Abstract
Invasive aspergillosis (IA) remains a devastating disease in immune compromised patients despite significant advances in our understanding of fungal virulence and host defense mechanisms. In this review, we summarize important research advances in the fight against IA with particular focus on early events in the interactions between Aspergillus fumigatus and the host that occur in the respiratory tract. Advances in understanding mechanisms of immune effector cell recruitment, antifungal effector mechanisms, and how the dynamic host-fungal interaction alters the local microenvironment to effect outcomes are highlighted. These advances illustrate exciting new therapeutic opportunities, but also emphasize the importance of understanding each unique fungus-host interaction for improving patient outcomes.
Introduction
Humans breathe 10–15 m3 of air daily, a volume that typically contains several hundred to several thousand airborne Aspergillus conidia (VandenBergh et al, 1999). For the most part, this lifelong encounter results in asymptomatic fungal clearance, facilitated in part by mucociliary clearance mechanisms. However, owing to their small size (i.e. 2–3 µm in diameter), inhaled conidia can reach terminal airways where they activate the respiratory innate immune system via soluble and membrane-bound receptors. Critical immune effector cells, including neutrophils, inflammatory monocytes, and macrophages, must be recruited and activated to thwart fungal invasion.
Inhalation of Aspergillus conidia results in its deposition into a new microenvironment. In immune competent murine models of invasive aspergillosis (IA), germination of Aspergillus conidia in the airways is rarely observed, although there appears to be some strain dependency that remains to be fully appreciated (Rizzetto et al, 2013). However, the large inocula typically used to establish a bronchopneumonia in these murine models results in significant inflammation in the lower airways of the lung. While large inocula of fungal conidia are plausible under specific exposure conditions (e.g., during mulching/gardening), it is unclear whether inocula capable of inducing robust airway inflammation in mice are responsible for disease development in susceptible patient populations. Yet, even a low number of inhaled fungal conidia likely induce localized microenvironment changes that can impact subsequent host immune responses in immune compromised patients. How this microenvironment impacts IA outcomes has not been defined and many open questions remain. For example, questions such as how the microenvironment alters fungal pathogen associated molecular pattern exposure (PAMP) and virulence and how the microenvironment alters immune signaling and antifungal activity of phagocytes such as macrophages, monocytes, and neutrophils remain under appreciated.
Neutrophils are one of the key inflammatory cells that mediate resistance against infection with A. fumigatus. This is highlighted by the observation that patients who become neutropenic after chemotherapy are at a higher risk for developing IA (Gerson et al, 1984). Animal models clearly demonstrate that timely neutrophil recruitment to the respiratory tract following A. fumigatus exposure is critical for resistance to invasive disease and control of fungal growth (Bonnett et al, 2006; Mehrad et al, 1999a). Moreover, appropriate neutrophil activation and antifungal activity are necessary for resistance to invasive A. fumigatus infection as both patients with chronic granulomatous disease (CGD) and mice that lack NADPH oxidase subunits are highly susceptible to infection (Morgenstern et al, 1997; Pollock et al, 1995). However, recent epidemiological studies suggest that the incidence of IA is also increasing in non-neutropenic patients (Steinbach et al, 2012). Interestingly, IA in chronic granulomatous disease or following corticosteroid immunosuppression is associated with excessive accumulation of functionally impaired neutrophils that contribute to tissue damage (Balloy et al, 2005; de Luca et al, 2014). Therefore, precise regulation and calibration of pulmonary inflammation and leukocyte recruitment may ameliorate disease in these contexts. Surprisingly, our understanding of neutrophil recruitment and activation in response to A. fumigatus challenge has been slow to emerge. The goal of this mini-review is to highlight new advances in the last 3 years regarding our understanding of how the immune system prevents host damage from A. fumigatus challenge. We focus on recognition of the fungus as it enters the airway and mechanisms of immune cell recruitment, activation, and antifungal effector activity. Fungal components that effect these interactions with the host are discussed, and we discuss emerging awareness of how changes in the tissue microenvironment, such as oxygen and nutrient levels, can alter the outcome of the host-pathogen interaction.
A last point of emphasis is that emerging studies strongly suggest, perhaps not surprisingly, that the immune response to a given strain of A. fumigatus is not stereotypical. Thus, understanding this strain-specific variation represents a major gap in knowledge that is particularly relevant to design immune-enhancing or immune-modulating strategies (a goal of personalized medicine) for patients afflicted by this menacing mold. Consequently, a dynamic infection microenvironment, strain heterogeneity, patient genetic variability, diverse underlying disease conditions, and a limited antifungal arsenal all contribute to the complex and significant challenge inherent in improving IA outcomes.
Aspergillus recognition and Innate Immune Activation
Pentraxin-3
Pentraxin-3 (Ptx3), a soluble collectin and acute phase reactant, binds to conidia and this interaction can be inhibited by soluble galactomannan in vitro (Garlanda et al, 2002). Pentraxin-3 regulates complement interactions with fungal conidia and promotes neutrophil conidial uptake by a complement-, CD18/CD11b (a.k.a complement receptor 3/Mac-1)-, and FcγRII-dependent mechanisms (Moalli et al, 2010). Pentraxin-3 binds the Toll-like receptor (TLR) 4 accessory protein myeloid differentiation protein 2 (MD-2) can modulate lung inflammation via the TLR4/MD-2/CD14 signal transducer TIR domain-containing adapter inducing interferon-β (TRIF) to promote fungal clearance (Bozza et al, 2014). Consistent with this model, Ptx3−/− and MD-2−/− mice are susceptible to conidial challenge compared to control mice (Bozza et al, 2014; Garlanda et al, 2002). In humans, receipt of a donor graft with a specific pentraxin-3 gene variant increases the likelihood of developing IA during hematopoietic cell transplantation (Cunha et al, 2014).
Fungal interactions with lung-resident leukocytes through CLRs
Alveolar macrophages can rapidly phagocytose conidia within airways and this engulfment process can be blocked in vitro by antibodies directed against dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN/CD209) (Serrano-Gomez et al, 2004). Resting conidia express FleA, a protein that binds to fucosylated molecules found in mucinous secretions (Kerr et al, 2016). Conidial phagocytosis by macrophages can be disrupted by soluble fucose moieties and ΔfleA conidia are not engulfed at a rate comparable to wild-type conidia. These data support the notion that FleA mediates conidial interactions with phagocytic cells, though the macrophage receptor(s) responsible for these interactions have not been functionally characterized. Interestingly, the ΔfleA strain is hypervirulent compared to its parental strain, which correlates with significantly greater germination and growth in the airways. Collectively, these data suggest that host recognition of A. fumigatus FleA contributes to fungal clearance and control in vivo (Kerr et al, 2016).
Although resident alveolar macrophages and other phagocytes rapidly phagocytose resting conidia, engulfed conidia do not trigger robust inflammatory responses prior to conidial swelling, the first step in germination. The conidial surface consists of a layer of hydrophobins that are encoded by the rodA and rodB genes in A. fumigatus and that conceal an underlying layer of fungal β-(1,3) glucan (Aimanianda et al, 2009). Conidial swelling coincides with the obligate exposure of particulate β-(1,3) glucan (Hohl et al, 2005) and other cell wall polysaccharides. These polysaccharides are actively recognized by the host to initiate the antifungal immune response.
