Abstract
Purpose
Hydrogen sulfide (H2S) is an endogenous gaseous signaling molecule with significant pathophysiological importance, but its role in retinal neovascular diseases is unknown. Hydrogen sulfide is generated from L-cysteine by cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), and/or 3-mercaptopyruvate sulfurtransferase (3-MST). The aim of this study was to investigate the role of H2S in retinal neovascularization (NV) in ischemia-induced retinopathy.
Methods
Studies were performed in a murine model of oxygen-induced retinopathy (OIR). Hydrogen sulfide was detected with a fluorescent assay. Western blots and immunohistochemistry were used to assess the changes of H2S-producing enzymes. Gene deletion and pharmacologic inhibition were used to investigate the role of H2S in retinal NV.
Results
Hydrogen sulfide production was markedly increased in retinas from OIR mice compared with those from room air (RA) controls. Cystathionine-β-synthase and CSE were significantly increased in OIR retinas, whereas 3-MST was not changed. Cystathionine-β-synthase was expressed throughout the neuronal retina and upregulated in neurons and glia during OIR. Cystathionine-γ-lyase was also localized to multiple retinal layers. Its immunoreactivity was prominently increased in neovascular tufts in OIR. Pharmacologic inhibition of CBS/CSE or genetic deletion of CSE significantly reduced retinal NV in OIR.
Conclusions
Our data indicate that the H2S-generating enzymes/H2S contributes to retinal NV in ischemia-induced retinopathy and suggest that blocking this pathway may provide novel therapeutic approaches for the treatment of proliferative retinopathy.
Keywords: hydrogen sulfide, neovascularization, oxygen-induced retinopathy
Ischemia-induced pathologic retinal neovascularization (NV) is a common cause of irreversible vision loss, occurring in conditions such as proliferative diabetic retinopathy and retinopathy of prematurity.1–3 Treatment for pathologic retinal NV includes panretinal laser photocoagulation, which destroys neurons to lower the total retinal metabolic demand and alleviate ischemia.3 Intravitreal injections of anti-vascular endothelial growth factor (VEGF) are also used to treat pathologic NV but their therapeutic effect is short-lived.4 In order to develop novel therapeutic strategies for ischemia-induced retinopathy, it is necessary to elucidate the mechanisms and identify novel mediators involved in the progression of retinal pathologic NV.
Hydrogen sulfide (H2S) is a crucial gaseous signaling molecule that is involved in many physiological and pathologic conditions.5–21 Three H2S-generating enzymes, cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST), are responsible for H2S production in various types of tissues.22,23 The role of H2S in retinal pathophysiological changes has been appreciated in the past decade. Studies discovered several protective properties of H2S in a variety of conditions, including protecting retina from light-induced degeneration by suppression of Ca2+ influx,10 attenuating excitotoxic neurotransmission,9 protecting retinal neuronal cell apoptosis against NMDA or retinal ischemia-reperfusion–induced injury via its antioxidative activity,11,12,24 inducing retinal vascular relaxation,25,26 and alleviating retinal oxidative stress and inflammation in a rat model of streptozotocin-induced diabetes.27 In spite of this progress, the function of H2S in neovascular disease in the retina is unknown, although increased plasma and vitreous H2S has been found in patients with proliferative diabetic retinopathy,28 and H2S exhibits proangiogenic property in organs where ischemic vessel growth is beneficial.15–20 In this study, we determined the role of H2S-generating enzymes/H2S pathway in retinal NV using a mouse model of oxygen-induced retinopathy.
Methods
Treatment of Animals
All procedures with animals were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by The University of Texas Medical Branch Institutional Animal Care and Use Committee. CSE−/− mice were generated as described previously.13,17 CSE−/− and CSE+/− littermates were obtained by crossing CSE+/− and CSE−/− mice, and CSE+/− and CSE+/+ littermates were obtained by crossing CSE+/− and CSE+/+ mice. Oxygen-induced retinopathy (OIR) was induced by exposing litters to 70% oxygen from postnatal day (P)7 to P12, followed by a return to room air from P12 to P17. Age-matched C57BL-6 mice were kept in room air. From P12 to P17, the animals were anesthetized and their retinas were collected for morphologic or molecular studies. In a subset of wild-type mice exposed to OIR, a frequently used CBS and CSE inhibitor, aminooxyacetic acid (AOAA)29 (Sigma-Aldrich Corp., St. Louis, MO, USA), or vehicle (saline) was injected (intraperitoneally [IP], 3 mg/kg/day) to littermate pups from P12 to P16. On P17, pups were weighed and killed, and eyes or retinas were prepared for morphologic or molecular studies.
Retinal H2S Detection
Retinal H2S was detected using previously described fluorescent methods with minor modifications.30 Briefly, isolated retinas were homogenized in a nondenaturing lysis buffer (50 mM Tris-HCl pH = 8.0, 150 mM NaCl, 1% Nonidet P-40, 1% Triton X-100, protease inhibitors) on ice for 20 minutes, followed by centrifugation at 14,000g for 15 minutes at 4°C. The protein concentration was determined by bicinchoninic acid (BCA) protein assay (Pierce Biotechnology, Rockford, IL, USA). The reaction mixture contained the protein cell extract, 50 mM Tris-HCl (Sigma-Aldrich Corp.), 1 mM L-cysteine (Sigma-Aldrich Corp.), 1 mM homocysteine (Sigma-Aldrich Corp.), 50 μM pyridoxal-5′-phosphate (MP Biomedicals, Santa Ana, CA, USA), and 10 μM H2S probe 7-azido-4-methylcoumarin (7-Az) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Reactions were incubated for 2 hours at 37°C and fluorescence was measured with a Synergy H1 Hybrid Reader (Bio-tek Instruments, Winooski, VT, USA) using ex = 365 nm, em = 450 nm. A linear standard curve was generated by adding freshly prepared sodium hydrosulfide (NaHS) to the fluorescent assay medium.
Plasma H2S Detection
Animals were anesthetized with isoflurane, and a syringe with 26-gauge needle was inserted into the heart to draw blood for H2S analysis. Plasma (20 μL) was collected after centrifuging and incubated with 7-Az for 15 minutes at 37°C, and then fluorescence was measured with a Synergy H1 Hybrid Reader. Fluorescent product was calculated by subtracting background fluorescence of plasma. Hydrogen sulfide detection was performed in duplicate for each blood sample.
