,, Usui Y,, Okunuki Y,, et al. Correlation of vascular endothelial growth factor with chemokines in the vitreous in diabetic retinopathy. Retina. 2010; 30: 339–344. [DOI] [PubMed] [Google Scholar]
- 43. Nawaz MI,, Van Raemdonck K,, Mohammad G,, et al. Autocrine CCL2, CXCL4, CXCL9 and CXCL10 signal in retinal endothelial cells and are enhanced in diabetic retinopathy. Exp Eye Res. 2013; 109: 67–76. [DOI] [PubMed] [Google Scholar]
- 44. Schoenberger SD,, Kim SJ,, Sheng J,, Rezaei KA,, Lalezary M,, Cherney E. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Invest Ophthalmol Vis Sci. 2012; 53: 5906–5911. [DOI] [PubMed] [Google Scholar]
- 45. Mori K,, Gehlbach P,, Ando A,, et al. Retina-specific expression of PDGF-B versus PDGF-A: vascular versus nonvascular proliferative retinopathy. Invest Ophthalmol Vis Sci. 2002; 43: 2001–2006. [PubMed] [Google Scholar]
- 46. Schoenberger S,, Kim S,, Shah R,, Sheng J,, Cherney E. Reduction of interleukin 8 and platelet-derived growth factor levels by topical ketorolac, 0.45%, in patients with diabetic retinopathy. JAMA Ophthalmol. 2014; 132: 32–37. [DOI] [PubMed] [Google Scholar]
- 47. Praidou A,, Klangas I,, Papakonstantinou E,, et al. Vitreous and serum levels of platelet-derived growth factor and their correlation in patients with proliferative diabetic retinopathy. Curr Eye Res. 2009; 34: 152–161. [DOI] [PubMed] [Google Scholar]
- 48. Freyberger H,, Brocker M,, Yakut H,, et al. Increased levels of platelet-derived growth factor in vitreous fluid of patients with proliferative diabetic retinopathy. Exp Clin Endocrinol Diabetes. 2000; 108: 106–109. [DOI] [PubMed] [Google Scholar]
- 49. Endo H,, Naito T,, Asahara T,, Kajima M,, Shiota H. Cytokines in the vitreous fluid of patients with proliferative diabetic retinopathy—vascular endothelial growth factor and platelet-derived growth factor are elevated in proliferative diabetic retinopathy. Nihon Ganka Gakkai Zasshi. 2000; 104: 711–716. [PubMed] [Google Scholar]
- 50. Emi M,, Katagiri T,, Harada Y,, et al. A novel metalloprotease/disintegrin-like gene at 17q21.3 is somatically rearranged in two primary breast cancers. Nat Genet. 1993; 5: 151–157. [DOI] [PubMed] [Google Scholar]
- 51. Takahashi E,, Sagane K,, Oki T,, Yamazaki K,, Nagasu T,, Kuromitsu J. Deficits in spatial learning and motor coordination in ADAM11-deficient mice. BMC Neurosci. 2006; 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takahashi E,, Sagane K,, Nagasu T,, Kuromitsu J. Altered nociceptive response in ADAM11-deficient mice. Brain Res. 2006; 1097: 39–42. [DOI] [PubMed] [Google Scholar]
- 53. Skeie JM,, Brown EN,, Martinez HD,, et al. Proteomic analysis of vitreous biopsy techniques. Retina. 2012; 32 (10): 2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.