Abstract
A 5-month-old female pit bull terrier dog evaluated for ataxia, progressive regurgitation, and recurrent aspiration pneumonia had markedly elevated creatine kinase activity, non-inflammatory generalized myopathy, and severe esophageal dysmotility. A narrow-field total laryngectomy was performed. The dog is doing well 30 months after surgery, and no longer has episodes of aspiration pneumonia, despite intermittent regurgitation. This case represents the first application of total laryngectomy for the prevention of chronic recurrent aspiration pneumonia in the dog.
Résumé
Laryngectomie totale pour la gestion d’une pneumonie par aspiration chronique chez un chien myopathique. Une chienne Pit Bull Terrier âgée de 5 mois évaluée pour de l’ataxie, de la régurgitation progressive et une pneumonie par aspiration récurrente présentait une activité de la créatine kinase particulièrement élevée, une myopathie généralisée non inflammatoire et un trouble de motilité de l’œsophage grave. Une laryngectomie totale à champ étroit a été réalisée. La chienne se porte bien 30 mois après la chirurgie et n’a plus d’épisodes de pneumonie par aspiration, malgré une régurgitation intermittente. Ce cas représente la première application d’une laryngectomie totale pour la prévention d’une pneumonie par aspiration chronique récurrente chez un chien.
(Traduit par Isabelle Vallières)
Since Theodor Billroth performed the first human laryngectomy for laryngeal carcinoma in 1873, the procedure has been modified and improved many times (1–6). Total laryngectomy has been used to prevent chronic aspiration in 4 human patients (7); however, application of the procedure for treatment of chronic aspiration in humans was limited due to complete loss of phonation and naso-laryngeal breathing, hyposmia, and atrophy of nasal mucosa (7,8). Various medical and surgical alternatives to laryngectomy have been used for chronic aspiration in humans, including dietary and behavioral modification, oral care and sitting posture, levodopa to improve swallow and cough reflex, tracheostomy with gas bag, laryngeal diversion, tracheoesophageal diversion, laryngeal diversion with a speech fistula, dynamic laryngotracheal closure, and a paced glottis closure (9–13). Conservative measures are generally preferred for concerns of voice preservation in humans. However, when medical management fails, total laryngectomy is the gold standard treatment of chronic and life-threatening aspiration pneumonia in humans (7). Experimental laryngectomy was first performed in the dog in the early 19th century (6,14), and was exclusively used for the surgical management of laryngeal cancer in dogs (15–17). To the best of our knowledge, a total laryngectomy has not been performed for the treatment and prevention of chronic recurrent aspiration pneumonia in the dog.
This report documents the successful management via total laryngectomy of a 5-month-old female pit bull terrier with progressive regurgitation and recurrent aspiration pneumonia secondary to a severe non-inflammatory congenital myopathy.
Case description
A 5-month-old female pit bull terrier dog was referred to the William R. Pritchard Veterinary Medical Teaching Hospital (VMTH), University of California, Davis, for evaluation of an abnormal gait. The dog was rescued 1 mo previously, and the previous medical history was unknown. At presentation, the dog weighed 16 kg with a body condition score (BCS) of 4/9. The pelvic limbs were held in an adducted position, and the pelvic limb musculature was palpably firm, with limited range of motion and apparent pain on gentle manipulation of the coxofemoral (CF) joints. The thoracic limbs had a wide-based stance with elbow abduction, but normal range of motion in all joints. On neurological examination, abnormalities were restricted to a short-strided gait in all 4 limbs, reduced flexor reflexes and delayed proprioception in the pelvic limbs. Bilateral CF luxation was noted on pelvic radiographs. A serum chemistry profile revealed hyperphosphatemia [2.2 mmol/L, reference range (RR): 0.84 to 1.68 mmol/L], hypercalcemia (3 mmol/L, RR: 2.4 to 2.8 mmol/L), and marked elevation of creatine kinase (CK) activity (74 272 IU/L, RR: 55 to 257 IU/L) with elevated alanine aminotransferase (ALT) (295 IU/L, RR: 21 to 72 IU/L) and aspartate aminotransferase (AST) (1339 IU/L, RR: 20 to 49 IU/L). The alkaline phosphatase (ALP) activity was mildly increased (97 IU/L, RR: 14 to 91 IU/L). The elevated CK and AST were consistent with a myopathy, and the elevated ALP activity and increased phosphorus were associated with osteoblastic activity during growth. A complete blood (cell) count (CBC) and urinalysis were unremarkable. Infectious disease titers for Neospora (direct fluorescent antibody) and Toxoplasma (latex agglutination test) were negative.
