Abstract
A partial budget model was developed to evaluate the economic value of Rumensin Controlled Release Capsule (CRC) boluses when administered before calving to reduce disease and increase milk production. After accounting for disease incidences in a herd and the percentage by which Rumensin CRC can reduce them, and the increase in milk production attributable to administration of Rumensin CRC, the return on investment (ROI) per lactation was 4:1. Another partial budget model was developed to estimate the economic value of propylene glycol (PG) to treat ketosis when diagnosed by 3 different cow-side tests or when administered to all cows without using any cow-side testing. After accounting for the sensitivity and specificity of each test, ROI per lactation ranged from 2:1 to 4:1. The ROI was 2:1 when no cow-side testing was used. In conclusion, prevention of diseases that occur in the postpartum period and treatment of ketosis after calving yielded a positive ROI that varies based on disease incidence and method of diagnosis.
Résumé
Valeur économique des ionophores et du propylèneglycol pour prévenir la maladie et traiter l’acétonémie au Canada. Un modèle de budget partiel a été développé pour évaluer la valeur économique des bolus de capsules à libération contrôlée (CLC) de Rumensin lors de l’administration avant le vêlage afin de réduire les maladies et d’accroître la production de lait. Après avoir tenu compte de l’incidence des maladies dans un troupeau et du pourcentage par lequel la CLC de Rumensin peut les réduire et de l’augmentation de la production de lait attribuable à l’administration de la CLC de Rumensin, le rendement du capital investi (RCI) par lactation était de 4:1. Un autre modèle de budget partiel a été développé pour estimer la valeur économique du propylèneglycol (PG) afin de traiter l’acétonémie lors du diagnostic par 3 tests différents pour les vaches ou lors de l’administration à toutes les vaches sans le recours à des tests auprès des vaches. Après avoir tenu compte de la sensibilité et de la spécificité de chaque test, le RCI par lactation s’échelonnait de 2:1 à 4:1. Le RCI était de 2:1 lorsqu’aucun test auprès des vaches n’était utilisé. En conclusion, la prévention des maladies qui se produit dans la période postpartum et le traitement de l’acétonémie après le vêlage a donné un RCI positif qui varie selon l’incidence de maladies et la méthode de diagnostic.
(Traduit par Isabelle Vallières)
Introduction
The “classic” transition period has been defined as the 3 wk prior to and following calving (1) and is a critical period in the lactation cycle of a dairy cow. During this phase cows start to experience a decrease in dry matter intake (DMI) (2) that reaches its nadir at calving and then gradually increases until a peak at 10 to 12 wk after calving (3). The peak in DMI is preceded by the peak in milk production at 7 to 9 wk following calving, and as a consequence cows will go through a period of negative energy balance (NEB). Cows respond to NEB by mobilizing their body fat to meet the energy requirements, resulting in an increase in blood ketones (4). The period of NEB is also associated with depression in immune function (5). As a result, almost half of the cows in this phase are affected by infectious or metabolic diseases (6), which in turn will influence their well-being and the profitability of the dairy enterprise.
One of the management approaches commonly used during the entire non-lactating period and extending into lactation is the administration of ionophores such as monensin to help reduce health problems and increase milk production (7). Administration of ionophores to dairy cattle resulted in an improvement in energy metabolism (8), milk production (9), and health, including a reduction in the risk of ketosis and displaced abomasum (DA), but not reproductive performance.
On the treatment side, one of the effective protocols used to treat ketosis is oral administration of propylene glycol (PG) (10,11). However, the number of cows that will or will not receive treatment depends on the underlying risk of hyperketonemia and the sensitivity and specificity of the cow-side test used to diagnose ketosis (6), which in turn can affect the outcome of a treatment program and impact its economic value.
The objective of this study was to model the economic value of using Rumensin Controlled Release Capsule (CRC) (Elanco Animal Health, Eli Lilly and Co., Greenfield, Indiana, USA) to help prevent post-parturient diseases in Canada, and to estimate the economic value of using PG in treating cows when diagnosed with ketosis using different cow-side tests, and when administered to all cows without using cow-side testing.
Materials and methods
The economic value of Rumensin CRC to reduce disease incidence and increase milk production was estimated using a partial budget model in which the increased revenues, decreased revenues, and increased expenses associated with its administration before calving were compared to when not administered. Following the same approach, a different partial budget model was developed to evaluate the economic value of treating ketotic cows with PG when diagnosed using 3 different cow-side tests and when given to all cows without diagnosis with cow-side testing. For both models, increased revenue was a result of an increase in milk production, decreased revenue was a result of an increase in disease incidence, whereas increased expenses were associated with extra labor used to administer treatments, cost of treatments, and cow-side testing in case of treatment with PG.