Western Blotting
Isolated retinas were homogenized in radioimmunoprecipitation assay (RIPA) buffer (Millipore, Billerica, MA, USA) supplemented with protease inhibitors (Roche Applied Science, Indianapolis, IN, USA). The lysates were centrifuged to clear debris, and protein concentration was determined by a BCA assay (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Proteins were then separated by SDS-PAGE and blotted onto a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked and incubated overnight at 4°C with primary antibodies against tubulin (1:10,000; Sigma-Aldrich Corp.), CSE (1:1000; PeproTech, Rocky Hill, NJ, USA), CBS (1:1000; Abnova; Walnut, CA, USA), or 3-MST (1:1000; Atlas Antibodies, Stockholm, Sweden). After washing, membranes were incubated with horseradish peroxidase–conjugated secondary antibody (1:3000; Cell Signaling, Danvers, MA, USA). Immunoreactive proteins were detected using the enhanced chemiluminescence system (Thermo Fisher Scientific). Protein levels were quantified via densitometry with ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA).
RNA Isolation and Quantitative Reverse Transcriptase–Polymerase Chain Reaction (qPCR)
Total RNA was extracted from mouse retinas using RNAqueous-4PCR kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Quantitative PCR was performed as described before.31 Primer sequences for mouse transcripts were as follows: angiopoietin-2 For-5′-ACA CCG AGA AGA TGG CAG TGT-3′; angiopoietin-2 Rev-5′-CTC CCG AAG CCC TCT TTG TA-3′; erythropoietin (EPO) For-5′-CCC CCA CGC CTC ATC TG-3′; EPO Rev-5′-TGC CTC CTT GGC CTC TAA GA-3′; VEGF For-5′-TAC CTC CAC CAT GCC AAG TG-3′; VEGF Rev-5′-TCA TGG GAC TTC TGC TCT CCT T-3′; hypoxanthine-guanine phosphoribosyltransferase (Hprt) For-5′-GAA AGA CTT GCT CGA GAT GTC ATG-3′; Hprt Rev-5′-CAC ACA GAG GGC CAC AAT GT-3′. The fold difference in various transcripts was calculated by the ΔΔCT method using Hprt as the internal control.
Immunostaining of Retinal Sections
Eyeballs were fixed in 4% paraformaldehyde for 1 hour on ice, equilibrated in 30% sucrose overnight, and embedded in optical cutting solution temperature compound (Tissue Tek; Sakura Finetek, Torrance, CA, USA). Retinas were cut into 10-μm sections, permeabilized with PBS containing Triton X-100 for 15 minutes at room temperature, and blocked with blocking buffer (BioGenex, San Ramon, CA, USA) for 1 hour. Sections were incubated with Alexa Fluor 594–labeled isolectin B4 (Griffonia simplicifolia; 1:200; Invitrogen) and anti-CSE (1:400) or anti-CBS (1:500) overnight at 4°C. After washing with PBS, sections were incubated with Alexa Fluor 488–labeled goat anti-rabbit secondary antibody (1:1000; Invitrogen) at room temperature for 1 hour. To costain CBS and glial fibrillary acidic protein (GFAP), CBS antibody–labeled sections (red) were incubated with GFAP antibody (1:100; Dako, Carpinteria, CA, USA) conjugated with Alexa Fluor 488 using Zenon Rabbit IgG labeling Kit (Invitrogen) at room temperature for 2 hours. Slides were mounted with mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich Corp.) and examined with a fluorescence microscope (Olympus, Center Valley, PA, USA).The mean fluorescence intensity of CBS staining within the outer nuclear layer (ONL) was measured using ImageJ software and expressed as normalized intensity by subtracting background fluorescence.32 At least four fields were analyzed for each sample.
Immunostaining on Whole-Mount Retinas
The immunostaining in retinas was performed as previously described.31 Briefly, after fixation in 4% paraformaldehyde, retinas were carefully dissected from the choroid and sclera, blocked, and permeabilized in PBS containing 5% goat serum and 1% Triton X-100 for 30 minutes. The retinas were incubated with Alexa 594–labeled isolectin B4 overnight at 4°C, washed with PBS, mounted on microscope slides in mounting medium (Vectashield; Vector Laboratories, Burlingame, CA, USA), and examined with a fluorescence microscope. Vascular obliteration area and neovascular tuft area were quantified in a semiautomated manner that has been described in detail.33
Statistical Analysis
The results are expressed as mean ± SEM. Group differences were evaluated by using post hoc Student's t-test, and results were considered significant at P < 0.05.
Results
H2S Production Is Increased in Retinas From Oxygen-Induced Retinopathy
Studies were performed in a mouse model of OIR, which has been extensively used to investigate the mechanisms of retinal NV as seen in the proliferative stages of ischemia-induced retinopathy such as retinopathy of prematurity and diabetic retinopathy.31 From P7 to P12, hyperoxia (70% oxygen) toxicity causes vaso-obliteration in the central retina. Upon return to room air, the avascular central region becomes hypoxic, upregulating ischemic, angiogenic, and inflammatory mediators that spur the pathologic NV that peaks at P17.34
To determine if H2S is implicated in OIR, we examined H2S production at P17 in retinas from OIR mice and room air (RA) control mice. Retinal lysates were incubated with fluorogenic probe 7-Az, which rapidly reacts with H2S and is reduced to produce the fluorescent 7-amino-4-methylcoumarin (AMC). The intensity of fluorescence linearly correlates with the amount of H2S. This method is now widely used to detect H2S production.30 We observed that there was an average of 111 nmol H2S produced per mg protein in RA control retinas. In comparison, the production of H2S was significantly increased to 448 nmol H2S per mg protein in OIR retinas (Fig. 1).
Figure 1.