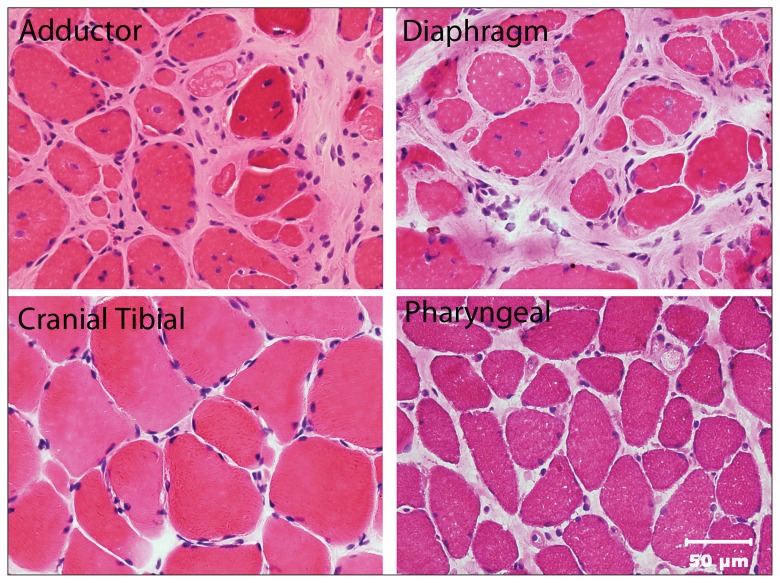
The dog was placed under general anesthesia for an electro-diagnostic evaluation by electromyography (EMG) and biopsies were collected from the cranial tibial, vastus lateralis, triceps, and adductor muscles. In addition, the dog underwent a bilateral femoral head ostectomy (FHO) and tenotomies of the pectineus muscle to improve the CF pain and ability to ambulate. The pectineus muscle was also biopsied during the FHO. The EMG was abnormal and characterized by diffuse complex repetitive discharges in the distal and proximal thoracic and pelvic limb, epaxial, head, and tongue muscles. All muscle biopsies revealed a non-inflammatory generalized myopathy with dystrophic features, most severe in the adductor (Figure 1) and pectineus muscles.
Figure 1.
Cryosections from the adductor, diaphragm, cranial tibial, and pharyngeal muscles stained with hematoxylin and eosin (H&E). Excessive variability in myofiber size is evident in the adductor, diaphragm, and pharyngeal muscles with marked endomysial fibrosis separating individual muscle fibers in the adductor and diaphragm muscles consistent with a severe degenerative myopathy. The cranial tibial muscle is relatively spared.
Bar = 50 μm for all images.