Cost of clinical diseases
Costs of DA and metritis were obtained from a recent study (12). The costs of mastitis and retained placenta were obtained from 2 other studies, respectively (13,14), whereas the costs of ketosis and clinical ketosis (CK) were estimated previously (28). Costs of clinical diseases retrieved from the literature were used after excluding milk losses, drug costs, and veterinary fees from total disease costs to avoid double counting in current calculations. Disease costs used in the current analysis are listed in Table 1.
Table 1.
Values of disease costs used in a partial budget model to evaluate the economic benefit of using Rumensin Controlled Release Capsule (CRC) to reduce disease incidence and increase milk production and to evaluate the economic benefit of using propylene glycol (PG) to treat cows with ketosis when diagnosed by different cow-side tests. All values listed are in Canadian dollars
| Disease | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Cost estimate | DA | MET | MAST | RP | CK | KET |
| Total loss | 905 | 507 | 572 | 403 | 233 | 203 |
| Losses excluding milk lossc | 833a,b | — | — | — | — | — |
| Losses excluding milk loss, veterinary fees, and drugs | — | 271a | 303a | 255a | 53a,b | — |
| Losses after excluding the cost of clinical disease risk (DA, MET, CK) attributable to ketosis | — | — | — | — | — | 127a |
| Losses after excluding the cost of clinical disease risk (DA, CK) and culling attributable to ketosis | — | — | — | — | — | 152b |
DA — displaced abomasum; MET — metritis; MAST — mastitis; RP — retained placenta; CK — clinical ketosis; cases of ketosis identified by clinical signs regardless of serum BHBA concentrations; KET — ketosis; defined as elevated serum BHBA ≥ 1400 μmol/L in either of the first 2 wk following calving and without showing clinical signs.
Values used in the partial budget model to estimate the economic value of Rumensin CRC to reduce disease incidence and increase milk production.
Values used in the partial budget model to estimate the economic value of PG to treat cows with ketosis.
The cost of milk loss only was excluded from the total cost of a DA because veterinary fees and drug costs are unique to the surgery.
Model for estimating the economic value of Rumensin CRC for disease reduction
A partial budget was developed in Excel (Microsoft office 2010; Microsoft, Redmond, Washington, USA) to estimate the economic value of Rumensin CRC boluses administered in the close-up period to decrease the incidence of disease after calving and to increase milk production. Inputs and assumptions used in the model are listed in Table 2.
Table 2.
Assumptions and inputs used in the partial budget model to evaluate the economic benefit of using Rumensin Controlled Release Capsule (CRC) to reduce disease incidence and increase milk production. Value listed for each clinical disease is the percentage by which CRC can reduce it whereas values in parentheses are the median incidences used as a default input for each disease in the model
| Item | Value | Reference |
|---|---|---|
| Effect of using CRC on disease reduction | ||
| Metritis | 16% (16.7%) | 17 |
| Ketosis | 50% (40%) | 20 |
| Mastitis | 9% (40%) | 17 |
| Clinical ketosis | 25% (9.6%) | 17 |
| Retained placenta | 8% (16.7%) | 17 |
| Displaced abomasum | 25% (4.8%) | 17 |
| Effect of using CRC on milk production | ||
| Increase production in BCS = 3.25 to 3.75a | 0.85 kg/d | 15 |
| Increase production in BCS ≥ 4b | 1.2 kg/d | 15 |
| Time period milk is increased | 90 days | 15 |
| Price of 1 bolus of CRC | $18 | |
| Milk price | $0.81 | 21 |
| Dairy efficiencyc | 2.3 | |
| Cost of 1 kg of TMR (dry matter basis) | $0.3 | 22 |
| Labor wages/h | $15 | 23 |
| CRC boluses administered/h | 15 | |
Cows with BCS between 3.25 and 3.75 are assumed to be 65% of the lactating animals in the default inputs used to build the partial budget model.
Cows with BCS ≥ 4 are assumed to be 10% of the lactating animals in the default inputs used to build the partial budget model.
Milk yield (kg) produced as a result of a cow consuming 1 additional kg of dry matter above maintenance requirements (24); this calculation accounts only for the concentration of energy required to produce marginal milk after considering a NEL 0.75 Mcal/kg of 4% milk and 1.73 Mcal/kg of feed in dry matter basis (25).
