Abstract
The objective of this study was to develop a model to estimate the cost of a case of subclinical ketosis (SCK) in Canadian dairy herds. Costs were derived from the default inputs, and included increased clinical disease incidence attributable to SCK, $76; longer time to pregnancy, $57; culling and death in early lactation attributable to SCK, $26; milk production loss, $44. Given these figures, the cost of 1 case of SCK was estimated to be $203. Sensitivity analysis showed that the estimated cost of a case of SCK was most sensitive to the herd-level incidence of SCK and the cost of 1 day open. In conclusion, SCK negatively impacts dairy herds and losses are dependent on the herd-level incidence and factors included in the calculation.
Résumé
Coût d’un cas d’acétonémie subclinique dans les troupeaux laitiers canadiens. L’objectif de cette étude consistait à développer un modèle pour estimer le coût d’un cas d’acétonémie subclinique (ASC) dans les troupeaux laitiers canadiens. Les coûts ont été dérivés des valeurs par défaut et comprenaient l’incidence accrue de maladie clinique attribuable à l’ASC, 76 $; un délai plus long avant la gestation, 57 $; la réforme et la mort au début de la lactation attribuable à l’ASC, 26 $; la perte de production laitière, 44 $. Compte tenu de ces chiffres, le coût de 1 cas d’ASC a été estimé à 203 $. Une analyse de sensibilité a montré que le coût estimé d’un cas d’ASC était le plus sensible à l’incidence de l’ASC au niveau du troupeau et au coût d’une journée ouvrable. En conclusion, l’ASC a un impact négatif sur les troupeaux laitiers et les pertes dépendent de l’incidence au niveau du troupeau et des facteurs inclus dans le calcul.
(Traduit par Isabelle Vallières)
Introduction
After calving, all lactating dairy cows go through an inevitable phase of negative energy balance (1) that results from the lag of dry matter intake behind milk production, rendering cows susceptible to metabolic diseases (2). Ketosis (clinical and subclinical), a widespread condition in dairy herds across North America, is one such metabolic disease (3). Subclinical ketosis (SCK) results in reduced milk production (4–6) and decreased reproductive performance (4,7) relative to similar cows not diagnosed with the condition. In addition, affected cows are more likely to develop other diseases including displaced abomasum (DA), clinical ketosis (CK), and metritis (5,8,9), and are more likely to be culled in early lactation (10). As a consequence, the negative effects of SCK will impact herd performance as a whole (11,12). Therefore, the impacts of SCK on health, reproductive performance, and production can be costly for each affected cow, and can affect profitability of a dairy enterprise.
There have been previous attempts to quantify economic losses associated with SCK. One Canadian study estimated a cost of $78 for a case of SCK (13). This figure might be an underestimate of the actual cost of SCK, not only because the input costs have increased over time, but also because the authors: i) accounted for only 2 diseases on which SCK can have an impact (DA and CK); ii) did not incorporate the increased risk of culling into their calculations; and iii) considered milk loss due to SCK for only 2 wk. Another study estimated that a case of SCK can cost €735 ($1031.00 CDN) (14). This figure might be an overestimation, as some overlapping effects such as production loss were double-counted. A recent comprehensive model estimated the average cost of a case of ketosis to be $289 US (15); however, it was based on conditions within the US dairy industry.
Therefore, there is a need for a model that estimates the cost per case of SCK in Canada as comprehensively as possible, based on currently available information. The objective was to estimate the losses that result from an individual case of SCK in Canadian dairy herds, including a sensitivity analysis to identify influencing input variables.
Materials and methods
A spreadsheet in Excel (Microsoft Office 2010; Microsoft, Redmond, Washington, USA) was developed to estimate the cost of an individual case of SCK. Subclinical ketosis was defined as elevated serum β-hydroxybutyrate (BHBA) ≥ 1400 μmol/L in either of the first 2 weeks following calving and in a cow not showing clinical signs. Economic losses due to SCK were calculated by summing the losses resulting from reduction in milk production, increased risk of clinical diseases (DA, CK, and metritis), increased risk of culling and death in early lactation, and reduced reproductive performance. The price and cost of different variables in addition to herd or cow data that were used as inputs in the current model are listed in Table 1.
Table 1.