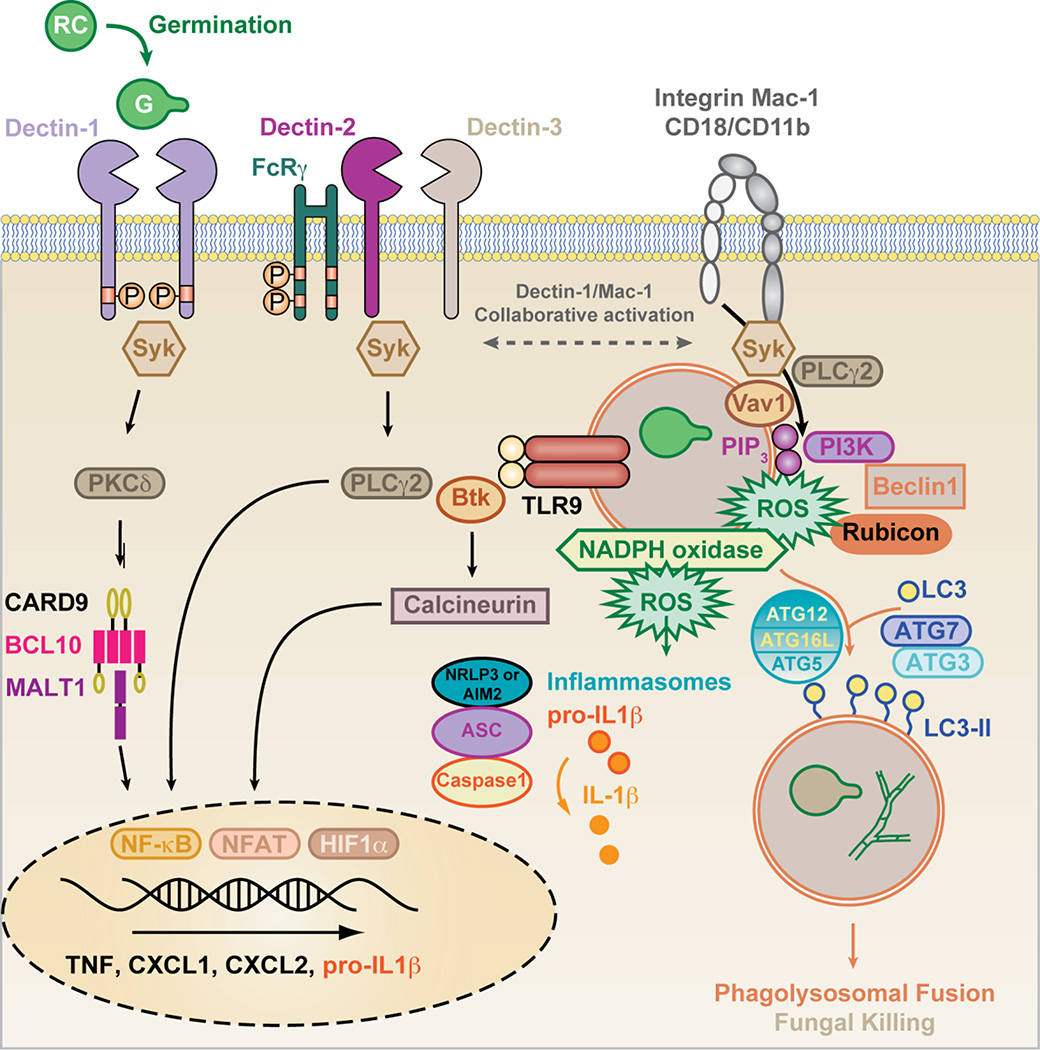
The C-type lectin receptor Dectin-1 (Clec7a) binds to β-glucans on germinating conidia (Gersuk et al, 2006; Hohl et al, 2005; Steele et al, 2005). Dectin-1 binding to β-glucan extrudes the regulatory phosphatases CD45 and CD148 from the vicinity of receptor-particulate β-glucan complexes (Goodridge et al, 2011) (Figure 1). These events induce Src-dependent phosphorylation of the ITAM-like motif found in the intracellular domain of Dectin-1 and facilitate the recruitment of the SHP-2 phosphatase (Deng et al, 2015). Spleen tyrosine kinase (Syk) docks to this scaffold and transduces signals via protein kinase C (PKC)-δ (Strasser et al, 2012) to CARD9, which complexes with B cell CLL/lymphoma 10 (BCL10) and Mucosaassociated lymphoid tissue lymphoma translocation protein 1 (MALT1) (Gross et al, 2006) in order to activate NF-κB-dependent cytokine production (i.e., IL-6, IL-12, IL-23, TNF, CXCL1/KC, and CXCL2/MIP-2) (Brown, 2011; Rogers et al, 2005). In macrophages and dendritic cells (DCs), the CARD9-Bcl10-Malt1 complex directs il1b transcription and caspase-1- and -8-dependent IL-1β release (Gringhuis et al, 2012; Gross et al, 2009) (Figure 1). Dectin-1/Syk/CARD9-dependent cytokines are critical for T helper cell differentiation into IL-17A producing cells, which are critical for antifungal immunity (Deng et al, 2015; LeibundGut-Landmann et al, 2007; Rivera et al, 2011).
Figure 1. Model of fungus-induced signaling and activation of effector systems in myeloid cells.
Germinating A. fumigatus conidia (SC, swollen conidia) expose primarily surface β-glucan as well as other ligands that can activate Syk via CLRs (i.e., Dectin-1 with a hemi-ITAM in the receptor tail), FcRγ-coupled (i.e., Dectin-2 that complexes with Dectin-3), and integrin receptors, i.e. Mac-1 (CD11b/CD18). Downstream PKCδ and CARD9 activation is critical for caspase-1 and -8 activity, NF-κB activation, and cytokine production. Syk-dependent PLCγ2 activation is linked to NADPH oxidase assembly in neutrophils. Following phagocytosis, A. fumigatus triggers macrophage TLR9-Btk signaling that is transduced into calcineurin-dependent NFAT activation. HIF-1α cooperates with NF-κB and NFAT to regulate inflammatory cytokine production (e.g., CXCL1) in myeloid cells.
Macrophage phagosomes that contain swollen conidia recruit Rubicon-, Beclin-1, UVRAG-, and VPS34-containing complexes that result in phosphatidylinositol-3-phosphate (PIP3) deposition and the assembly of functional NADPH oxidase. PIP3 and ROS mediate the assembly of ATG12/ATG5/ATG16L-dependent conjugation systems that facilitate ATG3- and ATG7-dependent lipidation of LC3. Lipidated LC3 inserts into phagosomal membrane and regulates the phagolysosomal fusion. The relative contribution of NADPH oxidase versus LC3-associated autophagy to fungal killing in myeloid cells has not been clearly defined in vivo. Rubicon not only associates with the class III phosphatidylinositol-3-kinase (i.e. VPS34 complex), but also stabilizes the NADPH oxidase complex, and negatively regulates CARD9-dependent NF-κB signal transduction. Please see text for additional detail.
In humans, mendelian defects in the card9 gene promote the spontaneous development of IA, notably at extrapulmonary sites (M. Lionakis and colleagues, personal communication; manuscript under review at the Journal of Allergy and Clinical Immunology). Similarly, immune competent CARD9−/− mice develop lethal invasive pulmonary aspergillosis after intratracheal challenge with resting A. fumigatus conidia (Jhingran et al, 2012). Consistent with a role in CARD9 activation, PKC-δ−/− mice display defective lung fungal I favor clearance (Li et al, 2016). In contrast to the card9 gene, mendelian defects in the clec7a gene have not been associated with the spontaneous development of IA in humans. However, a Dectin-1/Clec7a (Y238X) polymorphism is associated with increased susceptibility to IA during allogeneic hematopoietic cell transplantation (Cunha et al, 2010), in the context of immune damage associated with conditioning chemotherapy, hematopoietic cell engraftment, or graft versus host disease. In murine models, Dectin-1−/− mice show variable susceptibility to respiratory A. fumigatus challenge (Jhingran et al, 2012; Werner et al, 2009). This finding may reflect differences in mouse strain backgrounds or in fungal strains that were tested in these studies. Additional CARD9-coupled receptors may provide molecular redundancy with respect to A. fumigatus recognition and may promote host defense as described below.
Dectin-2 (Clec4n) forms complexes with Dectin-3 (Clec4d) to bind Candida α-mannans (Zhu et al, 2013) or with Mincle (Clec4e) to bind Malassezia glycolipids (Ishikawa et al, 2013; Yamasaki et al, 2009). Both complexes signal via the ITAM-coupled adaptor FcRγ (Sato et al, 2006) and can activate Syk- and CARD9-dependent signal transduction (Figure 1). A. fumigatus germination exposes Dectin-2 ligands on germlings and hyphae, activating cytokine responses in cultured murine bone marrow DCs (Jhingran et al, 2012) and in human plasmacytoid DCs in vitro (Loures et al, 2015). In a murine A. fumigatus corneal challenge model with mutant conidia that lack the hydrophoin layer, both Dectin-1- and Dectin-2-dependent signals are required for optimal neutrophil recruitment to the corneal stroma and for fungal killing (Carrion Sde et al, 2013). Notably, fungal killing of wild-type conidia in this model did not depend on Dectin-1 or Dectin-2 signaling function (Carrion Sde et al, 2013). A recent study illustrated that Dectin-2 can regulate autocrine IL-17A-IL-17RC signaling that increases neutrophil fungal killing capacity upon secondary ocular challenge with wild-type A. fumigatus conidia (Taylor et al, 2014). The relative contribution of dectin-1 and FcRγ-dependent C-type lectin receptors to CARD9-dependent host defense in the lung remains to be elucidated. Since CARD9 integrates signals from multiple pattern recognition receptors, such as RIG-I/MAVS (Poeck et al, 2010), Toll-like receptors (TLR) (Hara et al, 2007), or Nod2 (Hsu et al, 2007), it is possible that CLR-independent mechanisms may contribute to CARD9 activation following A. fumigatus challenge as well.
Fungal interactions with lung-resident leukocytes through TLR9
Recent work has found that transplant patients on calcineurin inhibitors are at elevated risk of invasive fungal infections, including Aspergillus spp. (Herbst et al, 2013). However, until recently, the mechanism was not well understood. Elegant work by Armstrong-James and colleagues demonstrated that calcineurin signaling is central in regulating early neutrophil airway influx to prevent IA (Herbst et al, 2015). Moreover, mice that are treated with cyclosporine A or that lack the calcineurin B subunit (CnB) in the myeloid compartment are highly susceptible to systemic Candida albicans challenge (Greenblatt et al, 2010). Tissue-resident macrophages phagocytose A. fumigatus conidia and activate nuclear factor of activated T cells (NFAT) in response to non-conventional TLR9 signaling, which is transduced by Bruton’s tyrosine kinase (Btk) rather than by MyD88 signaling. Btk activation drives phospholipase C (PLC)-γ2 activation, resulting in calcineurin and downstream NFAT activation (Figure 1). Calcineurin inhibition by FK506 diminishes macrophage and lung TNF expression, with smaller decreases in CXCL1, CXCL2, and CCL3 (MIP-1α) (Herbst et al, 2015). Earlier work has shown that TNF signaling is crucial in host defense against A. fumigatus through its regulation of neutrophil responses (Mehrad et al, 1999b; Schelenz et al, 1999). Thus, calcineurin activation downstream of TLR9 engagement contributes to host resistance to IA, a finding that is important in the management of solid organ and hematopoietic cell transplant patients maintained on calcineurin inhibitors.