The economic benefits of using Rumensin CRC are a function of both the reduction in economic impact of peri-parturient disease and an increase in revenue due to more efficient milk production during lactation. Reduction in economic impact of peri-parturient disease is achieved by a reduction in disease incidence after administration of Rumensin CRC. The percentage by which disease incidence is reduced is listed in Table 2. The reduced disease incidence was multiplied by the respective cost of each disease and then compared to costs when Rumensin CRC was not administered. Increased revenue due to increased milk production after administration of Rumensin CRC for cows within each body condition score (BCS) (range: 3.25 to 3.75 and ≥ 4 in a 5-point scale) was calculated based on a marginal increase in milk production for 90 d (15) by deducting the cost of extra feed consumed after administration of Rumensin CRC from the extra revenue obtained from selling more milk after using Rumensin CRC. Total cost of labor to administer Rumensin CRC was calculated based on hourly wages and the number of Rumensin CRC boluses administered/hour. The net revenue of using Rumensin CRC was compared to not using it and then the return on investment (ROI) was calculated using the following formula:
Because it is difficult to disentangle how much of the increase in milk production was due to the direct effect of Rumensin CRC or to the reduction in ketosis, a sensitivity analysis specific to milk production was performed. Hypothetical scenarios starting with an assumption that 100% of the milk increase (i.e., 0.85 kg/d and 1.2 kg/d for cows with a BCS ranging from 3.25 to 3.75 and with a BCS ≥ 4, respectively) (15) is attributed to the direct effect of Rumensin CRC, and then allowing for 10% of the increase in milk production to be due to reduction of ketosis (i.e., 90% of milk increase is due to the direct effect of administering Rumensin CRC) until assuming that 100% of the milk increase was attributed to reduction of ketosis. Another sensitivity analysis was performed to evaluate the effect of a change of 1 unit of an input at a time on the default ROI obtained from the base model. The following inputs were varied by adding/subtracting 50% of the default value used: price of Rumensin CRC, cost of 1 kg of feed, proportion of cows with BCS ranging from 3.25 to 3.75 and with a BCS ≥ 4, hourly labor wage, and the number of cows treated/hour. The ranges used for the following inputs were based on ranges reported previously by 1 study (16) for ketosis and by another study (14) for CK, metritis, retained placenta, mastitis, and DA. Finally, milk price was varied by adding/subtracting 10 cents to/from the default value used in the model.
Model for estimating the economic value of propylene glycol for treatment of ketosis
Another partial budget model was built to estimate the economic value of using oral PG to treat ketosis after being diagnosed using 1 of 3 different cow-side tests, and after treating all cows without cow-side testing. The 3 tests used were: Precision XTRA (Abbott Laboratories, Abbott Park, Illinois, USA) using a blood sample, Keto-Test (Elanco Animal Health) using a milk sample, or Ketostix (Bayer Animal Health, Shawnee Mission, Kansas, USA) using a urine sample (Table 3). The sensitivity and specificity of each method of diagnosis of ketosis was incorporated into calculations. Other inputs, assumptions, and calculations used in building the model are listed in Tables 3 and 4.
Table 3.
Assumptions and inputs used for each cow-side test to evaluate the economic benefit of using propylene glycol to treat cows with ketosis, and sensitivity and specificity of each cow-side test used to diagnose ketosis and the number of true positives, false positives, and false negatives within each testing method, assuming a herd size of 1 cow and a cumulative incidence of 40% for ketosis
| Precision XTRA | Keto-Test | Ketostixa | |
|---|---|---|---|
| Sensitivityb | 0.90 | 0.8 | 0.79 |
| Specificityb | 0.965 | 0.94 | 0.96 |
| True positive | 0.360 | 0.328 | 0.190 |
| False positive | 0.021 | 0.036 | 0.254 |
| False negative | 0.040 | 0.072 | 0.210 |
| Cost of test | $3.0 | $2.0 | $0.25 |
| Cows providing urine | — | — | 60% |
| Cost of 4 doses of PG | $12 | $12 | $12 |
| Total cost of PG | $4.57 | $4.37 | $5.33 |
| Total cost of labor | $0.76 | $0.73 | $0.89 |
Sensitivity and specificity of Ketostix is only applied to cows from which a urine sample can be obtained. The baseline used in this model was 60% of cows will provide a urine sample resulting in a sensitivity and specificity of 47.4% and 57.6% respectively, for Ketostix.
From reference 6.
Table 4.
Assumptions and inputs used in the partial budget model to evaluate the economic benefit of using propylene glycol (PG) to treat cows with ketosis when diagnosed by different cow-side tests
| Item | Value | Reference |
|---|---|---|
| Effect of PG on disease/culling reduction when administered to ketotic cows | ||
| Clinical ketosis | 46% | 10 |
| Ketosis | 50% | 10 |
| Displaced abomasum | 37.5% | 11 |
| Culling | 52.4% | 11 |
| LIRa of each event affecting cows with ketosis that PG can impact | ||
| Clinical ketosis | 6.2% | 28 |
| Ketosis | 40% | 26 |
| Displaced abomasum | 3.3% | 28 |
| Culling | 1.8% | 27 |
| Effect of PG on milk yield when administered to ketotic cows | ||
| Increase in milk production | 0.69 kg/d | 10 |
| Time period milk is increased | 30 d | 10 |
| Loss due to premature culling | $500 | 28 |
| Milk price | $0.81 | 21 |
| Dairy efficiencyb | 2.3 | |
| Cost of 1 kg of TMR (dry matter basis) | $0.3 | 22 |
| Labor wages/h | $15 | 23 |
| Number of cows administered with PG/h | 15 | |
| Number of times PG will be administered | 4 | 10 |
Lactational incidence risk = the number of cows affected with a certain event during a lactation divided by the number of cows that calved in the same time period.