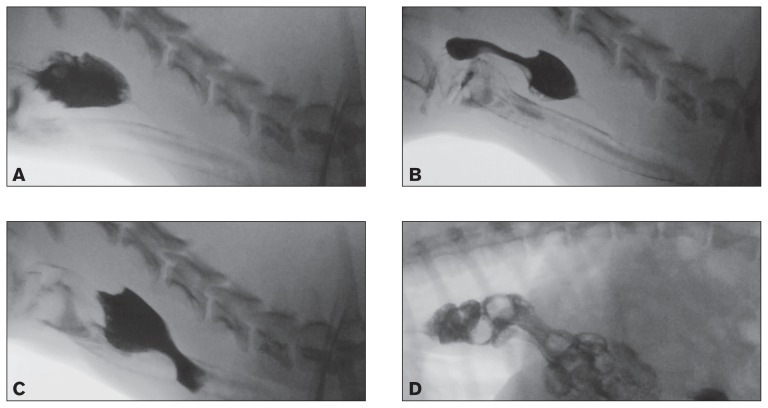
Following adoption, the dog was noted to have progressive dysphagia and regurgitation. Thoracic radiographs revealed a fluid-filled caudal thoracic esophagus and a videofluoroscopic swallow study revealed evidence of severe esophageal dysmotility in the cranial esophagus characterized by retention of large volumes of liquid barium and retrograde motility of the bolus into the pharynx (Figure 2A–C). In addition, the dog had multiple episodes of gastroesophageal reflux and a sliding (Type I) hiatal hernia (Figure 2D). Persistent regurgitation was managed with famotidine (Famotidine; Major Pharmaceuticals, Livonia, Michigan, USA), 1 mg/kg body weight (BW), PO, q24h, sucralfate (Carafate; Nostrum Laboratories, Kansas City, Missouri, USA), 1 g PO, q8h, and omeprazole (Omeprazole delayed release tablets; Dexcel Pharma Technologies, Yokneam, Israel), 1 mg/kg BW, PO, q24h. Feedings were changed to a commercial complete and balanced puppy food fed in small volumes 3 times daily. Although the dog was fed in a dog sitting position, regurgitation persisted. The dog underwent corrective surgery for the hiatal hernia characterized by a left-sided gastropexy, esophagopexy, and esophageal hiatal plication 2 mo later. The dog’s diaphragm was found to be markedly thickened secondary to the severe myopathy and the diaphragmatic defect was reduced. Two biopsies were obtained from the left crura of the diaphragm and the muscle was found to be severely affected with a dystrophic process similar to that observed in the other muscles (Figure 1). The frequency of regurgitation decreased for approximately 3 mo; however, clinical signs progressed shortly thereafter and the dog developed aspiration pneumonia that was treated with ampicillin/sulbactam (Unasyn; Pfizer, New York, New York, USA), 50 mg/kg BW, IV, q8h. A repeat videofluoroscopic swallow study revealed severe segmental esophageal dysmotility characterized by the absence of primary peristaltic waves and pooling of barium in the proximal esophagus. Liquid barium was subsequently refluxed through the upper esophageal sphincter into the pharynx and nasopharynx, and a large amount of barium was subsequently aspirated into the trachea. Persistence of gastroesophageal reflux and hiatal herniation was noted. Progression of the underlying myopathy with concurrent esophagitis secondary to reflux was considered the likely etiology. Esophagoscopy revealed that the proximal half of the esophagus was abnormally dilated and the gastroesophageal junction had rugal folds protruding above the diaphragmatic pinch. Significant reflux was observed with the application of gastric pressure. A 24-French percutaneous endoscopic gastrostomy (PEG) tube (EndoVive PEG tube; Boston Scientific, Marlborough, Massachusetts, USA) was placed to facilitate feeding, and this was replaced with a 24-Fr low-profile button (Bard Button; Bard Access Systems, Salt Lake City, Utah, USA) after 2 mo.
Figure 2.
A, B, C — Videofluoroscopic swallow study with liquid barium documenting evidence of aspiration characterized by barium coating the trachea and severe esophageal dysmotility with retrograde movement of the bolus in the proximal esophagus prior to movement of the bolus in an aborad direction. D — Videofluoroscopic swallow study with barium-soaked kibble documenting displacement of the cardia cranially to the diaphragmatic crus (sliding hiatal hernia) with gastroesophageal reflux.
Progressive regurgitation and recurrent aspiration pneumonia necessitated frequent visits and hospitalizations at the VMTH, with antimicrobial therapy consisting of ampicillin/sulbactam (Unasyn; Pfizer), 50 mg/kg BW, IV, q8h and enrofloxacin (Baytril; Bayer Healthcare, Shawnee Mission, Kansas, USA), 10 mg/kg BW by PEG tube q24h, with nebulization and coup-age necessary to manage the pneumonia. The dog’s quality of life was poor with frequent episodes of regurgitation, nasal reflux of ingesta, and difficulty sleeping due to partial airway obstruction. The dog lost 3 kg body weight and had a BCS of 2/9. Regurgitation persisted despite administration of cisapride (Cisapride, compounded product; Road Runner Pharmacy, Phoenix, Arizona, USA), 0.5 mg/kg BW, PO, q12h. A customized calorie-dense complete and balanced home-prepared diet of liquid consistency was formulated for the dog. Despite the administration of prokinetics and the liquid diet via the low profile gastrostomy device (LPGD), the dog continued to regurgitate and had 3 episodes of airway obstruction with loss of consciousness, requiring emergent intervention to re-establish airway patency. In light of the frequent episodes of regurgitation and subsequent aspiration with airway obstruction, a narrow field laryngectomy was elected when the dog was 14 mo of age.