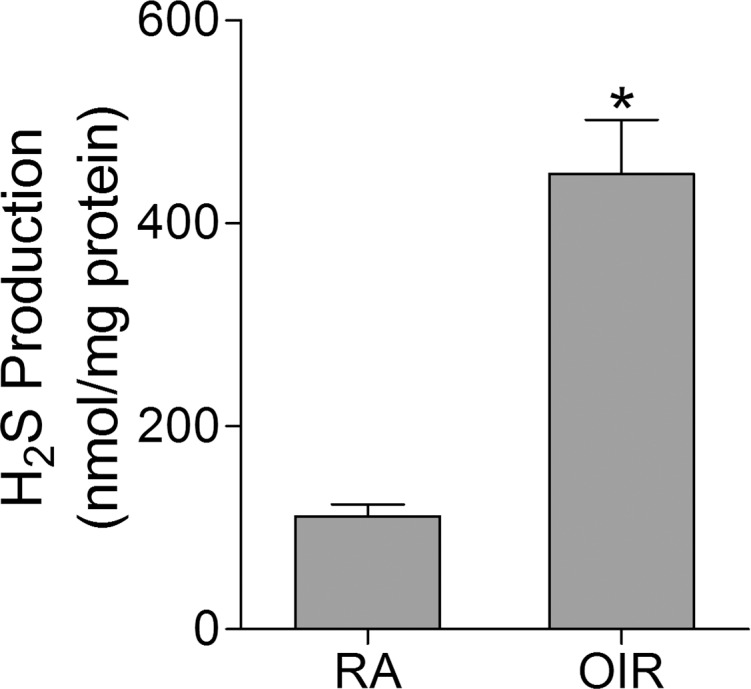
Hydrogen sulfide production is increased in the retinas of OIR. Mice were subjected to OIR or maintained in room air (RA) as control. Retinas were collected at P17, and H2S production was evaluated with a fluorescent assay, using NaHS to generate a linear standard curve (n = 6). *P = 0.0001 compared with RA control.
Expression of H2S-Producing Enzymes Is Altered in Retinas From Oxygen-Induced Retinopathy
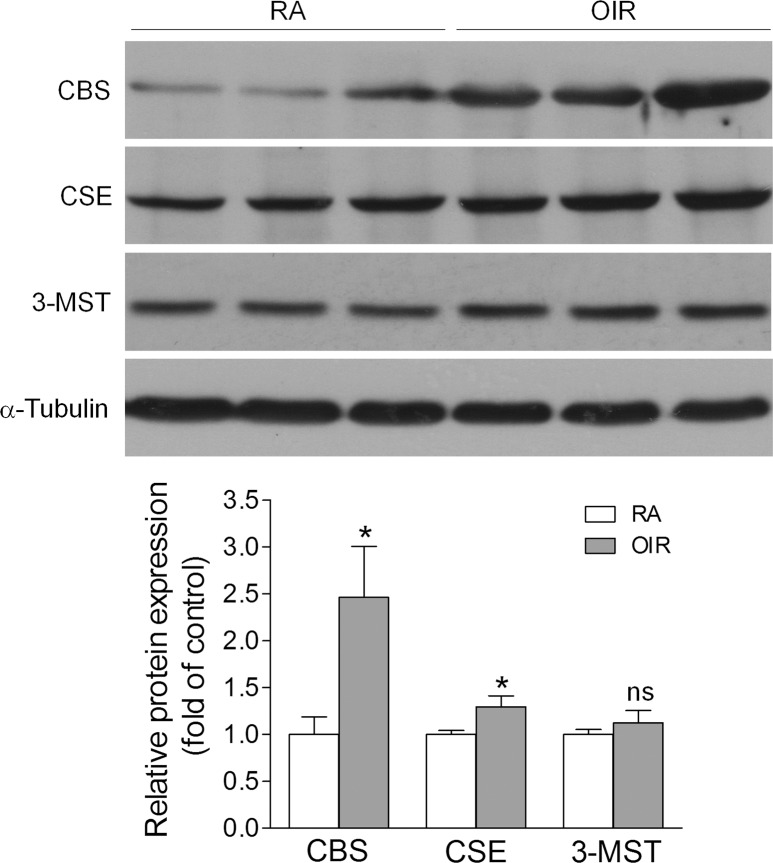
Hydrogen sulfide can be generated by CBS, CSE, and 3-MST.22,23 To investigate the mechanisms by which H2S production was upregulated in OIR, we measured the levels of CBS, CSE, and 3-MST by Western blot. We found that all three enzymes were expressed in the retina (Fig. 2). Compared with RA controls, CBS was significantly upregulated by approximately 2.5-fold and CSE was increased slightly in OIR retinas, whereas the level of 3-MST was not altered. These findings suggest that the increased production of H2S in OIR is mainly mediated by CBS and CSE.
Figure 2.
The expression of H2S-producing enzymes is altered in the retinas of OIR. Mice were subjected to OIR or maintained in RA as control. Retinas were collected at P17, and the protein levels of CBS, CSE, and 3-MST were evaluated by Western blot. The graphs represent the densitometric analysis of protein levels (n = 6–7). *P = 0.0265 (CBS) and 0.0365 (CSE) compared with RA control; ns, not significant.