Milk yield (kg) produced as a result of a cow consuming 1 additional kg of dry matter above maintenance requirements (24); this calculation accounts only for the energy required to produce marginal milk after considering a NEL concentration of 0.75 Mcal/kg of 4% milk and 1.73 Mcal/kg of feed in dry matter basis (25).
Two studies were referenced to evaluate the impact of PG on disease and culling reduction (10,11). One study (11) reported risk ratios for culling and developing DA in control cows. According to this study, control cows were 2.1 times more likely to be culled than cows treated with PG. Therefore, treated cows will be 0.476 (1/2.1) times less likely to be culled than control cows, which means that PG decreases culling by 52.4% (1 – 0.476). Using the same approach, the percentage of DA will decrease by 37.5% after administration of PG to ketotic cows. The other study (10) reported hazard ratios for developing CK and resolving ketosis for cows treated with PG. Following the same approach as above it can be calculated that CK will decrease by 46% (1 – 0.54), and ketosis will be resolved by 50% in cows treated with PG.
Sensitivity and specificity, in addition to the cost of each test used to diagnose ketosis, were obtained from a review by LeBlanc (6). Sensitivity and specificity of Ketostix were multiplied by the assumed percent of cows from which a urine sample can be easily obtained (60%). After assuming a cumulative incidence of ketosis, true positive, true negative, false negative, and false positive numbers for each test were calculated. It was assumed that cows will be treated orally with 300 g of PG for 4 d (11). Total cost of PG was calculated by summing true positives and false positives and then multiplying them by the cost of 1.2 kg of PG. Labor costs were calculated based on hourly wages and the number of cows that can be treated/hour.
The economic value of clinical disease/culling reduction as a consequence of using PG to treat ketosis was calculated for each cow-side test by multiplying disease reduction/culling in ketotic cows treated with PG and the respective cost of each clinical disease/culling. In addition, the cost of increased disease/culling risk in cows diagnosed as false negatives using each cow-side test was calculated by multiplying the number of false negatives resulting from each cow-side test, the attributable risk of clinical disease/culling due to ketosis, and the cost of each clinical disease/culling (28).
For 1 cow with ketosis both marginal increased (if treated) and decreased (if not treated) milk production after administering (if treated) or not administering (if not treated) PG were calculated. These values were multiplied by the number of true positives and false negatives of each cow-side test, respectively, to calculate the marginal revenue for increased milk production when treated and the opportunity cost for milk not produced if not treated.
The net revenue of using PG after diagnosing ketosis with each cow-side test was compared to that of not treating cows with ketosis and then ROI was calculated as described for Rumensin CRC. Finally, a sensitivity analysis was done to study the impact of the change of an input on the ROI of each diagnostic test.
Results
Model for estimating the economic value of Rumensin CRC for disease reduction
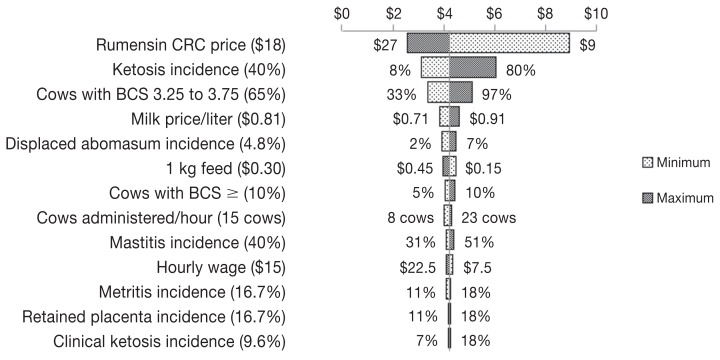
The ROI after using Rumensin CRC was 4:1. Sensitivity analysis varying how much of the increase in milk production could be due to the direct effect of Rumensin CRC or the reduction in the incidence of ketosis showed an ROI ranging from 4:1 when 100% of the increase in production is attributed to the administration of Rumensin CRC to 2:1 when 100% of the increase in production is attributed to the reduction of disease. Regarding the general sensitivity analysis, change in the price of Rumensin CRC and the incidence of ketosis had the greatest impact on ROI, followed by the percent of cows with a BCS of 3.25 to 3.75 (Figure 1).