Inputs used to calculate the cost of 1 case of subclinical ketosis (SCK; defined as elevated serum BHBA ≥ 1400 μmol/L in either of the first 2 weeks following calving in a cow not showing clinical signs)
| Item | Value | Reference |
|---|---|---|
| Price/cost | ||
| Average milk price in Canada ($/L) | 0.81 | (22) |
| Cost ($) of 1 kg of TMR (dry matter basis) to produce 1 L of milk | 0.3 | (20) |
| Average value of a cow in a herd ($) | 2100 | (20) |
| Cost of pregnant replacement heifer ($)a | 2500 | (23) |
| Cull cow value ($)b | 1680 | (23) |
| Cost of 1 day open beyond 100 days ($) | 3 | (21) |
| Herd/cow data | ||
| Herd annual turnover rate (%) | 35 | |
| Median time to pregnancy (d) in cows without SCK | 108 | (7) |
| Median time to pregnancy (d) in cows with SCK in 1st or 2nd wk postpartum | 124 | (7) |
| Median time to pregnancy (d) in cows with SCK in 1st and 2nd wk postpartum | 130 | (7) |
| Dairy efficiencyc | 2.3 | |
| Milk reduction (kg) for 30 days due to SCK | 65 | (4,5) |
Pregnant replacement heifer price is based on average weight of 454 kg and $250/cwt.
Cull cow value is based on average weight of 635 kg and $120/cwt.
Milk yield (kg) produced as a result of a cow consuming 1 additional kg of dry matter above maintenance requirements (24); this calculation accounts only for the concentration energy required to produce marginal milk after considering that NEL of 0.75 Mcal/kg of 4% milk and 1.73 Mcal/kg of feed on a dry matter basis (25).
Losses due to reduction in milk production attributable to subclinical ketosis
The estimate of milk loss due to SCK used in the current study was based on the first Dairy Herd Improvement (DHI) test, and was 2.15 kg/ketotic cow (4,5). This amounts to a loss of 65 kg/30 d for a case of SCK when assuming that, on average, the first test date is at 30 days in milk (DIM). The marginal loss of milk production ($) was calculated by subtracting the feed cost (dry matter basis) saved due to the reduction in milk production caused by SCK from the total loss ($) incurred due to the reduction in milk production associated with SCK.
Losses due to increased disease incidence attributable to subclinical ketosis
The overall loss due to increased clinical disease (CK, DA, and metritis) in cows with SCK is a function of the cost of a clinical case of each disease and the attributable risk (the increment in clinical disease incidence attributed to SCK) for the associated diseases.
The cost of CK was calculated using inputs listed in Table 2, and was $233. The costs of both DA ($707) and metritis ($396) were obtained from McArt et al (15). All disease costs except CK were in US dollars and converted to Canadian dollars using an exchange rate of 1.28. To calculate the attributable risk of increased disease incidence due to SCK, the lactational incidence risk (LIR; the number of cows affected with a certain disease during a lactation divided by the number of cows that calved in the same time period) of CK (3), DA, and metritis (8), and the increased risk of developing CK (5,16), DA (5,16,17), and metritis (5) due to SCK were obtained from previous studies. The Basal risk (clinical disease incidence for cows not affected with SCK) and the attributable risk (clinical disease incidence for cows affected with SCK) were then calculated (Table 3).
Table 2.