CBS and CSE Are Localized in Different Retinal Cells in Oxygen-Induced Retinopathy
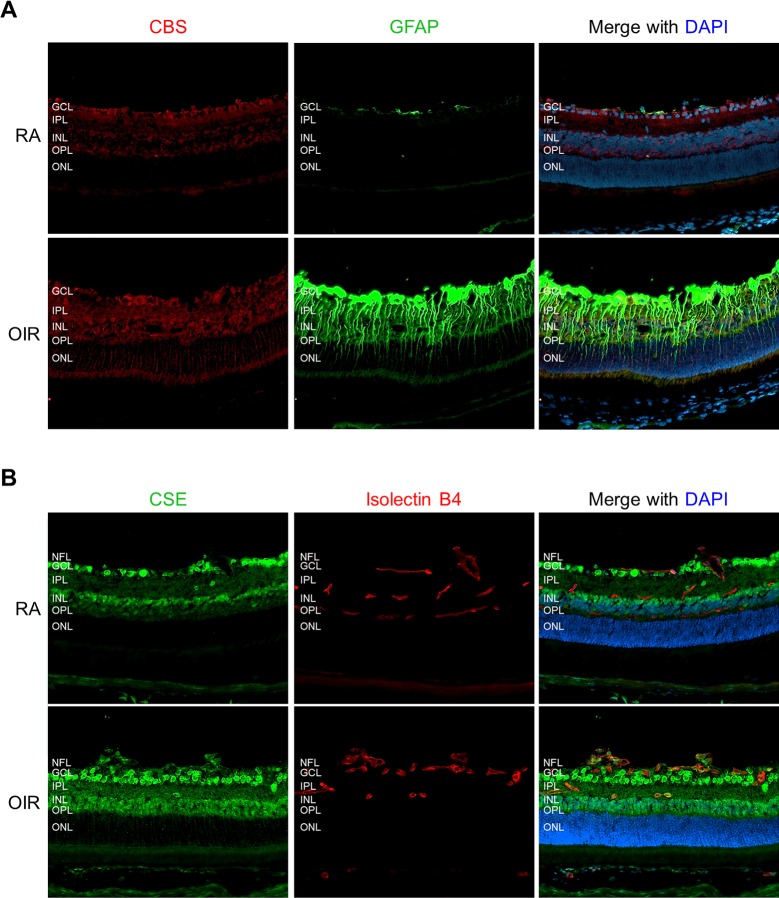
We next determined the retinal localization of CBS and CSE. In RA control mice, the immunoreactivity of CBS was mainly localized to the ganglion cell layer (GCL), inner plexiform layer (IPL), and inner nuclear layer (INL), and was weakly detected in the outer plexiform layer (OPL). In OIR retina, there was increased CBS immunoreactivity in all of these layers (Fig. 3A). More strikingly, CBS staining was prominently increased in the ONL. Quantitative analysis of the fluorescence intensity of CBS revealed ∼3-fold increase in the ONL of OIR retina compared with that of RA retina (Supplementary Fig. S1A). To specify the ONL cell type in which CBS was upregulated, we costained CBS with an antibody against the GFAP, which is a glial-specific protein and abundant in astrocytes. In Müller cells, it is normally expressed at a low level in the inner half of their cell body and endfeet but is massively upregulated under stress conditions.35,36 Consistently, we observed a robust increase of GFAP in Müller cells in OIR retinas (Fig. 3A). Moreover, CBS immunoreactivity in the ONL was colocalized with the distal processes of Müller cells that were GFAP positive (Fig. 3A), indicating that CBS was upregulated in Müller cells during OIR. In addition, retinal sections were stained with isolectin B4 to identify retinal vasculature (Supplementary Fig. S1B). The CBS immunoreactivity in retinal vessels was weak, and no difference was noticed between RA and OIR retinas.
Figure 3.
The expression of CBS and CSE is increased in the retinas of OIR. Retinal frozen sections from P17 RA or OIR mice were stained with antibodies for (A) CBS (red) and GFAP (green) or (B) CSE (green) and isolectin B4 (red). Nuclei were stained with DAPI (blue). Representative images were taken with fluorescence microscopy at ×200 magnification. NFL, nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer.
Cystathionine-γ-lyase was detected in the IPL, INL, and OPL and highly expressed in the GCL and nerve fiber layer (NFL) (Fig. 3B). Although CSE was absent in normal vessels of RA retinas, it was markedly upregulated in neovascular tufts of OIR retinas and its immunoreactivity was colocalized with endothelial cells (isolectin B4 positive). Interestingly, CSE staining was also identified in some large cells with the morphology of leukocytes within NV tufts, consistent with other reports that CSE is a component of leukocytes.37,38
Pharmacologic Inhibition of CBS/CSE Reduces Retinal Neovascularization
The upregulation of H2S production and H2S-producing enzymes CBS and CSE in OIR suggests a potential involvement of this pathway in retinal NV. To test this possibility, we pharmacologically inhibited CBS and CSE with aminooxyacetic acid (AOAA), which has classically been considered a selective CBS inhibitor but has also been shown to significantly inhibit CSE.29 Injection of AOAA (IP 3 mg/kg) resulted in moderate (∼25%) inhibition of H2S production in vivo (Supplementary Fig. S2). To study the effect of CBS/CSE inhibition on retinal NV, AOAA was injected daily from P12 to P16, during the relative hypoxic phase of OIR. Treatment with AOAA did not affect body weight (Fig. 4A), but significantly reduced the amount of retinal NV, from 13.4% in control mice to 9.5% in AOAA-treated littermates (Fig. 4B). These findings indicate that (CBS and CSE)/H2S pathway is involved in the pathogenesis of ischemia-induced retinopathy.
Figure 4.
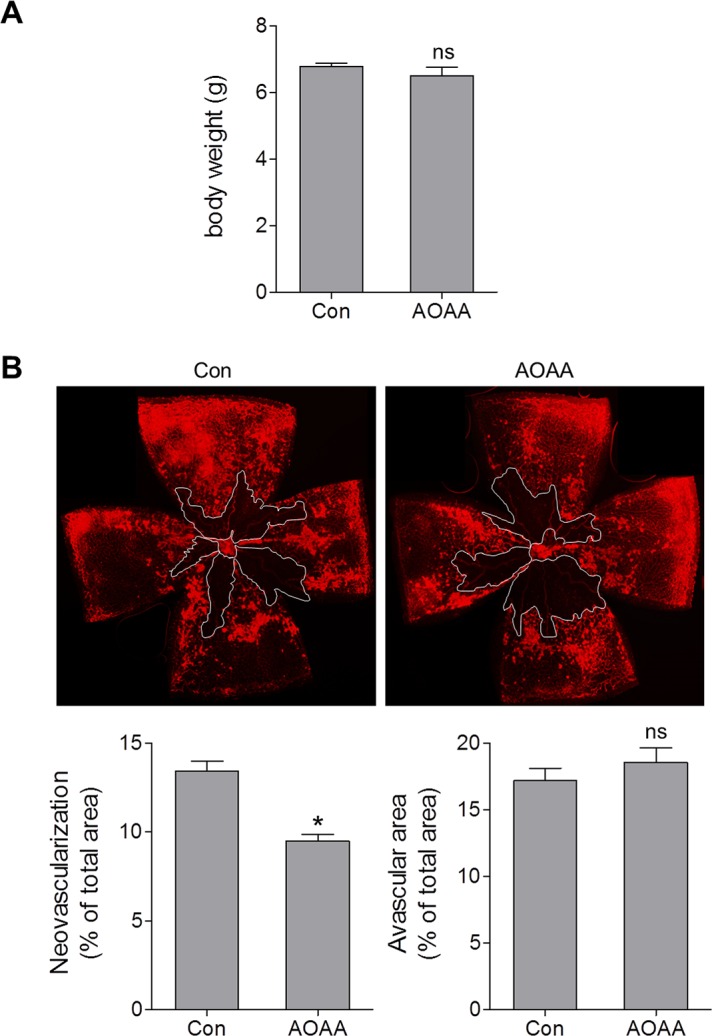
Blocking CBS/CSE decreases retinal neovascularization in OIR. Oxygen-induced retinopathy mice were injected with aminooxyacetic acid (AOAA) (IP, 3 mg/kg/day) or vehicle (Con) from P12 to P16. (A) Mouse body weight was measured at P17 (n = 6). (B) Retinas were collected at P17 and stained with isolectin B4, and representative images of the retinal flat mounts are shown. White lines outline the area of vaso-obliteration. Graphs represent neovascularization and avascular areas quantified using ImageJ software (n = 6). *P = 0.0002 compared with vehicle-treated mice; ns, not significant.