A narrow-field total laryngectomy was performed under general anesthesia by 2 otolaryngologists and a board-certified veterinary surgeon. The dog was placed in dorsal recumbency, the fur was clipped, and the skin was prepared with aseptic technique. A cushion was placed under the neck to elevate the cervical region into the surgical field. A 5-cm horizontal curvilinear incision was made 2 cm below the level of the cricoid cartilage. The sternohyoideus and sternothyroideus muscles were bluntly separated on the ventral midline and retracted to expose the larynx. Blunt and sharp dissection was utilized to expose the cricoid and thyroid cartilage of the larynx as well as the cranial aspect of the trachea (1st and 2nd tracheal rings). The entire circumference of the cranial trachea was dissected free from all surrounding tissues. The pharyngeal constrictor muscles (hyopharyngeus, thyropharyngeus, and cricopharyngeus) were separated from their attachments on the larynx as were the sternothyroideus and thyrohyoideus muscles. The cuff of the endotracheal tube was deflated, and the endotracheal tube was pulled cranially, to allow for a tracheal incision. A #15 blade was utilized to enter the trachea between the 2nd and 3rd tracheal rings. This incision was continued around the entire circumference of the trachea; a sterile endotracheal tube was introduced into the open end of the trachea, and the cuff was inflated to allow for a tight seal. Cranially, the larynx was freed from its attachments to the hyoid apparatus (disarticulated bilaterally) as well as the attachments to the tongue and hyoid bone, which were preserved. The larynx and cranial trachea were then removed. The pharyngeal mucosa was closed with 3-0 polydioxanone (PDS Suture; Ethicon, Somerville, New Jersey, USA) in a simple continuous pattern, followed by closure of the paired thyropharyngeal and cricopharyngeal muscles with 3-0 polydioxanone (Ethicon) in a simple continuous pattern. The cranial trachea was then brought into apposition with the skin to form a permanent tracheostomy. The severed end of the trachea was rotated to allow the entire circumference to be sutured to the skin edges forming an airtight seal. The trachea was sutured to the skin with 3-0 silk (Perma-Hand Silk Suture; Ethicon) because of its braided and non-absorbable features and optimal knot security qualities. Two closed-suction drains (Jackson-Pratt drain; Cardinal Health, Dublin, Ohio, USA) were placed in the surgical wound and the rest of the cervical incision was closed with staples. A light compression dressing was placed around the neck. The dog recovered uneventfully from anesthesia in the intensive care unit (ICU) and was discharged from the unit the following morning.
The dog was unable to swallow immediately following surgery secondary to the pharyngeal and esophageal myopathy, but otherwise did well in the post-operative period. The stoma was cleaned with sterile saline after nebulization with sterile water for 10 min 3 times daily for 10 d and a size #8 tracheostoma vent (Bivona; Smiths Medical ASD, Gary, Indiana, USA) was applied 14 d after surgery and held in place with a Posey foam tracheal tie (Posey Company, Arcadia, California, USA). The drains were removed on postoperative day 3 and the staples were removed on postoperative day 7. The dog is doing well 30 mo after surgery with a weight of 25 kg and a BCS of 8/9. There have been no episodes of aspiration pneumonia, even though the dog continues to regurgitate. The dog is managed with glycopyrrolate (Par Pharmaceutical, Woodcliff Lake, New Jersey, USA), 1 mg q8h to reduce saliva production, acid suppressants (famotidine, omeprazole), and gastrointestinal protectants (sucralfate) administered via PEG tube for the chronic gastroesophageal reflux and esophagitis, and lactated Ringer’s solution (500 mL SQ, q12h) to maintain hydration status. The dog has frequent episodes of regurgitation and nasal reflux of saliva and refluxed food due to her pharyngeal and esophageal myopathy. She wears the tracheostoma vent continuously to maintain a patent tracheal stoma. In addition, the dog is heat intolerant secondary to the inability to thermoregulate by panting; however, quality of life is considered excellent by the owners and clinicians. Neurological status has minimally progressed since initial presentation with increased muscle rigidity in the thoracic limbs.