Inputs used to calculate the cost of 1 case of clinical ketosis (defined as a case of ketosis manifested with clinical signs regardless of serum BHBA concentrations)
| Item | Value | Reference |
|---|---|---|
| Incidence of clinical ketosis (%) | 9.6 | (3) |
| 300 g of propylene glycol for 4 days ($) | 12 | (26) |
| Labour wages ($)/hour | 15 | (27) |
| Number of cows drenched/hour | 15 | |
| Death due to clinical ketosis (%) | 1.3 | (28) |
| Culling due to clinical ketosis (%) | 5 | (28) |
| Hourly veterinary fee ($) | 160 | |
| Time (hours) to diagnose and treat clinical ketosis by a veterinarian | 0.3 | |
| Cases of clinical ketosis examined and treated by veterinarians (%) | 10 | (30) |
| Milk production loss (kg) due to clinical ketosis | 255 | (31) |
| Average milk price in Canada ($/L) | 0.81 | (22) |
| Dairy efficiencya | 2.3 | |
| Cost ($) of 1 kg of TMR (dry matter basis) to produce 1 L of milk | 0.3 | (20) |
| Cost of pregnant replacement heifer ($) | 2500 | (23) |
| Cull cow price ($) | 1680 | (23) |
| Average value of a cow in a herd ($) | 2100 | (20) |
| Cost of 1 day open beyond 100 days ($) | 3.0 | (21) |
| Increase in days open for cows with clinical ketosis | 10 | (30) |
| Risk of aspiration pneumonia (%) with drenching | 0.003 | J.P. Goff, Iowa State University, Ames, Iowa, personal communication, 2015 |
| Risk of immediate death after aspiration pneumonia (%) | 0.0024 | (32) |
| Risk of survival after aspiration pneumonia (%) | 0.0009 | (32) |
| Risk of culling after survival and treatment (%) | 0.0003 | (32) |
Milk yield (kg) produced as a result of a cow consuming one additional kg of dry matter above maintenance requirements (24); this calculation accounts only for the concentration energy required to produce marginal milk after considering that NEL of 0.75 Mcal/kg of 4% milk and 1.73 Mcal/kg of feed in dry matter basis (25).
Table 3.
Inputs and calculations of epidemiological measures of the contribution of subclinical ketosis (SCK) to subsequent clinical diseases, used to estimate the cost of 1 case of SCK (Average odds ratios references in brackets)
| Event | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Measure | DA | CK | Metritis | SCK | Culling | Death |
| Increased odds due to SCK | 6.42 (5,16,17) | 5.48 (15,16) | 2.51 (5) | — | 1.8 (10) | — |
| LIR (%) | 4.8 (8) | 9.6 (3) | 16.7 (8) | 40.0 | 7.53a | — |
| Basal risk (%)b | 1.52 | 3.44 | 10.41 | — | 5.7 | — |
| Attributable risk (%)c | 3.3 | 6.2 | 6.3 | — | 1.82 | 2.2 |
| Cost of disease/eventd ($) | 29.73 | 14.36 | 31.88 | — | 7.48 | 18.48 |
DA — Displaced abomasum; CK — Clinical ketosis; LIR — Lactational incidence risk.
Percent of culling by 60 DIM (21.5%) multiplied by annual herd turnover rate (35%).
Event occurrence for cows not affected with SCK. Calculated by solving for x in this formula: LIR of a clinical disease/culling = (percent of cows with SCK × increased odds to develop that clinical disease/culling × x) + (percent of cows without SCK × x).
Incremental increase of event attributed to SCK. Calculated using this formula: Attributable risk = (percent of cows with SCK × increased odds to develop that clinical disease/culling × x) — (percent of cows with SCK × x).
Cost of increased disease incidence due to SCK is a function of the cost of a disease and the attributable risk. Cost of increased culling due to SCK is a function of cost of premature culling and the attributable risk. Cost of death due to SCK is a function of average value of a cow in a herd, LIR of SCK and attributable risk.
Losses due to culling and death attributable to subclinical ketosis
To calculate the loss resulting from culling due to SCK, the following steps were performed. First, the cost of premature culling was calculated based on a culling depreciation model (J. Fetrow, University of Minnesota, St. Paul, Minnesota, USA, personal communication, 2014) to estimate current predicted value of a cow in lactation based on the herd’s parity-specific culling risk and current lactation among other input variables. Second, the turnover rate in the first 60 DIM (LIR of culling in the first 60 DIM) was calculated by multiplying the average culling percent until 60 DIM (18,19) by an assumed annual herd turnover rate of 35%. Third, the risk of culling in early lactation for cows with SCK was obtained (10), and the basal and attributable risk of culling due to SCK were calculated. Finally, loss resulting from culling due to SCK is a function of the cost of premature culling and the attributable risk of culling due to SCK (Table 3).
Considering death associated with SCK, calculations were based on an attributable risk of death due to SCK of 2.2% (J.A. McArt, Cornell University, Ithaca, New York, USA, personal communication, 2015). Assuming an average value of $2100 for a lactating cow within a herd (20), loss from death due to SCK is a function of attributable risk of death due to SCK, average value of a lactating cow, and the incidence of SCK in a herd.