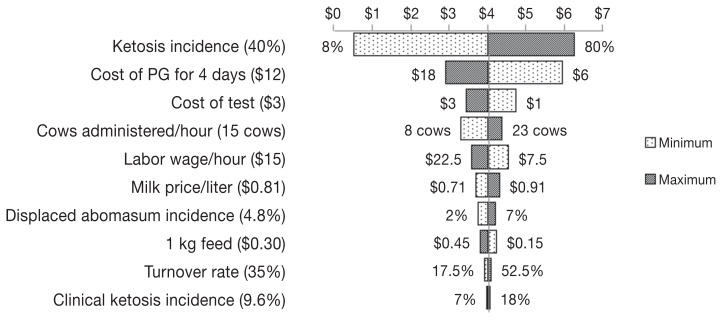
Figure 1.
A Tornado plot depicting the change in the return on investment after using Rumensin controlled release capsule (CRC) in Canada. Values in parentheses are baseline values used in building the model, while values on the sides of the bars are the minimum and maximum values used for sensitivity analysis. The line in the middle of the plot separating minimum and maximum bars represents the return on investment (indicated by $3.95 of return for $1 invested) resulting from using the baseline values of different inputs.
Model for estimating the economic value of propylene glycol for treatment of ketosis
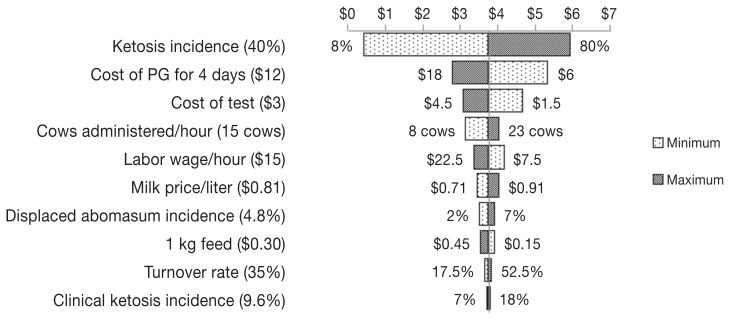
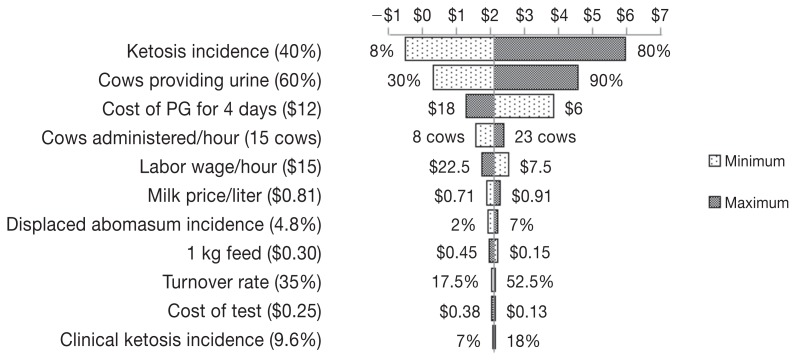
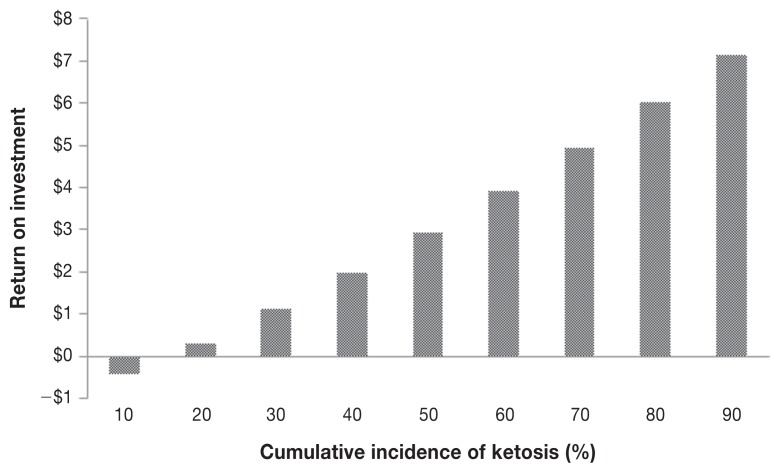
Returns on investment for treatment based on testing using Precision XTRA, Keto-Test, or Ketostix, and for treatment without testing were 4:1, 4:1, 2:1, and 2:1, respectively. Figures 2 to 4 show Tornado plots representing the sensitivity analysis for each of the cow-side tests. The incidence of ketosis and the cost of treatment were the most sensitive inputs for the 3 tests. In addition, the percent of cows providing a urine sample was among the sensitive inputs for the Ketostix test. Figure 5 shows the results of hypothetical scenarios of different incidences of ketosis where all cows were treated with PG without being tested.
Figure 2.