Discussion
Aspiration pneumonia is a common clinical problem in dogs with neurological disease, particularly neuromuscular disorders such as myasthenia gravis, polymyopathies, and polyneuropathies affecting the swallowing reflex (18–20). In 1 report, aspiration pneumonia was most frequently noted in dogs with esophageal disease, vomiting, and neurological disorders (21). In that study, 22 of 88 dogs died or were euthanatized, and 3 of the 22 dogs (14%) were euthanized because of continual or refractory aspiration pneumonia (21). Most dogs with myasthenia gravis have esophageal weakness and dysfunction, in addition to generalized weakness. In 1 study, 12 of 25 dogs (48%) with myasthenia gravis died or were euthanized shortly after admission to the hospital due to aspiration pneumonia (18). Given the high incidence of spontaneous remission of myasthenia gravis in dogs (22), a laryngectomy procedure should be reserved for those animals with the most intractable pneumonia and an irreversible primary disease that is refractory to medical therapy, regardless of cause. Currently, there is no established surgical treatment for prevention of chronic or recurrent aspiration pneumonia in dogs.
In humans, it has been suggested that surgery is indicated for those cases with chronic aspiration and severe associated medical problems, whereby a total laryngectomy is the only justifiable surgical intervention which can improve patient quality of life (7). We utilized this approach to prevent recurrent aspiration pneumonia in a dog and prevented chronic aspiration, markedly improved quality of life, and obviated the need for euthanasia. Documented complications of laryngectomy in dogs include collapse of the laryngeal stoma, tracheoesophageal fistula formation, pharyngeal dehiscence, megaesophagus, hypocalcemia secondary to iatrogenic hypoparathyroidism, pneumonia, and death (15–17). Two of these reports describe significant post-laryngectomy morbidity in dogs while another report demonstrated no significant complications during 18 mo of follow-up (15–17). Other complications related to permanent tracheostomy include skin fold occlusion of a tracheal stoma, hyposmia and olfactory mucosal changes, loss of thermoregulation through panting, and increased CNS temperature (23–25). Postoperative activity of such dogs should be limited, as upper respiratory ventilation is required for normal thermoregulation and restriction of outdoor activities in warm weather should be considered in some cases as well (17). These dogs are also unable to swim due to the risk of drowning.
Although this dog was cured of recurrent aspiration pneumonia, it remains unable to swallow because of the progressive myopathy. The dog has also developed hyposmia as reported by the owners because the dog can no longer smell the family cat entering the family room, and the dog used to chase the cat out of the room as soon as she was aware of its presence. The myopathy in this case is congenital and no specific treatments or cures are available, thus the necessity for the laryngectomy to prevent aspiration pneumonia. She is maintained on intermittent tube feedings administered through a PEG tube and subcutaneous fluids, and maintains an excellent quality of life according to her owners and clinicians. It is plausible that dogs with less severe dysphagia may be able to eat and drink orally after laryngectomy, without the previous risk of aspiration. By removing the larynx and separating the respiratory and digestive tracts, the risk of aspiration pneumonia is eliminated.
Chronic regurgitation and recurrent life-threatening aspiration pneumonia are challenging conditions to treat and result in death or euthanasia in many dogs, particularly in those with esophageal and neuromuscular disorders. Currently there is no established surgical management available for this disorder and many dogs are euthanized. This case report suggests that a narrow field laryngectomy can be safely and effectively performed for management of chronic aspiration in the dog, and should be considered as a viable alternative treatment for dogs with profound oropharyngeal dysphagia, chronic regurgitation, and aspiration pneumonia.