Biphasic neutrophil recruitment is necessary for host resistance to against IA
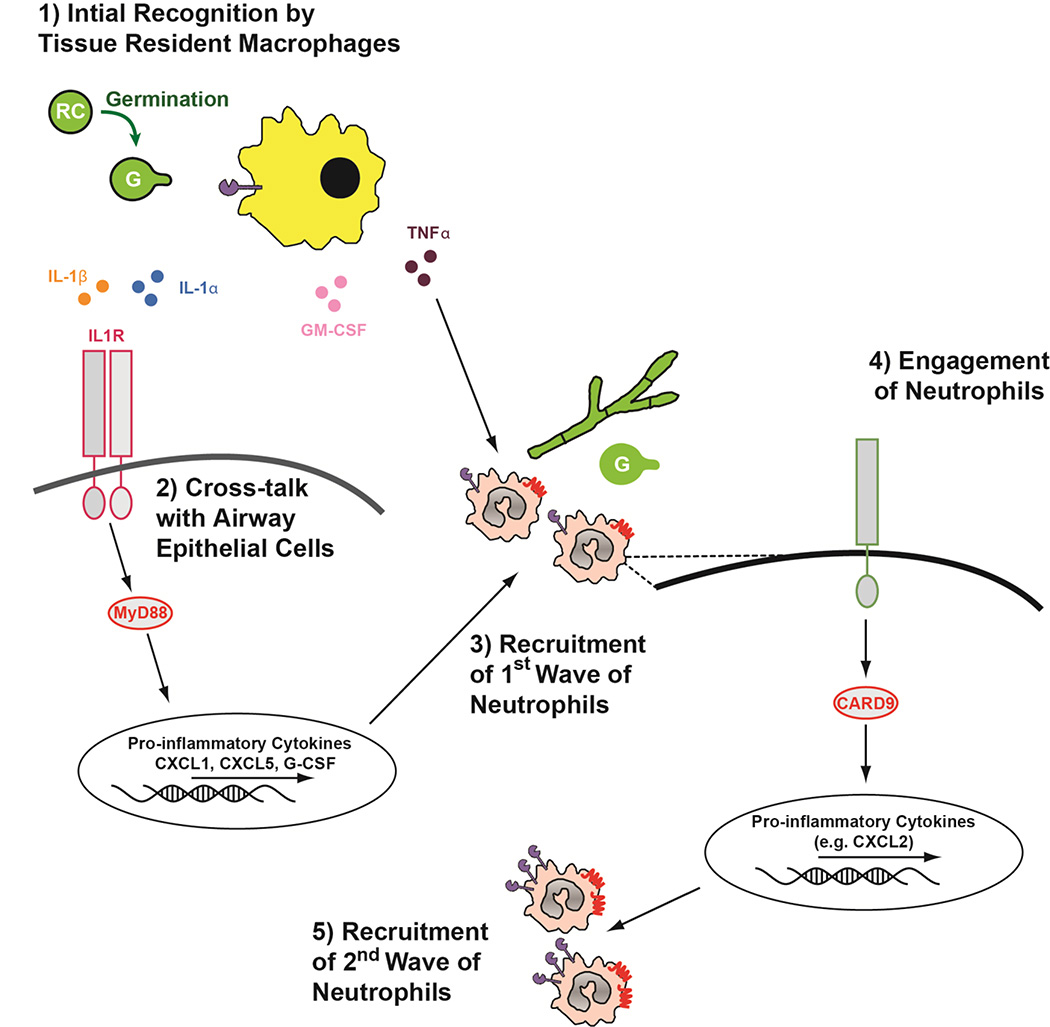
It is well established in both human patient populations and animal models that neutrophils are critical inflammatory cells necessary for host resistance against IA (Gerson, 1984, Mehrad, 1999, Bonnett, 2006). Early work demonstrated that anti-CXCR2 treated (Mehrad et al, 1999a) or Cxcr2−/− (Bonnett et al, 2006) mice are highly susceptible to IA. In mice, CXCR2 mediates chemotactic responses to CXCL1, CXCL2, and CXCL5, but the relative contribution of each of these ligands to CXCR2-dependent neutrophil trafficking has not been defined. Until recently, the precise inflammatory events regulating CXCR2-dependent recruitment of neutrophils to the lungs following A. fumigatus challenge remained ill defined. IL1RI/MyD88- and Card9-dependent signaling non-redundantly mediates optimal CXCR2-depenent recruitment of neutrophils to the lungs throughout the course of A. fumigatus infection in a murine model of Aspergillus bronchopneumonia (Jhingran et al, 2015) (Figure 2). Signaling by IL-1 cytokines is also critical in humans, because SNPs in the IL-1 gene cluster are associated with increased susceptibility to IPA (Wojtowicz et al, 2015) Sainz et al, 2008). Additionally, Card9 defects in humans can result in spontaneous development of IA (M. Lionakis and colleagues, personal communication; manuscript under review at the Journal of Allergy and Clinical Immunology). IL1RI/MyD88 signaling is critical for neutrophil recruitment during A. fumigatus-induced keratitis (Leal et al, 2010) and bronchopneumonia (Caffrey et al, 2015; Jhingran et al, 2015; Shepardson et al, 2014). Following challenge with A. fumigatus the early expression of CXCR2 ligands, specifically CXCL1 and to a lesser extent CXCL5, was highly dependent on IL-1RI/MyD88 signaling in radioresistant, CC10-expressing lung epithelial cells (Caffrey et al, 2015; Jhingran et al, 2015). Exposure to A. fumigatus induces high levels of both IL-1α and IL-1β protein early in the infection (Caffrey et al, 2015). Expression of the IL-1α and IL-1β cytokines is partially dependent on HIF-1α signaling in myeloid cells (Shepardson et al, 2014), likely alveolar macrophages (Bonnett et al, 2006) and CCR2+ monocytes (Caffrey et al, 2015; Espinosa et al, 2014). HIF-1α is stabilized after recognition of β-glucan by Dectin-1 (Cheng et al, 2014), which can regulate IL-1 expression (Leal et al, 2010; Steele et al, 2005). However, which IL-1 cytokine is responsible for signaling through IL-1RI to drive CXCR2 ligands is currently debated (Caffrey et al, 2015; Karki et al, 2015; Moretti et al, 2014).
Figure 2. Biphashic neutrophil recruitment to the lungs in response to Aspergillus fumigatus.
(1) Upon entry into the airway resting Aspergillus conidia (RC) rapidly begin germination. Upon swelling and germtube emergence (G) tissue resident monocytes and macrophages recognize Aspergillus exposed carbohydrate cell wall components through an array of pattern-recognition receptors. These activated monocytes and macrophage secrete numerous inflammatory cytokines, including IL-1α, IL-1β, GM-CSF, and TNF-α into the surrounding tissue to initiateneutrophil recruitment. TNF-α may directly enhance neutrophil recruitment to the lung, while (2) IL-1α and IL-1b will mediate cross-talk with airway epithelial cells that express the IL-1RI. Through IL-1RI/MyD88-dependent signaling events airway epithelial cells produce CXCL1, CXCL5, and G-CSF. (3) These pro-inflammatory mediators drive the first wave of CXCR2-dependent neutrophil recruitment to the lungs. (4) Upon entry into the lungs neutrophil interact with the Aspergillus germtubes, which results in Card9-dependent, but Dectin-1 and Dectine-2 independent, signaling which results in the expression of CXCL2 by neutrophils. (5) Finally, CXCL2 drives the accumulation of a second wave of neutrophils to the lungs through a CXCR2-dependent mechanism. Neutrophils found in the lungs at later time-point also express higher levels of the CXCR2 receptor and Dectin-1.
Subsequently, Card9 signaling drives a second phase of CXCL1 and CXCL2 expression that occurs in a MyD88-independent manner, which functions to mediate a second wave of neutrophil recruitment to infected airways (Jhingran et al, 2015; Jhingran et al, 2012) (Figure 2). This Card9-dependent response depends on Card9 expression in radiosensitive cells, with the majority of CXCL2-expressing cells being neutrophils (Jhingran et al, 2015). Interestingly, Card9 signaling at this point appears to be independent of Dectin-1 and Dectin-2 (Jhingran et al, 2015). Beyond the Card9-coupled CLRs discussed above, Card9 integrates signals from multiple pattern recognition receptors including RIG-I/Mda5/MAVS (Poeck et al, 2010), Toll-like receptors (TLR) (Hara et al, 2007), or Nod2 (Hsu et al, 2007). Therefore, it is possible that CLR-independent mechanisms may contribute to Card9 activation following A. fumigatus challenge. More work elucidating this late interaction of A. fumigatus with the host respiratory tract is needed and may have particular relevance to chronic forms of the disease with significant hyphal growth and tissue damage. In this context, strain heterogeneity in response to these infection environments may significantly skew or alter the host inflammatory response and consequently drive changes to the therapeutic strategy needed to protect the host from damage.
Regulation of CXCR2 expression on neutrophils
Interestingly, patients with autosomal dominant Hyper-IgE syndrome (AD-HIES/Job’s syndrome), a rare primary immunodeficiency due to mutations in STAT3, develop fungal pneumonias, including aspergillosis (Vinh et al, 2010). The molecular mechanism behind the increase in Aspergillus spp. infection in AD-HIES patients remains undefined, though AD-HIES neutrophils inhibit A. fumigatus hyphal growth and metabolism similar to control neutrophils (Vinh et al, 2010). The cytokine granulocyte colony-stimulating factor (G-CSF) drives STAT3 activation in immature bone marrow neutrophils, which increases CXCR2 expression and neutrophil mobilization (Nguyen-Jackson et al, 2010). Interestingly, leukocytes from AD-HIES patients express lower levels of the ELR+ chemokine receptors, CXCR1 and CXCR2 (Mintz et al, 2010).