A Tornado plot depicting the change in the return on investment after using propylene glycol (PG) to treat ketosis diagnosed by Precision XTRA due to the change of 1 input at a time from the minimum to the maximum value. Values in parentheses are baseline values used in building the model while values on the sides of the bars are the minimum and maximum values used for sensitivity analysis. The line in the middle of the plot separating minimum and maximum bars represents the return on investment (indicated by $ of return per $1 of investment) resulting from using the baseline values of different inputs.
Figure 4.
A Tornado plot depicting the change in the return on investment after using propylene glycol (PG) to treat ketosis diagnosed by Ketostix due to the change of 1 input at a time from the minimum to the maximum value. Values in parentheses are baseline values used in building the model while values on the sides of the bars are the minimum and maximum values used for sensitivity analysis. The line in the middle of the plot separating minimum and maximum bars represents the return on investment (indicated by $ of return per $1 of investment) resulting from using the baseline values of different inputs.
Figure 5.
Variation in return on investment (ROI) after treating all cows with propylene glycol (PG) without testing them at different herd incidence levels of ketosis. At an average herd incidence of 40% the return on investment is 2:1 which is similar to the ROI of Ketostix when used to diagnose and treat cows with ketosis using PG.
Discussion
The current model for estimating the economic value of Rumensin CRC for disease reduction showed an economic benefit due to helping prevent diseases and increase milk production in a dairy herd. The higher the incidence of ketosis in a herd, the higher the value of using Rumensin CRC is. Reproductive performance and culling were not included as parameters in the model because a meta-analysis showed no impact of monensin on either parameter (17). There are no previous reports of economic analysis of using Rumensin CRC to help reduce disease and increase milk production. However, 1 study reported an increase of $0.39/day per cow in return over feed (milk income minus feed cost) when monensin was added to lactating cow rations (18).
Return on investment resulting from the sensitivity analysis specific to milk production is positive for all scenarios (ranging from 4:1 to 2:1). However, ROI will be lower if all of the increase in milk production was due to the reduction in incidence of ketosis. In the overall sensitivity analysis (Figure 1), the price of Rumensin CRC was the most sensitive input because the capsule is administered to all cows in a herd and there is a wide range of ketosis incidence (8% to 80%) among herds (16). The sensitivity of the proportion of cows with a BCS between 3.25 and 3.75 can be explained by the larger number of cows within this BCS range in a dairy herd than cows with a BCS ≥ 4.
The model used to evaluate the economic benefits of using PG to treat ketosis diagnosed with different cow-side tests showed positive values when different scenarios were implemented. Diseases included in the model (DA and CK) were limited by the literature available that studied the effect of using PG to treat ketosis and the impact of this treatment on other diseases. McArt et al (11) found no difference in time to pregnancy between treated and control cows, hence reproductive performance was not included in the current model. The price of the Precision XTRA meter was not included in calculations because of its relatively low price ($40) that will be distributed over hundreds or thousands of cows.
Although the sensitivity of Ketostix is comparable to that of Keto-Test (79% and 82%, respectively), the low number of cows that are expected to provide a urine sample resulted in reduction of both the sensitivity and specificity of Ketostix, resulting in the lowest ROI among the 3 cow-side tests evaluated. However, when a sensitivity analysis was performed, the percent of cows providing urine was among the sensitive inputs, indicating that if a urine sample was obtained from a high percentage of cows the ROI will be comparable to that of Precision XTRA and Keto-Test (Figure 4). The lower sensitivity of Ketostix meant that the lowest number of true positive cows would be diagnosed among the 3 cow-side tests and therefore losses from diseases and culling after using PG will be the least. However, these values were offset by the opportunity cost of cows diagnosed as false negatives and therefore will not benefit from the PG treatments due to the low test sensitivity resulting in the lowest ROI.
Return on investment for both Precision XTRA and Keto-Test were approximately the same. Although the opportunity cost of cows diagnosed as false negatives after using Precision XTRA was lower than that of Keto-Test (because of the higher sensitivity of Precision XTRA), this difference was not large enough to result in a higher ROI for Precision XTRA. In addition, comparable prices of test strips for Precision XTRA and Keto-Test ($3 and $2, respectively) (6) contributed to both tests having comparable results.
Sensitivity analysis showed that the incidence of ketosis was a common sensitive input among the 3 evaluated tests because of the wide range of incidences used in the current model (8% to 80%) to represent North American dairy herds (16). Cost of treatment was sensitive for the 3 evaluated tests; for both Keto-Test and Ketostix the reason was the higher number of false positives diagnosed by both tests compared to Precision XTRA, while for Precision XTRA the reason was the increase in the number of true positive cows that were treated due to the high test sensitivity. One should consider that the sensitivities and specificities of different cow-side tests evaluated in the current model were relative to a laboratory measurement of serum β-hydroxybutyrate ≥ 1400 μmol/L (6) which means that they could vary at a different cutoff.