The ethical considerations and importance of comprehensive client communications surrounding the surgical management of a dog with a progressive myopathy cannot be overemphasized. The dog in this report did not have an obviously clinically progressive myopathy other than the involvement of the pharynx and esophagus. Prior to the laryngectomy procedure, the dog suffered 3 episodes of airway obstruction secondary to aspiration of regurgitated material with consequent loss of consciousness. Without the laryngectomy procedure, the dog would have undergone elective euthanasia as it was deemed inhumane for her to continue to aspirate and suffer respiratory obstruction. The internists and surgeons met with the owners on several occasions to discuss the ramification of the laryngectomy procedure as well as potential complications. The owners were given ample opportunity to discuss the options with their family members and elected to move forward with the procedure recognizing that euthanasia would be indicated if the dog’s quality of life was not dramatically improved. The dog has continued to thrive and its quality of life is deemed to be excellent by the owners and clinicians.
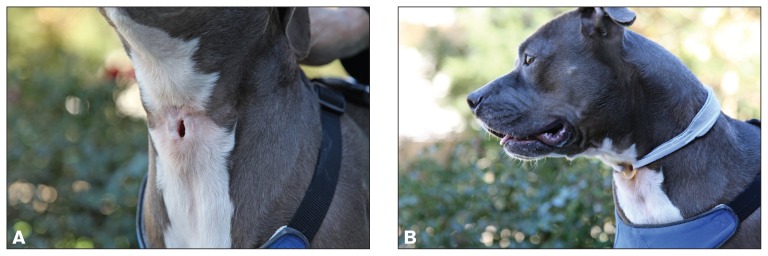
Figure 3.
A — Fully healed uncovered tracheostoma following laryngectomy in a pit bull terrier. B — The tracheostoma is kept patent with a soft, flexible silicon tracheostoma vent (Bivona; Smiths Medical ASD, Gary, Indiana, USA) that is secured in place with a foam tracheal tie (Posey Company, Arcadia, California, USA).
Acknowledgments
The authors thank John Doval for assistance with the images and Robin Fisher and Paula Howell for their technical support. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
The study was not supported by any grants or other funding.
References
- 1.Weir NF. Theodore Billroth: The first laryngectomy for cancer. J Laryngol Otol. 1973;87:1161–1169. [PubMed] [Google Scholar]
- 2.Stell PM. The first laryngectomy. J Laryngol Otol. 1975;89:353–358. doi: 10.1017/s0022215100080488. [DOI] [PubMed] [Google Scholar]
- 3.Rizzotto G, Succo G, Lucioni M, Pazzaia T. Subtotal laryngectomy with tracheohyoidopexy: A possible alternative to total laryngectomy. Laryngoscope. 2006;116:1907–1917. doi: 10.1097/01.mlg.0000236085.85790.d5. [DOI] [PubMed] [Google Scholar]
- 4.Majer EH. 100 Years of laryngectomy. Laryngol, Rhinol, Otol. 1975;54:3–9. [PubMed] [Google Scholar]
- 5.Bron L, Brossard E, Monnier P, Pasche P. Supracricoid partial laryngectomy with cricohyoidoepiglottopexy and cricohyoidopexy for glottic and supraglottic carcinomas. Laryngoscope. 2000;110:627–634. doi: 10.1097/00005537-200004000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz AW, Devine KD. Some historical notes about the first laryngectomies. Laryngoscope. 1959;69:194–201. doi: 10.1288/00005537-195902000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Cannon CR, McLean WC. Laryngectomy for chronic aspiration. Am J Otolaryngol. 1982;3:145–149. doi: 10.1016/s0196-0709(82)80046-x. [DOI] [PubMed] [Google Scholar]
- 8.Wei X, Fan X, Zhang J. [The long-term morphology of the nasal cavity after total laryngectomy]. J Clin Otorhinolaryngol. 2010;24:785–787. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. [PubMed] [Google Scholar]
- 9.Sasaki H, Sekizawa K, Yanai M, Arai H, Yamaya M, Ohrui T. New strategies for aspiration pneumonia. Intern Med. 1997;36:851–855. doi: 10.2169/internalmedicine.36.851. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Shen Z, Yao Z, Qiu H. [Surgical treatment for the chronic severe aspiration]. J Clinical Otorhinolaryngol. 2003;17:732–733. Lin Chuang Er Bi Yan Hou Ke Za Zhi. [PubMed] [Google Scholar]