Recently, it has been observed that the phenotype and antifungal function of neutrophil found after sublethal A. fumigatus challenge are altered. Neutrophils in the airways express elevated levels of Dectin-1/Clec7a and CXCR2 beginning 3 days after sublethal A. fumigatus challenge (Savers et al, 2016). Elevated levels of neutrophil Dectin-1/Clec7a and CXCR2 expression could also be seen in the peripheral blood and bone marrow at 10 days post-challenge and were associated with enhanced secondary neutrophil responses and subsequent protection against lethal A. fumigatus challenge (Savers et al, 2016). These data suggest that the proinflammatory environment induced by A. fumigatus likely has long-term effects that may alter neutrophil differentiation or mobilization in the bone marrow, a process that has been shown to underlie G-CSF signaling (Nguyen-Jackson et al, 2010). However, the role of the G-CSF in host defense against aspergillosis has not been explored. Our data demonstrate that G-CSF expression is highly dependent on IL-1RI/MyD88 signaling following A. fumigatus challenge (Caffrey et al, 2015). Therapeutically, G-CSF administration to neutropenic mice has been shown to enhance peripheral blood leukocytes and host resistance to fungal infections (Polak-Wyss, 1991; Uchida et al, 1992). Thus, much still remains to be uncovered about the regulation of this pathway, including whether G-CSF/STAT3 signaling can regulate neutrophil mobilization and maturation for the prevention of IA.
Regulation of neutrophil antifungal activity
In addition to the timely recruitment of neutrophils to the respiratory tract after A. fumigatus exposure, the appropriate activation of antifungal function is necessary for host resistance to IA. CGD patients have a defect in neutrophil NADPH oxidase activity and a 40% lifetime risk of IPA (Holland, 2010). Both in vitro and in vivo studies have shown that p47phox(−/−) neutrophils have decreased antifungal activity that is cell-intrinsic (Jhingran et al, 2012; Morgenstern et al, 1997; Pollock et al, 1995). Similarly, inflammatory monocytes and their descendant cells harness NADPH oxidase-dependent conidial clearance (Espinosa et al, 2014). Additionally, neutrophils from mice exposed to sub-lethal A. fumigatus inocula display enhanced reactive oxygen species formation, but the molecular mechanism behind this increase was not explored (Savers et al, 2016). During respiratory fungal challenge, neutrophil and monocyte NADPH oxidase activity is regulated in part by pulmonary granulocyte-macrophage colony-stimulating factor signaling (Kasahara et al, 2016). However, the mechanisms underlying the induction of antifungal effector activities against different Aspergillus morphotypes remain to be fully defined.
Neither Dectin-1 nor CARD9 are essential in a cell-intrinsic manner for conidial phagocytosis and killing by lung neutrophils and alveolar macrophages (Jhingran et al, 2015; Jhingran et al, 2012). Similarly, human neutrophils recognize A. fumigatus primarily via CD11b/CD18 rather than via Dectin-1 (Gazendam et al, 2016). In the lung, Syk signaling regulates neutrophil conidial uptake (Jhingran et al, 2012). Beyond CLRs, Syk coordinates the biological activities of CD11b/CD18 complexes (Mocsai et al, 2010). The CD11b/CD18 receptor contains a β-glucan binding site (Xia et al, 1999) and can also interact with pentraxin-3-opsonized conidia (Moalli et al, 2010), as described above. Furthermore, CD18 is essential for neutrophil NADPH oxidase activity via a Syk-dependent (and dectin-1-/CARD9-independent) signal transduction when neutrophils are challenged with killed A. fumigatus hyphae (Boyle et al, 2011; Leal et al, 2012). In contrast, other groups have reported that peritoneal-elicited or bone marrow neutrophils utilize Dectin-1 signaling to regulate the respiratory burst following exposure to A. fumigatus and C. albicans fungal components, or to zymosan, a β-glucan-enriched fungal ghost particle (Li et al, 2011; Werner et al, 2009). Collectively, these findings likely highlight molecular redundancy involved in activating and coupling Syk-dependent fungal recognition to the induction of leukocyte effector functions (Huang et al, 2012; Jhingran et al, 2012).
In addition to its direct effects on fungal viability, NADPH oxidase fosters the recruitment of the autophagy protein LC3 to peripheral blood mononuclear cell phagosomes that contain swollen A. fumigatus conidia (Kyrmizi et al, 2013). In CGD patients, defective LC3 recruitment to Aspergillus phagosomes can be ameliorated by pharmacologic blockade of IL-1 receptor signaling with anakinra restoring phagocytic killing of the conidia (de Luca et al, 2014).
In monocytes and macrophages, the formation of a complex that consists of VPS34, a class III phosphatidylinositol-4,5-bisphosphate 3-kinase, Beclin 1, UVRAG, and Rubicon, results in sustained PIP3 deposition in the early phagosomal membrane and initiates LC3-associated phagocytosis (Martinez et al, 2015). The ensuing recruitment of NOX2 (i.e. the catalytic p91 subunit of NADPH oxidase) into the NADPH oxidase complex and its activation ensures that both PIP3- and ROS-dependent signals facilitate the assembly of an ATG5/ATG12/ATG16L complex that in turn promotes ATG7- and ATG3-dependent lipidation and insertion of LC3 (i.e., LC3-II) into the phagosomal membrane (Martinez et al, 2015; Yang et al, 2012a). The newly formed LC3-associated phagosomes can fuse with LAMP1-containing lysosomes and undergo maturation (Figure 1).
Bone marrow-derived macrophages that lack Beclin-1, Rubicon, or ATG7 expression display defective A. fumigatus conidial clearance in vitro. Similarly, Rubicon−/− or conditional myeloid Beclin-1 or ATG7 (i.e., Beclin-1ΔLysM and ATG7ΔLysM) knockout mice exhibit slowed conidial clearance in the lung (Martinez et al, 2015). In addition to its role in mediating LC3-associated autophagy, Rubicon acts as a feedback inhibitor of CARD9-dependent signaling by disassembling the CARD9/BCL10/Malt1 complex (Yang et al, 2012b).
Conditional deletion of Atg5 in hematopoietic cells increases murine susceptibility to A. fumigatus challenge in an immune compromised pulmonary challenge model (Akoumianaki et al, 2016). Conidial melanins partially inhibit LC3-associated phagocytosis by impeding the assembly of a functional NADPH oxidase complex (Akoumianaki et al, 2016). This finding may partially explain the loss of virulence observed in conidial melanin mutants.
Despite the important fungicidal role of phagocyte NADPH oxidase, the majority of CGD patients are not diagnosed with IA during their lifetimes, implying the existence of alternate clearance mechanisms. Consistent with this observation, alveolar macrophages have the capacity to kill conidia effectively in the absence of detectable products of NADPH oxidase in vivo (Cornish et al, 2008). Human lactoferrin, an iron chelator, can act in in fungistatic manner and inhibit fungal germination in an NADPH oxidase-independent manner ex vivo (Zarember et al, 2007). Neutrophil-derived lipocalin-1 can sequester fungal siderophores and limit hyphal extension in the fungal keratitis model (Leal et al, 2013). Interleukin-6 controls the synthesis of host-derived iron chelators, heme- and siderophore-binding proteins that limit A. fumigatus iron acquisition in ocular tissues (Leal et al, 2013). Neutrophil calprotectin (a heterodimer of S100A8/S100A9 subunits) is an abundant cytoplasmic protein that contributes to nutritional immunity by sequestering essential ions (i.e., Zn++, Mn++) via chelation. Calprotectin limits extracellular hyphal growth in the eye in an ocular challenge model, but is dispensable for conidial killing in the lung following intratracheal conidial challenge (Clark et al, 2016). The role of nutrient sequestration may be less pronounced in the pulmonary immune response to A. fumigatus conidia, in part due to relative differences in attenuating conidial versus hyphal growth.
Neutrophil extracellular traps (NETs) can be observed in the murine lung when mice are challenged with pre-swollen conidia (Bruns et al, 2010), but are rarely observed when resting conidia, the infectious propagules, are administered to mice. The release of NETs depends on the size of encountered fungal particle(s) and phagocytosis of individual, small fungal cells (e.g., C. albicans blastoconidia or A. fumigatus conidia) directs neutrophil elastase and CD63 localization to the phagosome, temporally coincident with NADPH oxidase assembly on the phagosomal membrane (Branzk et al, 2014). The process of fungal cell phagocytosis activates Dectin-1 and this signaling pathway acts as a negative regulator of NETosis (Branzk et al, 2014). In contrast, large fungal particles that do not enter phagosomes trigger the nuclear translocation of neutrophil elastase. This process enables the proteolytic processing of histones that precedes chromatin decondensation required for NETosis. The role of NETosis in anti-Aspergillus defense in vivo remains controversial and is supported largely by circumstantial evidence (Bianchi et al, 2011). Ex vivo studies of conidial and hyphal killing by human neutrophils suggest that NETosis does not contribute fungistatic activity against either morphotype (Gazendam et al, 2016), though these experiments do not account for the inflammatory and tissue context found within the respiratory tract.
Finally, it is well established that transendothelial migration of neutrophils can enhance their effector functions (Kolaczkowska & Kubes, 2013; Swain et al, 2002). In vitro, human neutrophils that cross chemotactic gradients of N-formyl-methionyl-leucyl-phenylalanine (fMLP), leukotriene B4 (LTB4), or IL-8 have increased anti-Aspergillus activity compared to neutrophils that were exposed to a uniform concentration or not exposed at all (Jones et al, 2015). In murine neutrophils, we observed that bone marrow neutrophils isolated from Il1r1-deficient mice displayed decreased activity against A. fumigatus in an in vitro hyphal damage assay (Caffrey et al, 2015). Moreover, neutrophils that reached the airways of Myd88-deficient mice had decreased anti-conidial activity on a per cell basis early after A. fumigatus challenge, but this effect was dependent on neutrophil-extrinsic MyD88 expression (Jhingran et al, 2015). Interestingly, the decreased antihyphal activity of bone marrow neutrophils isolated from Il1r1-deficient mice is rescued by treatment with recombinant CXCL1 (Caffrey et al, 2015), suggesting exposure to a chemotactic signal enhances the antihyphal activity of murine neutrophils. It has also been demonstrated that airway neutrophils in Card9−/− mice and Syk−/− → C57BL/6 chimeric mice exhibit defects in antifungal activity (Jhingran et al, 2012). Thus, there is an integral connection between neutrophil migration to infected airways and the induction and magnitude of their antifungal effector functions. Recent work strongly suggests that migrating leukocytes are exposed to oxygen and nutrient gradients that can significantly impact their antimicrobial functions. In the ensuing section, we turn to the potential impact of the host microenvironment on effector cell functions.