Inhibition of CBS and CSE Does Not Change the Expression of Hypoxia-Driven Angiogenic Molecules in Ischemic Retina
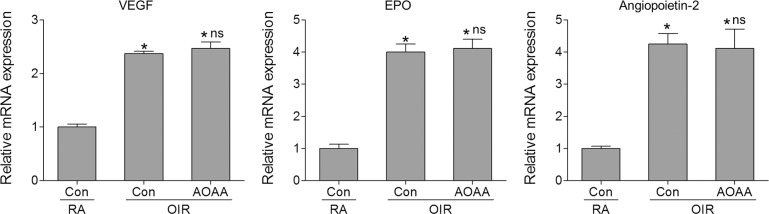
Hypoxia-inducible factor 1 (HIF-1)α–mediated upregulation of angiogenic molecules such as VEGF and EPO has an essential role in pathologic NV in ischemia-induced retinopathy.39–41 Disruption of HIF-1α in Müller cells reduces VEGF production in the retina and significantly alleviates NV in OIR.42 Since H2S has been shown to promote hypoxia-induced VEGF expression by enhancing HIF-1α expression and activity,14,43 we determined whether H2S was involved in the pathologic NV in OIR by regulating HIF-1α–mediated production of angiogenic molecules.14,43–46 We observed that CBS and CSE inhibition by AOAA did not affect the expression of VEGF, EPO, and angiopoietin-2 in OIR (Fig. 5), suggesting that (CBS and CSE)/H2S regulates retinal NV via a mechanism independent of HIF-1α–mediated expression of these angiogenic molecules in ischemia-induced retinopathy.
Figure 5.
Inhibition of CBS/CSE does not affect hypoxia-driven angiogenic gene expression in the retinas of OIR. Mice were subjected to OIR or maintained in RA as control. Oxygen-induced retinopathy mice were injected with aminooxyacetic acid (AOAA) (IP, 3 mg/kg/day) or vehicle (Con) from P12 to P16. At P17, retinas were collected and mRNA expression of VEGF, erythropoietin (EPO), and angiopoietin-2 was analyzed by quantitative PCR (n = 6). *P < 0.001 compared with RA control; ns, not significant compared with vehicle-treated OIR mice.
CSE Contributes to Retinal Neovascularization in Oxygen-Induced Retinopathy
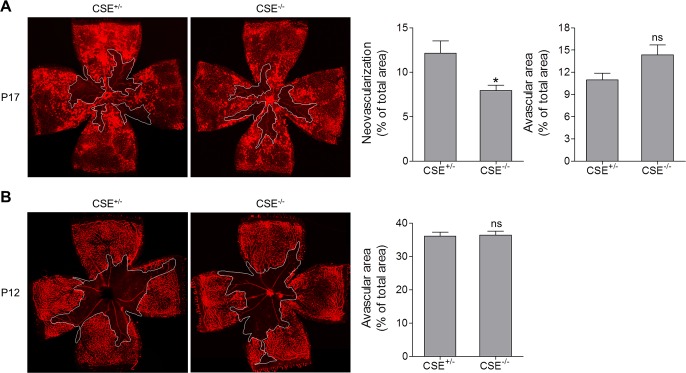
Cystathionine-γ-lyase is the main H2S-producing enzyme in the vasculature.22,23 Blocking CSE attenuates VEGF signaling in endothelial cells and markedly reduces microvessel formation in response to VEGF.17 The prominent increase of CSE expression in NV tufts suggests that the CSE/H2S pathway may have a role in retinal NV during ischemia-induced retinopathy. Therefore we generated littermate mice with wild-type (CSE+/+) and heterozygous CSE alleles (CSE+/−), or littermate mice with heterozygous (CSE+/−) and homozygous (CSE−/−) CSE deletion, and confirmed CSE deficiency in the retina (Supplementary Fig. S3A). Compared with CSE+/+ mice, H2S production in the plasma of CSE−/− mice was reduced by ∼50% (Supplementary Fig. S3B), which is consistent with the study of Yang et al.13 After examination of retinal vasculature in the OIR model, we observed that CSE+/− retinas displayed phenotypes identical to those of CSE+/+ retinas. There was no difference in vascular dropout area and NV tuft area between CSE+/+ and CSE+/− mice (Supplementary Fig. S4). However, CSE−/− OIR mice exhibited a 35% reduction of neovascular area compared with their CSE± OIR littermates at P17 (Fig. 6A), although the two genotypes had similar avascular area at P12 after exposure to hyperoxia (Fig. 6B). These data suggest that CSE-derived H2S contributes to pathologic retinal NV during ischemia-induced retinopathy. CSE−/− OIR mice also had slightly increased avascular area at P17 compared with CSE+/− OIR mice (Fig. 6A).
Figure 6.