Hypothetical scenarios depicted in Figure 5 (no treatment) show that the ROI when herd incidence of ketosis is 40% and all cows are treated without being tested will be comparable to the ROI after testing with Ketostix and then treating. As the incidence of ketosis increases in a herd the ROI of treating without testing will increase. Such a result agrees with a recent study (19) in which authors found that the strategy of treating all cows without testing is the most cost-effective approach if the incidence of ketosis is > 50%. One can therefore conclude that herd incidence and diagnostic procedures should be considered before implementing any monitoring or prevention programs.
In conclusion, return on investment for Rumensin CRC was positive and its magnitude of impact depends primarily on the herd incidence of ketosis. The impact of propylene glycol depends on ketosis incidence, and on the method used to diagnose ketosis. However, there was no economic difference between treating cows orally with PG after diagnosis of ketosis using either Precision XTRA or Keto-Test. Using PG to treat all cows without testing for ketosis is comparable to testing with Ketostix and then treating if herd incidence of ketosis is 40%. Treating without testing should be practiced cautiously as there are no reports that studied the impact of PG on health when not needed. The choice of a diagnostic cow-side test should be based on the herd incidence of ketosis, farm settings that can facilitate the use of one test of over another, and the farmer’s goal (e.g., presence of headlocks will facilitate the use of Precision XTRA, whereas testing in the milking parlour allows the efficient use of Keto-Test). CVJ
Figure 3.
A Tornado plot depicting the change in the return on investment after using propylene glycol (PG) to treat ketosis diagnosed by Keto-Test due to the change of 1 input at a time from the minimum to the maximum value. Values in parentheses are baseline values used in building the model while values on the sides of the bars are the minimum and maximum values used for sensitivity analysis. The line in the middle of the plot separating minimum and maximum bars represents the return on investment (indicated by $ of return per $1 of investment) resulting from using the baseline values of different inputs.
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
This study was funded by Elanco Animal Health, Greenfield, Indiana, USA.
References
- 1.Grummer RR. Impact of changes in organic nutrient metabolism on feeding the transition dairy cow. J Anim Sci. 1995;73:2820–2833. doi: 10.2527/1995.7392820x. [DOI] [PubMed] [Google Scholar]
- 2.Bertics SJ, Grummer RR, Cadorniga-Valino C, Stoddard EE. Effect of prepartum dry matter intake on liver triglyceride concentration and early lactation. J Dairy Sci. 1992;75:1914–1922. doi: 10.3168/jds.S0022-0302(92)77951-X. [DOI] [PubMed] [Google Scholar]
- 3.Bauman DE, Currie WB. Partitioning of nutrients during pregnancy and lactation: A review of mechanisms involving homeostasis and homeorhesis. J Dairy Sci. 1980;63:1514–1529. doi: 10.3168/jds.s0022-0302(80)83111-0. [DOI] [PubMed] [Google Scholar]
- 4.Herdt TH. Ruminant adaptation to negative energy balance. Influences on the etiology of ketosis and fatty liver. Vet Clin North Am Food Anim Pract. 2000;16:215–230. doi: 10.1016/s0749-0720(15)30102-x. [DOI] [PubMed] [Google Scholar]
- 5.Goff JP. Major advances in our understanding of nutritional influences on bovine health. J Dairy Sci. 2006;89:1292–1301. doi: 10.3168/jds.S0022-0302(06)72197-X. [DOI] [PubMed] [Google Scholar]
- 6.LeBlanc S. Monitoring metabolic health of dairy cattle in the transition period. J Reprod Dev. 2010;56( Suppl):S29–35. doi: 10.1262/jrd.1056s29. [DOI] [PubMed] [Google Scholar]
- 7.Duffield TF, Bagg RN. Use of ionophores in lactating dairy cattle: A review. Can Vet J. 2000;41:388–394. [PMC free article] [PubMed] [Google Scholar]
- 8.Duffield TF, Rabiee AR, Lean IJ. A meta-analysis of the impact of monensin in lactating dairy cattle. Part 1. Metabolic effects. J Dairy Sci. 2008;91:1334–1346. doi: 10.3168/jds.2007-0607. [DOI] [PubMed] [Google Scholar]
- 9.Duffield TF, Rabiee AR, Lean IJ. A meta-analysis of the impact of monensin in lactating dairy cattle. Part 2. Production effects. J Dairy Sci. 2008;91:1347–1360. doi: 10.3168/jds.2007-0608. [DOI] [PubMed] [Google Scholar]