Consequences of a Dynamic Infection Microenvironment on Fungal Immunity
While our understanding of Aspergillus immune recognition and immune effector cell recruitment has moved forward, the contribution of the tissue inflammatory microenvironment in promoting and regulating these responses represents a new frontier with significant therapeutic potential. Inflammatory responses are strongly associated with decreases in oxygen availability that contribute to decreases in pH and to significant alterations in the availability of micronutrients (e.g., iron) and macronutrients (e.g., glucose) (Eltzschig & Carmeliet, 2011; Naquet et al, 2016; Nizet & Johnson, 2009). Consequently, immune competent hosts have robust regulatory mechanisms to deal with this physiological bacchanal in response to microbes such as A. fumigatus. These responses are critical to prevent inhibition of cellular and tissue functions, yet are underexplored in the context of invasive fungal infections. Moreover, how specific immunomodulatory drugs alter the tissue microenvironment and the critical physiological regulatory mechanisms that are essential to prevent host damage remains an important area of investigation.
Evidence for the importance of maintaining a tissue environment conducive to robust and beneficial immune effector cell function is illustrated by recent elegant studies in bacterial pathogenesis, rheumatoid arthritis, inflammatory bowel disease, various cancers, and acute lung injury. For example, Campbell et al. observed that a neutrophil-driven hypoxic microenvironment in the GI tract is essential for resolution of colitis (Campbell et al, 2014). Depletion of neutrophils or their respiratory burst eliminated the protective response. Intriguingly, pharmacological stabilization of the transcription factor HIF-1α restored protection. These data strongly suggest a critical role for HIF-1α in regulating tissue microenvironment homeostasis to promote beneficial immune system function that prevent or mitigate host damage.
Recently, the role of HIF-1α in response to A. fumigatus murine airway challenge was reported (Shepardson et al, 2014). It was observed that treatment of mice with a high corticosteroid dose resulted in a significant decrease in HIF-1α mRNA and nuclear protein levels in lung homogenates compared to untreated animals. Thus, in a clinically relevant murine model that is conducive to massive fungal proliferation and host mortality, lung HIF-1α levels and activation are reduced. These data suggest a potential role for HIF-1α in mediating resistance to A. fumigatus pulmonary challenge. In support of this hypothesis, immune competent mice lacking HIF-1α in the myeloid compartment (i.e., HIF-1αΔLysM) challenged with A. fumigatus displayed severe IA characterized by substantial fungal proliferation that ultimately led to mortality (Shepardson et al, 2014).
With regard to the underlying mechanism of HIF1α-mediated fungal resistance, a seminal study on HIF-1α’s role in innate immunity revealed a significant decrease in macrophage-mediated killing of group B Streptococcus in the absence of HIF-1α (Cramer et al, 2003). Subsequent studies revealed a role for HIF-1α in macrophage-mediated killing of group A Streptococcus (GAS) and Pseudomonas aeruginosa. Similar results with neutrophil bactericidal activity against GAS have been reported. Thus, it was reasonable to hypothesize that HIF-1α mediated macrophage and/or neutrophil antifungal activity as well. Surprisingly, loss of HIF-1α did not alter macrophage, monocyte, or neutrophil A. fumigatus conidiacidal activities in vitro, ex vivo, and in vivo (Shepardson et al, 2014). Thus, current data suggest that during infection with A. fumigatus, myeloid HIF-1α activity is not critical for early antifungal effector mechanisms.
Further highlighting the importance of the timing of effector cell recruitment, loss of HIF-1α in myeloid cells resulted in a significant decrease in neutrophil numbers in the airways and lung parencyma (Shepardson et al, 2014). Rescue of pulmonary neutrophil recruitment to wild-type levels through exogenous provision of CXCL1, a CXCR2 ligand, restored full protection of myeloid HIF-1α-deficient mice to challenge with A. fumigatus. These data suggest a critical role for HIF-1α in contributing to the induction of cytokines and chemokines needed for optimal neutrophil recruitment and possibly cell survival at the site of infection (Figure 1). Besides CXCL1, other known pro-inflammatory cytokines were also reduced in the airways of myeloid HIF-1α deficient mice, suggesting a critical role for myeloid HIF-1α in regulating proinflammatory cytokine production in response to A. fumigatus challenge. In further support of these data, Fliesser and colleagues observed marked reductions in the mRNA levels of proinflammatory cytokine encoding genes in HIF-1α silenced human dendritic cells compared to controls (Fliesser et al, 2015). Moreover, a strong reduction in IL-1α protein levels were observed in the HIF-1α silenced human dendritic cells exposed to A. fumigatus and hypoxia. Examination of HIF-1α protein levels in DCs with reduced levels of Dectin-1 revealed an important partial Dectin-1 dependency for maintaining HIF-1α protein levels in the face of A. fumigatus challenge under the examined in vitro conditions.
Though clearly much remains to be learned about HIF-1α-mediated resistance to A. fumigatus, the initial reports suggest that augmenting HIF-1α activity in specific immune compromised patient populations may help improve IA outcomes. Ongoing efforts to therapeutically target HIF-1α are ongoing in other pathosystems (Bhandari and Nizet 2014). However, the underlying mechanism of the striking fungal-mediated murine mortality in the myeloid HIF-1α-deficient mice remains unclear. Defining this mechanism is an important research direction to tap the potential of targeting HIF-1α for antifungal therapeutic benefit. For example, high-dose steroid treatment in the IA murine model did not seem to dramatically alter total HIF-1α protein in the lungs, rather, HIF-1α nuclear localization was strongly reduced via an unknown mechanism. Therefore, if this observation holds true in steroid-treated patient populations at risk for IA, inducing nuclear HIF-1α localization may be an important therapeutic target rather than increasing HIF-1α levels per se.
With regard to HIF-1α downstream effectors, recent genome-wide association studies in humans have identified mutations in a HIF-1α transcriptional target gene, vascular endothelial growth factor (VEGF), that are associated with increased risk for IA in specific transplant patient populations (Lupianez et al, 2015). VEGF is a critical signaling protein involved in angiogenesis and is strongly induced by hypoxia. A. fumigatus produces secondary metabolites such as gliotoxin that can directly inhibit angiogenesis (Ben-Ami et al, 2009). However, promotion of angiogenesis in murine models of IPA can improve outcomes through inhibition of the fungal mediated anti-angiogenic mechanisms and enhanced neutrophil recruitment (Ben-Ami et al, 2013). In further support of a critical role for HIF-1α-mediated signaling in microvascular remodeling, angiogenesis, and tissue damage homeostasis, von-Hippel-Lindau (VHL) haplodeficient mice were observed to be remarkably resistant to A. fumigatus proliferation (Jiang et al, 2013). VHL haplodeficient endothelial cells had increased angiogenic activity and were resistant to serum deprivation induced cell death. VHL directly controls levels of HIF-1α and is critical for hypoxia signaling and angiogenic responses (Ivan et al, 2001; Jaakkola et al, 2001). Taken together, myeloid HIF-1α signaling likely plays roles beyond the regulation of effector cell recruitment that mediate protection against IA that remain to be fully defined. Moreover, these results present a seminal example for how manipulation of the infection microenvironment, through understanding of basic molecular mechanisms, can potentially be harnessed therapeutically to improve IPA outcomes.
Aspergillus strain variation and the innate immune response
The impact of different A. fumigatus strains on host defense is an emerging area of great interest, particularly in the context of considering the specific fungal-host interaction to optimize IA patient care. Do all strains of A. fumigatus require the same host defense mechanisms for protection? Data suggest the answer to this question is no; the A. fumigatus strain matters. For example, it remains an open question why there are different observations on the dependency of IL-1α or IL-1β to initiate IL-1RI/MyD88 signaling. One possibility is that different A. fumigatus strains under investigation preferentially induce one IL-1 cytokine over the other, since the inflammatory response differs rather dramatically between strains (Amarsaikhan et al, 2014; O'Dea et al, 2014; Rizzetto et al, 2013). While the extent of A. fumigatus strain variability on the host immune response remains unknown, these initial reports strongly suggest that more work (elucidating the role of Aspergillus spp. strain variation on host interactions and infectious outcomes) in this area is warranted. What fungal effectors and mechanisms drive these responses is unclear and their identification could yield novel therapeutic approaches. For example, strain variation may include differential expression of fungal immunomodulatory factors that are detected by the host immune system. For example, the polysaccharide galactosaminogalactan (GAG), a recently uncovered virulence factor in Aspergillus spp., can modulate the host inflammatory response. Purified GAG has been shown to be sufficient to induce the IL-1 antagonist, IL-1RA, which enhances the A. fumigatus pathogenesis and correlates with decreased neutrophil accumulation in the lungs (Gresnigt et al, 2014). More recent work demonstrates that while GAG overexpression in Aspergillus nidulans increases its virulence; this finding was not the result of altering neutrophil recruitment to the lungs through modulating IL-1Ra or IL-1β levels (Lee et al, 2015). The extent to which GAG production and secretion varies within a single or different Aspergillus species remains unclear but almost certainly varies.