CSE deletion results in decreased neovascularization at P17. CSE+/− and CSE−/− littermates were subjected to OIR. Retinas were harvested and stained with isolectin B4 at (A) P17 and (B) P12. Representative images of retinal flat mounts are shown, and white lines outline the area of vaso-obliteration. Graph represents neovascularization and avascular area in CSE+/− and CSE−/− mice (n = 11). *P = 0.0202 compared with CSE+/−; ns, not significant.
Discussion
Hydrogen sulfide is a member of the gasotransmitter family, along with nitric oxide (NO) and carbon monoxide (CO).47 It has been shown that H2S can promote angiogenesis in organs including the heart, hindlimb, and tumor48,49; however, the role of H2S in retinal NV is yet unclear. Here we demonstrate that H2S-generating enzymes (CBS and CSE) and H2S production are increased in the retinas from OIR mice, and that blocking CBS and CSE significantly reduces retinal NV during ischemia-induced retinopathy. Given that H2S induces angiogenesis via VEGF-dependent and VEGF-independent mechanisms,14,15,17,43,50,51 our data suggest that blockade of H2S-generating enzymes may provide an alternative therapeutic approach to prevent vision loss due to retinal NV in ischemia-induced retinopathy, even when anti-VEGF agents are not effective. Moreover, CBS and CSE inhibition may offer some advantages since it specifically reduces retinal pathologic NV but has minimal negative effect on vascular repair, unlike VEGF trap, which reduces retinal NV but blocks retinal revascularization and increases avascular area.31
As a reducing agent and an antioxidant molecule that modulates many downstream signaling events, H2S has been widely known to protect various cell types under stress/pathologic conditions.5–8,52 In the central nervous system, H2S protects neuronal cell damage in stroke, traumatic brain injury, and spinal cord injury via multiple actions including dilation of cerebral vessels, inhibition of neuronal inflammation, prevention of activation of apoptotic pathways, and counteraction of glutamate-mediated excitotoxicity.53 In the cardiovascular system, H2S prevents against atherosclerosis,54 promotes vasorelaxation,13 and preserves endothelial mitochondrial function during hyperglycemia.15,52 In the retina, H2S attenuates excitotoxic neurotransmission,9 prevents light-induced excitotoxicity,10 reduces neuronal injury after ischemia/repefusion,11,12 and alleviates retinal oxidative stress and inflammation in diabetes.27 While plenty of evidence indicates that promoting H2S production is beneficial, pathologic roles of H2S are also suggested. Hydrogen sulfide may participate in secondary neuronal injury of many CNS diseases by promoting calcium overload.53 Upregulation of the CBS–H2S pathway has been found to promote the development of neuropathic pain55 and tumorigenesis.48 Our study showing that the CBS/CSE-derived H2S is involved in retinal NV during ischemia-induced retinopathy represents the first case in which overactivating H2S was harmful in the retina. Overall, H2S exhibits complex roles depending on the cellular and disease contexts. Consequently, while our study suggests that blocking H2S production at the proliferative stage of ischemia-induced retinopathy can be beneficial, cautions are warranted when using this strategy. Reduction of H2S production at an early stage of ischemia-retinopathy might lessen its neuronal and vascular protective effects and therefore accelerate the progression of disease. Additionally, local inhibition of H2S-generating enzymes is preferred since systemic blockade of H2S may cause impairment of cardiovascular functions.
Three H2S-generating enzymes (CBS, CSE, and 3-MST) have been identified,22,23 all of which are expressed in the retina. The expression of CBS and CSE is upregulated in OIR, suggesting that these two enzymes are implicated in pathologic changes in OIR. In contrast to CBS and CSE, the level of 3-MST is not changed. By inhibiting H2S production with a pharmacologic inhibitor for both CBS and CSE or genetic deletion of CSE, we showed that H2S is a novel player in retinal NV. Hydrogen sulfide is known to induce angiogenesis by inducing endothelial cell proliferation, migration, and tube formation.48 During OIR, H2S overproduction by upregulation of CSE in neovascular tufts or CBS in glia and neurons may promote retinal pathophysiological angiogenesis by modifying the angiogenic abilities of endothelial cells. Mechanistically, H2S may activate a variety of pathways including Akt activation,48 synergistic interaction with nitric oxide–induced angiogenic response,48 sulfhydration and activation of KATP channels,17,56 upregulation of VEGF receptor (VEGFR)2 and neuropilin (NRP)-1 via stabilization of the transcription factor specificity protein 1,50 and VEGFR2 activation by reducing an inhibitory disulfide bond in its intracellular region that disrupts signal transduction.51 In addition to its direct effect to stimulate the angiogenic ability of endothelial cells, H2S-mediated retinal NV may indirectly involve its actions on other retinal cell types. Since H2S has been shown to promote hypoxia-induced VEGF expression by enhancing HIF-1α expression and activity14,43 and CBS is upregulated in multiple retinal cell types, we reasoned that upregulation of H2S may enhance VEGF expression in OIR. We measured several HIF-1α–driven angiogenic molecules including VEGF in OIR retinas, but CBS/CSE inhibitor did not reduce the expression of these molecules. Future studies comparing retinal gene expression profiles between vehicle and CBS/CSE inhibitor-treated OIR retinas are needed to identify its potential downstream targets.
In summary, our data provide the first evidence that H2S-generating enzymes/H2S pathway is activated and involved in pathologic retinal NV during ischemia-induced retinopathy. The limitation of the study is that we were not able to specify the role of CBS in retinal NV because CBS-deficient mice exhibit retinal ischemia and vascular defects during development,57 which precludes the use of these mice to study pathologic changes in OIR, and because there is no selective pharmacologic CBS inhibitor.29 Further studies conditionally deleting CBS in retinal neurons and glia will be needed to explore the precise contribution of CBS-derived H2S in retinal NV. Since NV also occurs in age-related macular degeneration, neovascular glaucoma, and corneal injury, our study calls for further investigation of the H2S pathways to determine if targeting this pathway is beneficial in the treatment of a variety of diseases.
Supplementary Material
Acknowledgments
Supported by the Retina Research Foundation (WZ, MM); National Science Foundation CBET-1053978 (MM); National Institutes of Health Grant EY022694, American Heart Association 11SDG4960005, and the John Sealy Memorial Endowment Fund for Biomedical Research (WZ); American Heart Association 15POST22450025 (HL); and National Institutes of Health Grant GM107846 (CS).