Losses due to reduced reproductive performance attributable to subclinical ketosis
The cost of 1 d open beyond 100 DIM was assumed to be $3 (21). All information on the difference in time to pregnancy between cows diagnosed with SCK and cows without SCK was retrieved from a previous report (7). From this, an average cost of days open for cows with SCK was estimated.
Sensitivity analysis
Holding all other inputs constant, by changing the value of 1 input from its baseline (default) level, the change in the cost of a case of SCK was calculated. In an attempt to standardize the change in inputs for sensitivity analysis, the following inputs were altered by adding or subtracting 50% of the baseline: cost of 1 d open, cost of 1 kg of feed, labor costs, average value of a cow in a herd, cost of a replacement heifer, price of a cull cow, and the turnover rate. The range used for the incidence of SCK was based on Duffield (29), and the range in incidence of CK, metritis, and displaced abomasum was based on Guard (30). Finally, the range used for milk price was based on adding or subtracting $0.10/L from the baseline used in the analysis.
Results
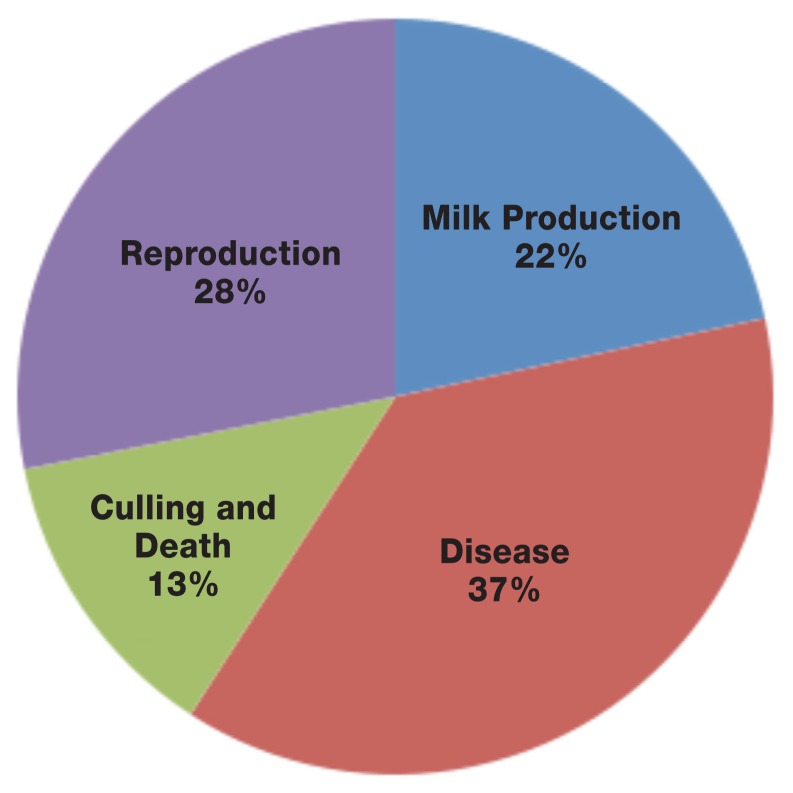
Loss resulting from SCK in the current model was $203 per case. The breakdown of the cost of 1 case of SCK is shown in Figure 1. The calculated estimate is lower than the $289.00 US ($370.00 CDN) average cost of a case of ketosis reported by McArt et al (15). Although milk price in Canada is higher than in the US, several differences beyond milk price affected the estimate. For example, reproductive loss due to SCK was based on extended time to pregnancy for subclinically ketotic cows, while in the US study it was based on an increased risk of ovarian dysfunction and pregnancy to first insemination (15). In addition, our calculations modelled SCK separately from clinical ketosis. While McArt et al (15) did not include the cost of clinical ketosis separately, they still factored treatment costs and other downstream impacts in the total cost of ketosis. However, if the cost of CK and SCK calculated in this study are summed together they will yield a comparable cost of $430.00 CDN to that obtained by McArt et al (15).
Figure 1.
A pie chart showing the breakdown of the cost of 1 case of subclinical ketosis.