Another possible mechanism for how different A. fumigatus isolates induce different host responses is the generation of unique microenvironments within the respiratory tract. Following challenge with A. fumigatus there is significant induction of hypoxia and its intensity differs in models of neutropenia-induced and corticosteroid-mediated immunosuppression (Grahl et al, 2011b), but whether hypoxia quantitatively and temporally differs due to the infecting A. fumigatus isolate has not been addressed. Hypoxia dramatically alters cell death pathways and the local redox status of the tissue microenvironment. Hypoxia-induced conditions may favor the release of one effector cytokine over another, as discussed below for IL-1α and IL-1β. The mechanisms of IL-1α and IL-1β secretion differ substantially depending on the type of cell death, cellular redox status, and protease activation. IL-1α release depends on necrotic cell death (England et al, 2014), calpain activity (Zheng et al, 2013), and increased oxidative stress (i.e., high intracellular H2O2 levels) (McCarthy et al, 2013), but the exact events that stimulate its release following A. fumigatus challenge remain undefined. In contrast, IL-1β release is dependent on pryoptotic cell death and on caspase-1 and/or caspase-8 activation, typically via multisubunit inflammasomes (Lamkanfi & Dixit, 2014). A. fumigatus is known to activate NLRP3- (Karki et al, 2015; Moretti et al, 2014; Said-Sadier et al, 2010) and AIM2- (Karki et al, 2015) containing inflammasomes in order to mature and secrete IL-1β. Hypoxia reduces the local pH within the pulmonary tract, which is also known to modulate the activity of the NLRP3 inflammasome and IL-1β release (Torres et al, 2014).
With regard to the fungal hypoxia response, growth in hypoxic conditions is essential for virulence (Chung et al, 2014; Grahl et al, 2012a; Grahl et al, 2011a; Grahl et al, 2012b; Shepardson et al, 2013; Willger et al, 2008). An underappreciated point is that tissue hypoxia can alter fungal production of immunomodulating polysaccharides that in turn promote inflammation and host damage. For example, A. fumigatus strains exposed to low oxygen conditions increase the thickness of their cell wall and expose higher levels of β-glucan (Shepardson et al, 2013). This observation is also observed in Candida albicans strains (Marakalala et al, 2013). Thus, significant work elucidating the interactions between A. fumigatus and the host respiratory tract is needed to understand how A. fumigatus proliferates within the airways, alters the local microenvironment, and modulates host immunity.
Conclusions and Future Directions
Many exciting advances in our understanding of immune mediated protection against A. fumigatus have occurred in the last few years, highlighted by robust animal model studies and new genetic associations in human populations. A major challenge moving forward remains how to harness these advances for therapeutic development. In this regard, fungal and host genetic variability is a daunting challenge that is now being fully appreciated. Moreover, the diversity of underlying disease conditions that predispose individuals to IA remains a significant challenge. Subtle changes in the infection microenvironment, unique to each fungal-host interaction, may prove to significantly impact infection outcomes. Consequently, much more research is needed to understand, in specific patient populations, which key immune defense mechanisms are perturbed, and why, in order to develop new therapeutic strategies. In this regard, with any proposed new intervention, the impact on fungal virulence must also be taken into consideration. Thus, exciting opportunities exist to further our knowledge of A. fumigatus host interactions driven by rapid advances in technology that are opening up the proverbial black box of the fungal-host interaction in a given patient. As precise mechanisms of the host-pathogen interaction are more fully appreciated, one can envision full genotypic and phenotypic analyses of invading pathogen and patient in combination with infection microenvironment profiling to design optimum therapy for positive outcomes. The vision is bold but achievable with increased research efforts and collaboration between scientists, physicians, patients, and funding agencies.
Acknowledgments
Acknowledgments and Funding:
This work was funded in part by NIH R01 AI093808 and R21 AI105617 grants to TMH and in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 to MSKCC. JJO and RAC were supported in part by institutional start-up funds and in part through the Dartmouth Lung Biology Center for Molecular, Cellular, and Translational Research Grant P30 GM106394 (PI: Bruce A. Stanton) and Center for Molecular, Cellular, and Translational Immunological Research Grant P30 GM103415 (PI: William R. Green). Work was funded also in part by NIH R01 AI081838. RAC and TMH are Investigators in the Pathogenesis of Infectious Diseases supported by the Burroughs Wellcome Fund. The funders had no role in the preparation or publication of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, Clavaud C, Paris S, Brakhage AA, Kaveri SV, Romani L, Latge JP. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460:1117–1121. doi: 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- Akoumianaki T, Kyrmizi I, Valsecchi I, Gresnigt MS, Samonis G, Drakos E, Boumpas D, Muszkieta L, Prevost MC, Kontoyiannis DP, Chavakis T, Netea MG, van de Veerdonk FL, Brakhage AA, El-Benna J, Beauvais A, Latge JP, Chamilos G. Aspergillus Cell Wall Melanin Blocks LC3-Associated Phagocytosis to Promote Pathogenicity. Cell host & microbe. 2016;19:79–90. doi: 10.1016/j.chom.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Amarsaikhan N, O'Dea EM, Tsoggerel A, Owegi H, Gillenwater J, Templeton SP. Isolate-dependent growth, virulence, and cell wall composition in the human pathogen Aspergillus fumigatus. PloS one. 2014;9:e100430. doi: 10.1371/journal.pone.0100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balloy V, Huerre M, Latge JP, Chignard M. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infection and immunity. 2005;73:494–503. doi: 10.1128/IAI.73.1.494-503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami R, Albert ND, Lewis RE, Kontoyiannis DP. Proangiogenic growth factors potentiate in situ angiogenesis and enhance antifungal drug activity in murine invasive aspergillosis. The Journal of infectious diseases. 2013;207:1066–1074. doi: 10.1093/infdis/jis940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami R, Lewis RE, Leventakos K, Kontoyiannis DP. Aspergillus fumigatus inhibits angiogenesis through the production of gliotoxin and other secondary metabolites. Blood. 2009;114:5393–5399. doi: 10.1182/blood-2009-07-231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M, Niemiec MJ, Siler U, Urban CF, Reichenbach J. Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J Allergy Clin Immunol. 2011;127:1243–1252. e1247. doi: 10.1016/j.jaci.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Bonnett CR, Cornish EJ, Harmsen AG, Burritt JB. Early neutrophil recruitment and aggregation in the murine lung inhibit germination of Aspergillus fumigatus Conidia. Infection and immunity. 2006;74:6528–6539. doi: 10.1128/IAI.00909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle KB, Gyori D, Sindrilaru A, Scharffetter-Kochanek K, Taylor PR, Mocsai A, Stephens LR, Hawkins PT. Class IA phosphoinositide 3-kinase beta and delta regulate neutrophil oxidase activation in response to Aspergillus fumigatus hyphae. J Immunol. 2011;186:2978–2989. doi: 10.4049/jimmunol.1002268. [DOI] [PubMed] [Google Scholar]
- Bozza S, Campo S, Arseni B, Inforzato A, Ragnar L, Bottazzi B, Mantovani A, Moretti S, Oikonomous V, De Santis R, Carvalho A, Salvatori G, Romani L. PTX3 binds MD-2 and promotes TRIF-dependent immune protection in aspergillosis. Journal of immunology. 2014;193:2340–2348. doi: 10.4049/jimmunol.1400814. [DOI] [PubMed] [Google Scholar]
- Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nature immunology. 2014;15:1017–1025. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD. Innate antifungal immunity: the key role of phagocytes. Annual review of immunology. 2011;29:1–21. doi: 10.1146/annurev-immunol-030409-101229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns S, Kniemeyer O, Hasenberg M, Aimanianda V, Nietzsche S, Thywissen A, Jeron A, Latge JP, Brakhage AA, Gunzer M. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS pathogens. 2010;6:e1000873. doi: 10.1371/journal.ppat.1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey AK, Lehmann MM, Zickovich JM, Espinosa V, Shepardson KM, Watschke CP, Hilmer KM, Thammahong A, Barker BM, Rivera A, Cramer RA, Obar JJ. IL-1alpha signaling is critical for leukocyte recruitment after pulmonary Aspergillus fumigatus challenge. PLoS pathogens. 2015;11:e1004625. doi: 10.1371/journal.ppat.1004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, Bayless AJ, Scully M, Saeedi BJ, Golden-Mason L, Ehrentraut SF, Curtis VF, Burgess A, Garvey JF, Sorensen A, Nemenoff R, Jedlicka P, Taylor CT, Kominsky DJ, Colgan SP. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion Sde J, Leal SM, Jr, Ghannoum MA, Aimanianda V, Latge JP, Pearlman E. The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. Journal of immunology. 