Disclosure: D. Gersztenkorn, None; C. Coletta, None; S. Zhu, None; Y. Ha, None; H. Liu, None; H. Tie, None; J. Zhou, None; C. Szabo, CBS Therapeutic, Inc. (I); W. Zhang, None; M. Motamedi, None
References
- 1. Al-Shabrawey M,, Elsherbiny M,, Nussbaum J,, Othman A,, Megyerdi S,, Tawfik A. Targeting neovascularization in ischemic retinopathy: recent advances. Expert Rev Ophthalmol. 2013; 8: 267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phelps DL. Retinopathy of prematurity. Pediatr Rev. 1995; 16: 50–56. [DOI] [PubMed] [Google Scholar]
- 3. Tsilimbaris MK,, Kontadakis GA,, Tsika C,, Papageorgiou D,, Charoniti M. Effect of panretinal photocoagulation treatment on vision-related quality of life of patients with proliferative diabetic retinopathy. Retina. 2013; 33: 756–761. [DOI] [PubMed] [Google Scholar]
- 4. Morello CM. Etiology and natural history of diabetic retinopathy: an overview. Am J Health Syst Pharm. 2007; 64: S3–S7. [DOI] [PubMed] [Google Scholar]
- 5. Mosharov E,, Cranford MR,, Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry. 2000; 39: 13005–13011. [DOI] [PubMed] [Google Scholar]
- 6. Kimura Y,, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004; 18: 1165–1167. [DOI] [PubMed] [Google Scholar]
- 7. Kimura Y,, Goto Y,, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2010; 12: 1–13. [DOI] [PubMed] [Google Scholar]
- 8. Diwakar L,, Ravindranath V. Inhibition of cystathionine-gamma-lyase leads to loss of glutathione and aggravation of mitochondrial dysfunction mediated by excitatory amino acid in the CNS. Neurochem Int. 2007; 50: 418–426. [DOI] [PubMed] [Google Scholar]
- 9. Njie-Mbye YF,, Opere CA,, Chitnis M,, Ohia SE. Hydrogen sulfide: role in ion channel and transporter modulation in the eye. Front Physiol. 2012; 3: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mikami Y,, Shibuya N,, Kimura Y,, Nagahara N,, Yamada M,, Kimura H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J Biol Chem. 2011; 286: 39379–39386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Biermann J,, Lagrèze WA,, Schallner N,, Schwer CI,, Goebel U. Inhalative preconditioning with hydrogen sulfide attenuated apoptosis after retinal ischemia/reperfusion injury. Mol Vis. 2011; 17: 1275–1286. [PMC free article] [PubMed] [Google Scholar]
- 12. Osborne NN,, Ji D, Abdul Majid AS, Fawcett RJ, Sparatore A, Del Soldato P. ACS67, a hydrogen sulfide-releasing derivative of latanoprost acid, attenuates retinal ischemia and oxidative stress to RGC-5 cells in culture. Invest Ophthalmol Vis Sci. 2010; 51: 284–294. [DOI] [PubMed] [Google Scholar]
- 13. Yang G,, Wu L,, Jiang B,, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008; 322: 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bir SC,, Kolluru GK,, McCarthy P,, et al. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1alpha and vascular endothelial growth factor-dependent angiogenesis. J Am Heart Assoc. 2012; 1: e004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coletta C,, Papapetropoulos A,, Erdelyi K,, et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A. 2012; 109: 9161–9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cai WJ,, Wang MJ,, Moore PK,, Jin HM,, Yao T,, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007; 76: 29–40. [DOI] [PubMed] [Google Scholar]
- 17. Papapetropoulos A,, Pyriochou A,, Altaany Z,, et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2009; 106: 21972–21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang MJ,, Cai WJ,, Li N,, Ding YJ,, Chen Y,, Zhu YC. The hydrogen sulfide donor NaHS promotes angiogenesis in a rat model of hind limb ischemia. Antioxid Redox Signal. 2010; 12: 1065–1077. [DOI] [PubMed] [Google Scholar]
- 19. Polhemus DJ,, Kondo K,, Bhushan S,, et al. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ Heart Fail. 2013; 6: 1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szabo C,, Papapetropoulos A. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br J Pharmacol. 2011; 164: 853–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang G. Hydrogen sulfide in cell survival: a double-edged sword. Expert Rev Clin Pharmacol. 2011; 4: 33–47. [DOI] [PubMed] [Google Scholar]
- 22. Li L,, Moore PK. Putative biological roles of hydrogen sulfide in health and disease: a breath of not so fresh air? Trends Pharmacol Sci. 2008; 29: 84–90. [DOI] [PubMed] [Google Scholar]
- 23. Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007; 6: 917–935. [DOI] [PubMed] [Google Scholar]
- 24. Sakamoto K,, Suzuki Y,, Kurauchi Y,, Mori A,, Nakahara T,, Ishii K. Hydrogen sulfide attenuates NMDA-induced neuronal injury via its anti-oxidative activity in the rat retina. Exp Eye Res. 2014; 120: 90–96. [DOI] [PubMed] [Google Scholar]
- 25. Winther AK,, Dalsgaard T,, Hedegaard ER,, Simonsen U. Involvement of hydrogen sulfide in perivascular and hypoxia-induced inhibition of endothelin contraction in porcine retinal arterioles. Nitric Oxide. 2015; 50: 1–9. [DOI] [PubMed] [Google Scholar]
- 26. Takir S,, Ortakoylu GZ,, Toprak A,, Uydes-Dogan BS. NaHS induces relaxation response in prostaglandin F(2alpha) precontracted bovine retinal arteries partially via K(v) and K(ir) channels. Exp Eye Res. 2015; 132: 190–197. [DOI] [PubMed] [Google Scholar]
- 27. Si YF,, Wang J,, Guan J,, Zhou L,, Sheng Y,, Zhao J. Treatment with hydrogen sulfide alleviates streptozotocin-induced diabetic retinopathy in rats. Br J Pharmacol. 2013; 169: 619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ran R,, Du L,, Zhang X,, et al. Elevated hydrogen sulfide levels in vitreous body and plasma in patients with proliferative diabetic retinopathy. Retina. 2014; 34: 2003–2009. [DOI] [PubMed] [Google Scholar]
- 29. Asimakopoulou A,, Panopoulos P,, Chasapis CT,, et al. Selectivity of commonly used pharmacological inhibitors for cystathionine beta synthase (CBS) and cystathionine gamma lyase (CSE). Br J Pharmacol. 2013; 169: 922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thorson MK,, Majtan T,, Kraus JP,, Barrios AM. Identification of cystathionine beta-synthase inhibitors using a hydrogen sulfide selective probe. Angew Chem Int Ed Engl. 2013; 52: 4641–4644. [DOI] [PubMed] [Google Scholar]
- 31. Zhang W,, Yokota H,, Xu Z,, et al. Hyperoxia therapy of pre-proliferative ischemic retinopathy in a mouse model. Invest Ophthalmol Vis Sci. 2011; 52: 6384–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi BY,, Kim JH,, Kim HJ,, et al. Pyruvate administration reduces recurrent/moderate hypoglycemia-induced cortical neuron death in diabetic rats. PLoS One. 2013; 8: e81523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stahl A,, Connor KM,, Sapieha P,, et al. Computer-aided quantification of retinal neovascularization. Angiogenesis. 2009; 12: 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stahl A,, Connor KM,, Sapieha P,, et al. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010; 51: 2813–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Narayanan SP,, Suwanpradid J,, Saul A,, et al. Arginase 2 deletion reduces neuro-glial injury and improves retinal function in a model of retinopathy of prematurity. PLoS One. 2011; 6: e22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kolb H. Glial cells of the Retina by Helga Kolb. Available at: http://webvision.med.utah.edu/book/part-ii-anatomy-and-physiology-of-the-retina/glial-cells-of-the-retina/. Accessed May 12, 2016.