Discussion
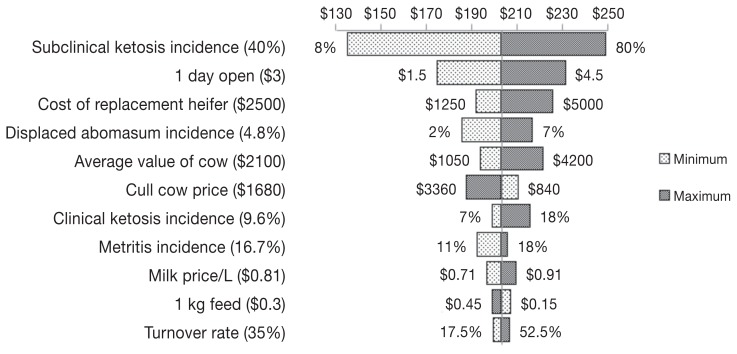
While there is an association between clinical disease incidence and SCK (5,8,9), it is not necessarily true in all herds that clinical disease incidence will increase if the incidence of SCK increases. That being the case, in contrast to McArt et al (15) we modelled clinical disease incidence (DA, CK, and metritis) separately from SCK (i.e., we did not tie the incidence of SCK to the incidence of the associated clinical diseases). Yet, and due to the method of calculation used, an increase in herd incidence of SCK was associated with the increase of the cost of a case of SCK due to its direct impact on the basal and attributable risk of other clinical diseases and culling. However, sensitivity analysis did not show the variation in clinical disease incidences to be influential (Figure 2).
Figure 2.
A Tornado plot to depict sensitivity analysis for estimation of the cost of a case of subclinical ketosis. The plot depicts the change in the cost of a case of SCK due to an increase or decrease of the value of an input. Values in parentheses are baseline values used in building the model while values on the sides of the bars are the minimum and maximum values used for sensitivity analysis. The line in the middle of the plot separating minimum and maximum bars represents loss due to a case of SCK using the baseline (default) values of inputs ($203).
The estimate obtained in the current study was lower than the loss of €735 ($1031.00 CDN) for a case of SCK reported in another recent study (14). The current study attempted to limit the overestimation and double counting in cost estimate calculations by accounting only for the attributable risk of clinical disease incidence due to SCK, but there might still have been some overlap of costs that were impossible to disentangle from interrelated diseases in the fresh period.
Loss calculated in the current study, however, is higher than what was calculated in an earlier Canadian study (13) that estimated the cost of a case of SCK to be $78. The authors of that study only estimated a loss of milk for 2 wk, while in the current study milk loss was estimated to be over 30 d. The authors also used a lower risk for developing disease than used herein; for example, a 3-fold increase in the risk of DA and CK was used, compared to a 6.5 and a 5.5-fold increase in the odds of developing each disease, respectively, in the current study. In addition, culling was not accounted for and milk price in Canada now is 2.7 times higher. It is worth mentioning that the authors of the older study also overestimated the losses due to developing other diseases, as they included basal disease incidence in their calculations. This means that losses due to SCK were actually lower than reported.
Losses in the current model were based on using a cut-point for blood BHBA concentrations of 1400 μmol/L. The cut-point was chosen based on the negative impact SCK can have on health and production of lactating dairy cattle (5). Losses will vary by changing the cut-point value, whether SCK is diagnosed in the first or second week after calving, or both. Modeling such thresholds and patterns would have added value to the model; however, this was not done because such a model is prohibitively complex, and data to support the differential impacts of SCK at that level of detail are sparse. Sensitivity analysis showed that the incidence of SCK was the input to which the estimates were most sensitive (Figure 2). The sensitivity was a result of the wide variation in the incidence of SCK among Canadian dairy herds.
In conclusion, the average cost of a case of SCK generated by our model was $203, and varied depending on the herd-level incidence of SCK. Given previous studies testing cows at least weekly over the first 2 wk postpartum has established a typical cumulative incidence of SCK to be 40%; herd-level costs of SCK are substantial. This analysis supports the importance of implementing prevention, monitoring, and treatment programs during the transition period. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
This study was funded by Elanco Animal Health (Greenfield, Indiana, USA).