2013;191:2581–2588. doi: 10.4049/jimmunol.1300748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJ, van der Veer BM, Deen PM, Logie C, O'Neill LA, Willems P, van de Veerdonk FL, van der Meer JW, Ng A, Joosten LA, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG. mTOR-and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D, Barker BM, Carey CC, Merriman B, Werner ER, Lechner BE, Dhingra S, Cheng C, Xu W, Blosser SJ, Morohashi K, Mazurie A, Mitchell TK, Haas H, Mitchell AP, Cramer RA. ChIP-seq and in vivo transcriptome analyses of the Aspergillus fumigatus SREBP SrbA reveals a new regulator of the fungal hypoxia response and virulence. PLoS Pathog. 2014;10:e1004487. doi: 10.1371/journal.ppat.1004487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HL, Jhingran A, Sun Y, Vareechon C, de Jesus Carrion S, Skaar EP, Chazin WJ, Calera JA, Hohl TM, Pearlman E. Zinc and Manganese Chelation by Neutrophil S100A8/A9 (Calprotectin) Limits Extracellular Aspergillus fumigatus Hyphal Growth and Corneal Infection. Journal of immunology. 2016;196:336–344. doi: 10.4049/jimmunol.1502037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish EJ, Hurtgen BJ, McInnerney K, Burritt NL, Taylor RM, Jarvis JN, Wang SY, Burritt JB. Reduced nicotinamide adenine dinucleotide phosphate oxidase-independent resistance to Aspergillus fumigatus in alveolar macrophages. J Immunol. 2008;180:6854–6867. doi: 10.4049/jimmunol.180.10.6854. [DOI] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha C, Aversa F, Lacerda JF, Busca A, Kurzai O, Grube M, Loffler J, Maertens JA, Bell AS, Inforzato A, Barbati E, Almeida B, Santos e Sousa P, Barbui A, Potenza L, Caira M, Rodrigues F, Salvatori G, Pagano L, Luppi M, Mantovani A, Velardi A, Romani L, Carvalho A. Genetic PTX3 deficiency and aspergillosis in stem-cell transplantation. N Engl J Med. 2014;370:421–432. doi: 10.1056/NEJMoa1211161. [DOI] [PubMed] [Google Scholar]
- Cunha C, Di Ianni M, Bozza S, Giovannini G, Zagarella S, Zelante T, D'Angelo C, Pierini A, Pitzurra L, Falzetti F, Carotti A, Perruccio K, Latge JP, Rodrigues F, Velardi A, Aversa F, Romani L, Carvalho A. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient-and donor-dependent mechanisms of antifungal immunity. Blood. 2010;116:5394–5402. doi: 10.1182/blood-2010-04-279307. [DOI] [PubMed] [Google Scholar]
- de Luca A, Smeekens SP, Casagrande A, Iannitti R, Conway KL, Gresnigt MS, Begun J, Plantinga TS, Joosten LA, van der Meer JW, Chamilos G, Netea MG, Xavier RJ, Dinarello CA, Romani L, van de Veerdonk FL. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3526–3531. doi: 10.1073/pnas.1322831111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Ma S, Zhou H, Zang A, Fang Y, Li T, Shi H, Liu M, Du M, Taylor PR, Zhu HH, Chen J, Meng G, Li F, Chen C, Zhang Y, Jia XM, Lin X, Zhang X, Pearlman E, Li X, Feng GS, Xiao H. Tyrosine phosphatase SHP-2 mediates C-type lectin receptor-induced activation of the kinase Syk and anti-fungal TH17 responses. Nature immunology. 2015;16:642–652. doi: 10.1038/ni.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Carmeliet P. Hypoxia and inflammation. The New England journal of medicine. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England H, Summersgill HR, Edye ME, Rothwell NJ, Brough D. Release of interleukin-1alpha or interleukin-1beta depends on mechanism of cell death. The Journal of biological chemistry. 2014;289:15942–15950. doi: 10.1074/jbc.M114.557561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa V, Jhingran A, Dutta O, Kasahara S, Donnelly R, Du P, Rosenfeld J, Leiner I, Chen CC, Ron Y, Hohl TM, Rivera A. Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS pathogens. 2014;10:e1003940. doi: 10.1371/journal.ppat.1003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesser M, Morton CO, Bonin M, Ebel F, Hunniger K, Kurzai O, Einsele H, Loffler J. Hypoxia-inducible factor 1alpha modulates metabolic activity and cytokine release in anti-Aspergillus fumigatus immune responses initiated by human dendritic cells. Int J Med Microbiol. 2015;305:865–873. doi: 10.1016/j.ijmm.2015.08.036. [DOI] [PubMed] [Google Scholar]
- Garlanda C, Hirsch E, Bozza S, Salustri A, De Acetis M, Nota R, Maccagno A, Riva F, Bottazzi B, Peri G, Doni A, Vago L, Botto M, De Santis R, Carminati P, Siracusa G, Altruda F, Vecchi A, Romani L, Mantovani A. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–186. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- Gazendam RP, van Hamme JL, Tool AT, Hoogenboezem M, van den Berg JM, Prins JM, Vitkov L, van de Veerdonk FL, van den Berg TK, Roos D, Kuijpers TW. Human Neutrophils Use Different Mechanisms To Kill Aspergillus fumigatus Conidia and Hyphae: Evidence from Phagocyte Defects. Journal of immunology. 2016;196:1272–1283. doi: 10.4049/jimmunol.1501811. [DOI] [PubMed] [Google Scholar]
- Gerson SL, Talbot GH, Hurwitz S, Strom BL, Lusk EJ, Cassileth PA. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Annals of internal medicine. 1984;100:345–351. doi: 10.7326/0003-4819-100-3-345. [DOI] [PubMed] [Google Scholar]
- Gersuk GM, Underhill DM, Zhu L, Marr KA. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. Journal of immunology. 2006;176:3717–3724. doi: 10.4049/jimmunol.176.6.3717. [DOI] [PubMed] [Google Scholar]
- Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, Bose N, Chan AS, Magee AS, Danielson ME, Weiss A, Vasilakos JP, Underhill DM. Activation of the innate immune receptor Dectin-1 upon formation of a 'phagocytic synapse'. Nature. 2011;472:471–475. doi: 10.1038/nature10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahl N, Dinamarco TM, Willger SD, Goldman GH, Cramer RA. Aspergillus fumigatus mitochondrial electron transport chain mediates oxidative stress homeostasis, hypoxia responses and fungal pathogenesis. Mol Microbiol. 2012a;84:383–399. doi: 10.1111/j.1365-2958.2012.08034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahl N, Puttikamonkul S, Macdonald JM, Gamcsik MP, Ngo LY, Hohl TM, Cramer RA. In vivo hypoxia and a fungal alcohol dehydrogenase influence the pathogenesis of invasive pulmonary aspergillosis. PLoS Pathogens. 2011a;7:e1002145. doi: 10.1371/journal.ppat.1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahl N, Puttikamonkul S, Macdonald JM, Gamcsik MP, Ngo LY, Hohl TM, Cramer RA. In vivo hypoxia and a fungal alcohol dehydrogenase influence the pathogenesis of invasive pulmonary aspergillosis. PLoS pathogens. 2011b;7:e1002145. doi: 10.1371/journal.ppat.1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahl N, Shepardson KM, Chung D, Cramer RA. Hypoxia and fungal pathogenesis: to air or not to air? Eukaryot Cell. 2012b;11:560–570. doi: 10.1128/EC.00031-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt MB, Aliprantis A, Hu B, Glimcher LH. Calcineurin regulates innate antifungal immunity in neutrophils. The Journal of experimental medicine. 2010;207:923–931. doi: 10.1084/jem.20092531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresnigt MS, Bozza S, Becker KL, Joosten LA, Abdollahi-Roodsaz S, van der Berg WB, Dinarello CA, Netea MG, Fontaine T, De Luca A, Moretti S, Romani L, Latge JP, van de Veerdonk FL. A polysaccharide virulence factor from Aspergillus fumigatus elicits anti-inflammatory effects through induction of Interleukin-1 receptor antagonist. PLoS pathogens. 2014;10:e1003936. doi: 10.1371/journal.ppat.1003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TB. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nature immunology. 2012;13:246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- Gross O, Gewies A, Finger K, Schafer M, Sparwasser T, Peschel C, Forster I, Ruland J. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature. 2006;442:651–656. doi: 10.1038/nature04926. [DOI] [PubMed] [Google Scholar]
- Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- Hara H, Ishihara C, Takeuchi A, Imanishi T, Xue L, Morris SW, Inui M, Takai T, Shibuya A, Saijo S, Iwakura Y, Ohno N, Koseki H, Yoshida H, Penninger JM, Saito T. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors. Nature immunology. 2007;8:619–629. doi: 10.1038/ni1466. [DOI] [PubMed] [Google Scholar]
- Herbst S, Shah A, Carby M, Chusney G, Kikkeri N, Dorling A, Bignell E, Shaunak S, Armstrong-James D. A new and clinically relevant murine model of solid-organ transplant aspergillosis. Dis Model Mech. 2013;6:643–651. doi: 10.1242/dmm.010330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst S, Shah A, Mazon Moya M, Marzola V, Jensen B, Reed A, Birrell MA, Saijo S, Mostowy S, Shaunak S, Armstrong-James D. Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus. EMBO Mol Med. 2015;7:240–258. doi: 10.15252/emmm.201404556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl TM, Van Epps HL, Rivera A, Morgan LA, Chen PL, Feldmesser M, Pamer EG. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS pathogens. 2005;1:e30. doi: 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SM. Chronic granulomatous disease. Clin Rev Allergy Immunol. 2010;38:3–10. doi: 10.1007/s12016-009-8136-z. [DOI] [PubMed] [Google Scholar]
- Hsu YM, Zhang Y, You Y, Wang D, Li H, Duramad O, Qin XF, Dong C, Lin X. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nature immunology. 2007;8:198–205. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