- 37. Wang XH,, Wang F,, You SJ,, et al. Dysregulation of cystathionine gamma-lyase (CSE)/hydrogen sulfide pathway contributes to ox-LDL-induced inflammation in macrophage. Cell Signal. 2013; 25: 2255–2262. [DOI] [PubMed] [Google Scholar]
- 38. Manna P,, Gungor N,, McVie R,, Jain SK. Decreased cystathionine-gamma-lyase (CSE) activity in livers of type 1 diabetic rats and peripheral blood mononuclear cells (PBMC) of type 1 diabetic patients. J Biol Chem. 2014; 289: 11767–11778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ozaki H,, Yu AY,, Della N,, et al. Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci. 1999; 40: 182–189. [PubMed] [Google Scholar]
- 40. Grimm C,, Wenzel A,, Groszer M,, et al. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002; 8: 718–724. [DOI] [PubMed] [Google Scholar]
- 41. Chen J,, Connor KM,, Aderman CM,, Willett KL,, Aspegren OP,, Smith LE. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2009; 50: 1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin M,, Chen Y,, Jin J,, et al. Ischaemia-induced retinal neovascularisation and diabetic retinopathy in mice with conditional knockout of hypoxia-inducible factor-1 in retinal Muller cells. Diabetologia. 2011; 54: 1554–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu X,, Pan L,, Zhuo Y,, Gong Q,, Rose P,, Zhu Y. Hypoxia-inducible factor-1alpha is involved in the pro-angiogenic effect of hydrogen sulfide under hypoxic stress. Biol Pharm Bull. 2010; 33: 1550–1554. [DOI] [PubMed] [Google Scholar]
- 44. Wang J,, Wu K,, Bai F,, et al. Celecoxib could reverse the hypoxia-induced Angiopoietin-2 upregulation in gastric cancer. Cancer Lett. 2006; 242: 20–27. [DOI] [PubMed] [Google Scholar]
- 45. Liu H,, Zhang W,, Kennard S,, Caldwell RB,, Lilly B. Notch3 is critical for proper angiogenesis and mural cell investment. Circ Res. 2010; 107: 860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bunn HF,, Gu J,, Huang LE,, Park JW,, Zhu H. Erythropoietin: a model system for studying oxygen-dependent gene regulation. J Exp Biol. 1998; 201: 1197–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kohn C,, Dubrovska G,, Huang Y,, Gollasch M. Hydrogen sulfide: potent regulator of vascular tone and stimulator of angiogenesis. Int J Biomed Sci. 2012; 8: 81–86. [PMC free article] [PubMed] [Google Scholar]
- 48. Hellmich MR,, Coletta C,, Chao C,, Szabo C. The therapeutic potential of cystathionine beta-synthetase/hydrogen sulfide inhibition in cancer. Antioxid Redox Signal. 2015; 22: 424–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qipshidze N,, Metreveli N,, Mishra PK,, Lominadze D,, Tyagi SC. Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. Int J Biol Sci. 2012; 8: 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saha S,, Chakraborty PK,, Xiong X,, et al. Cystathionine beta-synthase regulates endothelial function via protein S-sulfhydration. FASEB J. 2016; 30: 441–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tao BB,, Liu SY,, Zhang CC,, et al. VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal. 2013; 19: 448–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Suzuki K,, Olah G,, Modis K,, et al. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci U S A. 2011; 108: 13829–13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang JF,, Li Y,, Song JN,, Pang HG. Role of hydrogen sulfide in secondary neuronal injury. Neurochem Int. 2014; 64: 37–47. [DOI] [PubMed] [Google Scholar]
- 54. Streeter E,, Ng HH,, Hart JL. Hydrogen sulfide as a vasculoprotective factor. Med Gas Res. 2013; 3: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gui Y,, Li A,, Qiu B,, et al. Endogenous CBS-H2S pathway contributes to the development of CCI-induced neuropathic pain. Neurochem Res. 2016; 41: 1381–1389. [DOI] [PubMed] [Google Scholar]
- 56. Paul BD,, Snyder SH. H(2)S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. 2012; 13: 499–507. [DOI] [PubMed] [Google Scholar]
- 57. Tawfik A,, Al-Shabrawey M,, Roon P,, et al. Alterations of retinal vasculature in cystathionine-Beta-synthase mutant mice, a model of hyperhomocysteinemia. Invest Ophthalmol Vis Sci. 2013; 54: 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.