References
- 1.Herdt TH. Ruminant adaptation to negative energy balance. Influences on the etiology of ketosis and fatty liver. Vet Clin North Am Food Anim Pract. 2000;16:215–230. doi: 10.1016/s0749-0720(15)30102-x. [DOI] [PubMed] [Google Scholar]
- 2.Gerloff BJ. Dry cow management for the prevention of ketosis and fatty liver in dairy cows. Vet Clin North Am Food Anim Pract. 2000;16:283–292. doi: 10.1016/s0749-0720(15)30106-7. [DOI] [PubMed] [Google Scholar]
- 3.Carson ME. MSc Thesis. Guelph, Ontario: University of Guelph; 2008. The association of selected metabolites in peripartum dairy cattle with health and production. [Google Scholar]
- 4.Chapinal N, Carson ME, LeBlanc SJ, et al. The association of serum metabolites in the transition period with milk production and early-lactation reproductive performance. J Dairy Sci. 2012;95:1301–1309. doi: 10.3168/jds.2011-4724. [DOI] [PubMed] [Google Scholar]
- 5.Duffield TF, Lissemore KD, McBride BW, Leslie KE. Impact of hyperketonemia in early lactation dairy cows on health and production. J Dairy Sci. 2009;92:571–580. doi: 10.3168/jds.2008-1507. [DOI] [PubMed] [Google Scholar]
- 6.Ospina PA, Nydam DV, Stokol T, Overton TR. Associations of elevated nonesterified fatty acids and beta-hydroxybutyrate concentrations with early lactation reproductive performance and milk production in transition dairy cattle in the northeastern United States. J Dairy Sci. 2010;93:1596–1603. doi: 10.3168/jds.2009-2852. [DOI] [PubMed] [Google Scholar]
- 7.Walsh RB, Walton JS, Kelton DF, LeBlanc SJ, Leslie KE, Duffield TF. The effect of subclinical ketosis in early lactation on reproductive performance of postpartum dairy cows. J Dairy Sci. 2007;90:2788–2796. doi: 10.3168/jds.2006-560. [DOI] [PubMed] [Google Scholar]
- 8.Chapinal N, Carson M, Duffield TF, et al. The association of serum metabolites with clinical disease during the transition period. J Dairy Sci. 2011;94:4897–4903. doi: 10.3168/jds.2010-4075. [DOI] [PubMed] [Google Scholar]
- 9.Ospina PA, Nydam DV, Stokol T, Overton TR. Evaluation of nonesterified fatty acids and beta-hydroxybutyrate in transition dairy cattle in the northeastern United States: Critical thresholds for prediction of clinical diseases. J Dairy Sci. 2010;93:546–554. doi: 10.3168/jds.2009-2277. [DOI] [PubMed] [Google Scholar]
- 10.Roberts T, Chapinal N, Leblanc SJ, Kelton DF, Dubuc J, Duffield TF. Metabolic parameters in transition cows as indicators for early-lactation culling risk. J Dairy Sci. 2012;95:3057–3063. doi: 10.3168/jds.2011-4937. [DOI] [PubMed] [Google Scholar]
- 11.Chapinal N, Leblanc SJ, Carson ME, et al. Herd-level association of serum metabolites in the transition period with disease, milk production, and early lactation reproductive performance. J Dairy Sci. 2012;95:5676–5682. doi: 10.3168/jds.2011-5132. [DOI] [PubMed] [Google Scholar]
- 12.Ospina PA, Nydam DV, Stokol T, Overton TR. Association between the proportion of sampled transition cows with increased nonesterified fatty acids and beta-hydroxybutyrate and disease incidence, pregnancy rate, and milk production at the herd level. J Dairy Sci. 2010;93:3595–3601. doi: 10.3168/jds.2010-3074. [DOI] [PubMed] [Google Scholar]
- 13.Geishauser T, Leslie KE, Kelton DF, Duffield TF. Monitoring for subclinical ketosis in dairy herds. Compend Contin Educ Proc Vet. 2001;23:s65–s71. [Google Scholar]
- 14.Esslemont JR. The costs of ketosis in dairy cows. Proc 27th World Buiatrics Congress; Lisbon, Portugal. 2012. p. 156. [Google Scholar]
- 15.McArt JA, Nydam DV, Overton MW. Hyperketonemia in early lactation dairy cattle: A deterministic estimate of component and total cost per case. J Dairy Sci. 2015;98:2043–2054. doi: 10.3168/jds.2014-8740. [DOI] [PubMed] [Google Scholar]
- 16.Seifi HA, LeBlanc SJ, Leslie KE, Duffield TF. Metabolic predictors of post-partum disease and culling risk in dairy cattle. Vet J. 2011;188:216–220. doi: 10.1016/j.tvjl.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 17.LeBlanc SJ, Leslie KE, Duffield TF. Metabolic predictors of displaced abomasum in dairy cattle. J Dairy Sci. 2005;88:159–170. doi: 10.3168/jds.S0022-0302(05)72674-6. [DOI] [PubMed] [Google Scholar]
- 18.Dechow CD, Goodling RC. Mortality, culling by sixty days in milk, and production profiles in high- and low-survival Pennsylvania herds. J Dairy Sci. 2008;91:4630–4639. doi: 10.3168/jds.2008-1337. [DOI] [PubMed] [Google Scholar]
- 19.Godden SM, Stewart SC, Fetrow JF, et al. The relationship between herd rBST-supplementation and other factors with risk for removal for cows in Minnesota Holstein dairy herds. Proc Four-State Dairy Nutrition and Management; Dubuque, Iowa. 2003. pp. 55–64. [Google Scholar]
- 20.Guideline for estimating dairy cow production costs in Manitoba. 2015. [Last accessed May 3, 2016]. Available from: http://www.gov.mb.ca/agriculture/business-and-economics/financial-management/pubs/cop_dairy_cow.pdf.