- 10.McArt JA, Nydam DV, Ospina PA, Oetzel GR. A field trial on the effect of propylene glycol on milk yield and resolution of ketosis in fresh cows diagnosed with subclinical ketosis. J Dairy Sci. 2011;94:6011–6020. doi: 10.3168/jds.2011-4463. [DOI] [PubMed] [Google Scholar]
- 11.McArt JA, Nydam DV, Oetzel GR. A field trial on the effect of propylene glycol on displaced abomasum, removal from herd, and reproduction in fresh cows diagnosed with subclinical ketosis. J Dairy Sci. 2012;95:2505–2512. doi: 10.3168/jds.2011-4908. [DOI] [PubMed] [Google Scholar]
- 12.McArt JA, Nydam DV, Overton MW. Hyperketonemia in early lactation dairy cattle: A deterministic estimate of component and total cost per case. J Dairy Sci. 2015;98:2043–2054. doi: 10.3168/jds.2014-8740. [DOI] [PubMed] [Google Scholar]
- 13.Overton M, Rollin E. Mastitis in the Vital 90™ Days...What’s the Real Cost?. Proceedings of The High Plains Dairy Conference; Lubbock, Texas, USA. 2014. pp. 41–49. [Google Scholar]
- 14.Guard CL. The costs of common diseases in dairy cattle. Proc CVC Continuous Education Convention; San Diego, California, USA. 2008. [Google Scholar]
- 15.Duffield TF. DVSc Thesis. Guelph, Ontario: University of Guelph; 1997. Effects of a monensin controlled release capsule on energy metabolism, health and production in lactating dairy cattle. [Google Scholar]
- 16.Duffield TF. Subclinical ketosis in lactating dairy cattle. Vet Clin North Am Food Anim Pract. 2000;16:231–253. doi: 10.1016/s0749-0720(15)30103-1. [DOI] [PubMed] [Google Scholar]
- 17.Duffield TF, Rabiee AR, Lean IJ. A meta-analysis of the impact of monensin in lactating dairy cattle. Part 3. Health and reproduction. J Dairy Sci. 2008;91:2328–2341. doi: 10.3168/jds.2007-0801. [DOI] [PubMed] [Google Scholar]
- 18.McLaren CJ, Lissemore KD, Duffield TF, Leslie KE, Kelton DF, Grexton B. The association of herd milk production and management with a return-over-feed index in Ontario dairy herds. J Dairy Sci. 2005;88:419–425. doi: 10.3168/jds.S0022-0302(05)72703-X. [DOI] [PubMed] [Google Scholar]
- 19.McArt JA, Nydam DV, Oetzel GR, Guard CL. An economic analysis of hyperketonemia testing and propylene glycol treatment strategies in early lactation dairy cattle. Prev Vet Med. 2014;117:170–179. doi: 10.1016/j.prevetmed.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Duffield TF, Sandals D, Leslie KE, et al. Efficacy of monensin for the prevention of subclinical ketosis in lactating dairy cows. J Dairy Sci. 1998;81:2866–2873. doi: 10.3168/jds.S0022-0302(98)75846-1. [DOI] [PubMed] [Google Scholar]
- 21.Dairy Farmers of Ontario. The Milk Producer Magazine. Markets. [Last accessed May 16, 2016]. Available from: https://www.milk.org/Corporate/view.aspx?content=aboutus/MilkProducerMagazine.
- 22.Guideline for estimating dairy cow production costs in Manitoba. 2015. [Last accessed May 16, 2016]. Available from: http://www.gov.mb.ca/agriculture/business-and-economics/financial-management/pubs/cop_dairy_cow.pdf.
- 23.OMAFRA. Dairy Farm Wage Rate. 2015. [Last accessed May 16, 2016]. Available from: http://www.omafra.gov.on.ca/english/livestock/dairy/facts/wagerate.htm.
- 24.Britt JS, Thomas RC, Speer NC, Hall MB. Efficiency of converting nutrient dry matter to milk in Holstein herds. J Dairy Sci. 2003;86:3796–3801. doi: 10.3168/jds.S0022-0302(03)73987-3. [DOI] [PubMed] [Google Scholar]
- 25.National Research Council. Nutrient Requirements of Dairy Cattle. 7th revised ed. Washington, DC: Natl Acad Press; 2001. [Google Scholar]
- 26.McArt JA, Nydam DV, Oetzel GR. Epidemiology of subclinical ketosis in early lactation dairy cattle. J Dairy Sci. 2012;95:5056–5066. doi: 10.3168/jds.2012-5443. [DOI] [PubMed] [Google Scholar]
- 27.Roberts T, Chapinal N, Leblanc SJ, Kelton DF, Dubuc J, Duffield TF. Metabolic parameters in transition cows as indicators for early-lactation culling risk. J Dairy Sci. 2012;95:3057–3063. doi: 10.3168/jds.2011-4937. [DOI] [PubMed] [Google Scholar]
- 28.Gohary K, Overton MW, von Massow M, LeBlanc SJ, Lissemore KD, Duffield TF. The cost of a case of subclinical ketosis in Canadian dairy herds. Can Vet J. 2016;57:728–732. [PMC free article] [PubMed] [Google Scholar]