- 11.De Vito MA, Wetmore RF, Pransky SM. Laryngeal diversion in the treatment of chronic aspiration in children. Int J Pediatric Otorhinolaryngol. 1989;18:139–145. doi: 10.1016/0165-5876(89)90066-9. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Dulguerov P. Laryngeal diversion and tracheotracheal speech fistula for chronic aspiration. Ann Otol Rhinol Laryngol. 2000;109:602–604. doi: 10.1177/000348940010900613. [DOI] [PubMed] [Google Scholar]
- 13.Broniatowski M, Grundfest-Broniatowski S, Tyler DJ, et al. Dynamic laryngotracheal closure for aspiration: A preliminary report. Laryngoscope. 2001;111:2032–2040. doi: 10.1097/00005537-200111000-00031. [DOI] [PubMed] [Google Scholar]
- 14.Lapidot A, Catalfumo F, Gelot R, Liskow A. Total laryngectomy and autogenous reconstruction observations in an experimental series in canines. J Surg Oncol. 1973;5:259–266. doi: 10.1002/jso.2930050309. [DOI] [PubMed] [Google Scholar]
- 15.Block G, Clarke K, Salisbury SK, DeNicola DB. Total laryngectomy and permanent tracheostomy for treatment of laryngeal rhabdomyosarcoma in a dog. J Am Anim Hosp Assoc. 1995;31:510–513. doi: 10.5326/15473317-31-6-510. [DOI] [PubMed] [Google Scholar]
- 16.Crowe DT, Goodwin MA. Total laryngectomy for laryngeal mast cell tumor in a dog. J Am Anim Hosp Assoc. 1986;22:809–816. [Google Scholar]
- 17.Henderson RA, Powers RD, Perry L. Development of hypoparathyroidism after excision of laryngeal rhabdomyosarcoma in a dog. J Am Anim Hosp Assoc. 1991;198:639–643. [PubMed] [Google Scholar]
- 18.Dewey CW, Bailey CS, Shelton GD, Kass PH, Cardinet GH., 3rd Clinical forms of acquired myasthenia gravis in dogs: 25 cases (1988–1995) J Vet Intern Med. 1997;11:50–57. doi: 10.1111/j.1939-1676.1997.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 19.Ryckman LR, Krahwinkel DJ, Sims MH, Donnell RL, Moore PF, Shelton GD. Dysphagia as the primary clinical abnormality in two dogs with inflammatory myopathy. J Am Vet Med Assoc. 2005;226:1519–23. doi: 10.2460/javma.2005.226.1519. [DOI] [PubMed] [Google Scholar]
- 20.Khorzad R, Whelan M, Sisson A, Shelton GD. Myasthenia gravis in dogs with an emphasis on treatment and critical care management. J Vet Emerg Crit Care. 2011;21:193–208. doi: 10.1111/j.1476-4431.2011.00636.x. [DOI] [PubMed] [Google Scholar]
- 21.Kogan DA, Johnson LR, Sturges BK, Jandrey KE, Pollard RE. Etiology and clinical outcome in dogs with aspiration pneumonia: 88 cases (2004–2006) J Am Vet Med Assoc. 2008;233:1748–1755. doi: 10.2460/javma.233.11.1748. [DOI] [PubMed] [Google Scholar]
- 22.Shelton GD, Lindstrom JM. Spontaneous remission in canine myasthenia gravis: Implications for assessing human MG therapies. Neurology. 2001;57:2139–2141. doi: 10.1212/wnl.57.11.2139. [DOI] [PubMed] [Google Scholar]
- 23.Baker MA, Chapman LW, Nathanson M. Control of brain temperature in dogs: Effects of tracheostomy. Resp Physiol. 1974;22:325–333. doi: 10.1016/0034-5687(74)90081-4. [DOI] [PubMed] [Google Scholar]
- 24.Hedlund CS, Tangner CH, Montgomery DL, Hobson HP. A procedure for permanent tracheostomy and its effects on tracheal mucosa. Vet Surg. 1982;11:13–17. [Google Scholar]
- 25.Hedlund CS, Tangner CH, Waldron DR, Hobson HP. Permanent tracheostomy: Perioperative and long-term data from 34 cases. J Am Anim Hosp Assoc. 1988;24:585–591. [Google Scholar]