- 21.Groenendaal H, Galligan DT, Mulder HA. An economic spreadsheet model to determine optimal breeding and replacement decisions for dairy cattle. J Dairy Sci. 2004;87:2146–2157. doi: 10.3168/jds.S0022-0302(04)70034-X. [DOI] [PubMed] [Google Scholar]
- 22.Dairy Farmers of Ontario. The Milk Producer magazine. Markets. [Last accessed May 3, 2016]. Available from: https://www.milk.org/Corporate/view.aspx?content=aboutus/MilkProducerMagazine.
- 23.Ontario Stockyards Inc. 2015. [Last accessed May 3, 2016]. Available from: http://www.ontariostockyards.on.ca/pages/reports.
- 24.Britt JS, Thomas RC, Speer NC, Hall MB. Efficiency of converting nutrient dry matter to milk in Holstein herds. J Dairy Sci. 2003;86:3796–3801. doi: 10.3168/jds.S0022-0302(03)73987-3. [DOI] [PubMed] [Google Scholar]
- 25.National Research Council. Nutrient Requirements of Dairy Cattle. Washington, DC: Natl Acad Press; 2001. 7th revised ed. [Google Scholar]
- 26.McArt JA, Nydam DV, Ospina PA, Oetzel GR. A field trial on the effect of propylene glycol on milk yield and resolution of ketosis in fresh cows diagnosed with subclinical ketosis. J Dairy Sci. 2011;94:6011–6020. doi: 10.3168/jds.2011-4463. [DOI] [PubMed] [Google Scholar]
- 27.OMAFRA. Dairy Farm Wage Rate. 2015. [Last accessed May 3, 2016]. Available from: http://www.omafra.gov.on.ca/english/livestock/dairy/facts/wagerate.htm.
- 28.Gardner IA, Hird DW, Utterback WW, et al. Mortality, morbidity, case-fatality, and culling rates for California dairy cattle as evaluated by the national animal health monitoring system, 1986–87. Prev Vet Med. 1990;8:157–170. [Google Scholar]
- 29.Duffield TF. Metabolic disorders of ruminants: Subclinical ketosis in lactating dairy cattle. Vet Clin North Am Food Anim Pract. 2000;16:231–253. doi: 10.1016/s0749-0720(15)30103-1. [DOI] [PubMed] [Google Scholar]
- 30.Guard CL. The costs of common diseases in dairy cattle. Proc Central Veterinary Conference (CVC); San Diego, California. 2008. [Google Scholar]
- 31.Gröhn YT, McDermott JJ, Schukken YH, Hertl JA, Eicker SW. Analysis of correlated continuous repeated observations: Modelling the effect of ketosis on milk yield in dairy cows. Prev Vet Med. 1999;39:137–153. doi: 10.1016/s0167-5877(98)00145-7. [DOI] [PubMed] [Google Scholar]
- 32.Braun U, Schweizer G, Feller B, Pospischil A. Aspriation pneumonia in 40 cows after oral treatment. Schweiz Arch Tierheilkd. 2007;149:363–365. doi: 10.1024/0036-7281.149.8.363. [DOI] [PubMed] [Google Scholar]