- Huang H, Ostroff GR, Lee CK, Agarwal S, Ram S, Rice PA, Specht CA, Levitz SM. Relative contributions of dectin-1 and complement to immune responses to particulate beta-glucans. Journal of immunology. 2012;189:312–317. doi: 10.4049/jimmunol.1200603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Itoh F, Yoshida S, Saijo S, Matsuzawa T, Gonoi T, Saito T, Okawa Y, Shibata N, Miyamoto T, Yamasaki S. Identification of distinct ligands for the C-type lectin receptors Mincle and Dectin-2 in the pathogenic fungus Malassezia. Cell host & microbe. 2013;13:477–488. doi: 10.1016/j.chom.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jhingran A, Kasahara S, Shepardson KM, Junecko BA, Heung LJ, Kumasaka DK, Knoblaugh SE, Lin X, Kazmierczak BI, Reinhart TA, Cramer RA, Hohl TM. Compartment-specific and sequential role of MyD88 and CARD9 in chemokine induction and innate defense during respiratory fungal infection. PLoS pathogens. 2015;11:e1004589. doi: 10.1371/journal.ppat.1004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhingran A, Mar KB, Kumasaka DK, Knoblaugh SE, Ngo LY, Segal BH, Iwakura Y, Lowell CA, Hamerman JA, Lin X, Hohl TM. Tracing conidial fate and measuring host cell antifungal activity using a reporter of microbial viability in the lung. Cell Rep. 2012;2:1762–1773. doi: 10.1016/j.celrep.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Hsu JL, Tian W, Yuan K, Olcholski M, Perez Vde J, Semenza GL, Nicolls MR. Tie2-dependent VHL knockdown promotes airway microvascular regeneration and attenuates invasive growth of Aspergillus fumigatus. J Mol Med (Berl) 2013;91:1081–1093. doi: 10.1007/s00109-013-1063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CN, Dimisko L, Forrest K, Judice K, Poznansky MC, Markmann JF, Vyas JM, Irimia D. Human Neutrophils Are Primed by Chemoattractant Gradients for Blocking the Growth of Aspergillus fumigatus. The Journal of infectious diseases. 2015 doi: 10.1093/infdis/jiv419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R, Man SM, Malireddi RK, Gurung P, Vogel P, Lamkanfi M, Kanneganti TD. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell host & microbe. 2015;17:357–368. doi: 10.1016/j.chom.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara S, Jhingran A, Dhingra S, Salem A, Cramer RA, Hohl TM. Role of Granulocyte-Macrophage Colony-Stimulating Factor Signaling in Regulating Neutrophil Antifungal Activity and the Oxidative Burst During Respiratory Fungal Challenge. The Journal of infectious diseases. 2016;213:1289–1298. doi: 10.1093/infdis/jiw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr SC, Fischer GJ, Sinha M, McCabe O, Palmer JM, Choera T, Yun Lim F, Wimmerova M, Carrington SD, Yuan S, Lowell CA, Oscarson S, Keller NP, Fahy JV. FleA Expression in Aspergillus fumigatus Is Recognized by Fucosylated Structures on Mucins and Macrophages to Prevent Lung Infection. PLoS pathogens. 2016;12:e1005555. doi: 10.1371/journal.ppat.1005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- Kyrmizi I, Gresnigt MS, Akoumianaki T, Samonis G, Sidiropoulos P, Boumpas D, Netea MG, van de Veerdonk FL, Kontoyiannis DP, Chamilos G. Corticosteroids block autophagy protein recruitment in Aspergillus fumigatus phagosomes via targeting dectin-1/Syk kinase signaling. Journal of immunology. 2013;191:1287–1299. doi: 10.4049/jimmunol.1300132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Leal SM, Jr, Cowden S, Hsia YC, Ghannoum MA, Momany M, Pearlman E. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS pathogens. 2010;6:e1000976. doi: 10.1371/journal.ppat.1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SM, Jr, Roy S, Vareechon C, Carrion S, Clark H, Lopez-Berges MS, Di Pietro A, Schrettl M, Beckmann N, Redl B, Haas H, Pearlman E. Targeting iron acquisition blocks infection with the fungal pathogens Aspergillus fumigatus and Fusarium oxysporum. PLoS pathogens. 2013;9:e1003436. doi: 10.1371/journal.ppat.1003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SM, Jr, Vareechon C, Cowden S, Cobb BA, Latge JP, Momany M, Pearlman E. Fungal antioxidant pathways promote survival against neutrophils during infection. The Journal of clinical investigation. 2012;122:2482–2498. doi: 10.1172/JCI63239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Liu H, Barker BM, Snarr BD, Gravelat FN, Al Abdallah Q, Gavino C, Baistrocchi SR, Ostapska H, Xiao T, Ralph B, Solis NV, Lehoux M, Baptista SD, Thammahong A, Cerone RP, Kaminskyj SG, Guiot MC, Latge JP, Fontaine T, Vinh DC, Filler SG, Sheppard DC. The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps. PLoS pathogens. 2015;11:e1005187. doi: 10.1371/journal.ppat.1005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nature immunology. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- Li X, Cullere X, Nishi H, Saggu G, Durand E, Mansour MK, Tam JM, Song XY, Lin X, Vyas JM, Mayadas T. PKC-delta activation in neutrophils promotes fungal clearance. J Leukoc Biol. 2016 doi: 10.1189/jlb.4A0915-405R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Utomo A, Cullere X, Choi MM, Milner DA, Jr, Venkatesh D, Yun SH, Mayadas TN. The beta-glucan receptor Dectin-1 activates the integrin Mac-1 in neutrophils via Vav protein signaling to promote Candida albicans clearance. Cell host & microbe. 2011;10:603–615. doi: 10.1016/j.chom.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loures FV, Rohm M, Lee CK, Santos E, Wang JP, Specht CA, Calich VL, Urban CF, Levitz SM. Recognition of Aspergillus fumigatus hyphae by human plasmacytoid dendritic cells is mediated by dectin-2 and results in formation of extracellular traps. PLoS pathogens. 2015;11:e1004643. doi: 10.1371/journal.ppat.1004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupianez CB, Canet LM, Carvalho A, Alcazar-Fuoli L, Springer J, Lackner M, Segura-Catena J, Comino A, Olmedo C, Rios R, Fernandez-Montoya A, Cuenca-Estrella M, Solano C, Lopez-Nevot MA, Cunha C, Oliveira-Coelho A, Villaescusa T, Fianchi L, Aguado JM, Pagano L, Lopez-Fernandez E, Potenza L, Luppi M, Lass-Florl C, Loeffler J, Einsele H, Vazquez L, Jurado M, Sainz J. Polymorphisms in Host Immunity-Modulating Genes and Risk of Invasive Aspergillosis: Results from the AspBIOmics Consortium. Infection and immunity. 2015;84:643–657. doi: 10.1128/IAI.01359-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marakalala MJ, Vautier S, Potrykus J, Walker LA, Shepardson KM, Hopke A, Mora-Montes HM, Kerrigan A, Netea MG, Murray GI, Maccallum DM, Wheeler R, Munro CA, Gow NA, Cramer RA, Brown AJ, Brown GD. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS pathogens. 2013;9:e1003315. doi: 10.1371/journal.ppat.1003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Malireddi RK, Lu Q, Cunha LD, Pelletier S, Gingras S, Orchard R, Guan JL, Tan H, Peng J, Kanneganti TD, Virgin HW, Green DR. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat Cell Biol. 2015;17:893–906. doi: 10.1038/ncb3192. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- McCarthy DA, Clark RR, Bartling TR, Trebak M, Melendez JA. Redox control of the senescence regulator interleukin-1alpha and the secretory phenotype. The Journal of biological chemistry. 2013;288:32149–32159. doi: 10.1074/jbc.M113.493841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrad B, Strieter RM, Moore TA, Tsai WC, Lira SA, Standiford TJ. CXC chemokine receptor-2 ligands are necessary components of neutrophil-mediated host defense in invasive pulmonary aspergillosis. Journal of immunology. 1999a;163:6086–6094. [PubMed] [Google Scholar]
- Mehrad B, Strieter RM, Standiford TJ. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. Journal of immunology. 1999b;162:1633–1640. [PubMed] [Google Scholar]
- Mintz R, Garty BZ, Meshel T, Marcus N, Katanov C, Cohen-Hillel E, Ben-Baruch A. Reduced expression of chemoattractant receptors by polymorphonuclear leukocytes in Hyper IgE Syndrome patients. Immunol Lett. 2010;130:97–106. doi: 10.1016/j.imlet.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Moalli F, Doni A, Deban L, Zelante T, Zagarella S, Bottazzi B, Romani L, Mantovani A, Garlanda C. Role of complement and Fc{gamma} receptors in the protective activity of the long pentraxin PTX3 against Aspergillus fumigatus. Blood. 2010;116:5170–5180. doi: 10.1182/blood-2009-12-258376. [DOI] [PubMed] [Google Scholar]
- Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti S, Bozza S, Oikonomou V, Renga G, Casagrande A, Iannitti RG, Puccetti M, Garlanda C, Kim S, Li S, van de Veerdonk FL, Dinarello CA, Romani L. IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. PLoS pathogens. 2014;10:e1004462. doi: 10.1371/journal.ppat.1004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern DE, Gifford MA, Li LL, Doerschuk CM, Dinauer MC. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. The Journal of experimental medicine. 1997;185:207–218. doi: 10.1084/jem.185.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naquet P, Giessner C, Galland F. Metabolic adaptation of tissues to stress releases metabolites influencing innate immunity. Current opinion in immunology. 2016;38:30–38. doi: 10.1016/j.coi.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Nguyen-Jackson H, Panopoulos AD, Zhang H, Li HS, Watowich SS. STAT3 controls the neutrophil migratory response to CXCR2 ligands by direct activation of G-CSF-induced CXCR2 expression and via modulation of CXCR2 signal transduction. Blood. 2010;115:3354–3363. doi: 10.1182/blood-2009-08-240317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dea EM, Amarsaikhan N, Li H, Downey J, Steele E, Van Dyken SJ, Locksley RM, Templeton SP. Eosinophils are recruited in response to chitin exposure and enhance Th2-mediated immune pathology in Aspergillus fumigatus infection. Infection and immunity. 2014;82:3199–3205. doi: 10.1128/IAI.01990-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeck H, Bscheider M, Gross O, Finger K, Roth S, Rebsamen M, Hannesschlager N, Schlee M, Rothenfusser S, Barchet W, Kato H, Akira S, Inoue S, Endres S, Peschel C, Hartmann G, Hornung V, Ruland J. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nature immunology. 2010;11:63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- Polak-Wyss A. Protective effect of human granulocyte colony-stimulating factor (hG-CSF) on Cryptococcus and Aspergillus infections in normal and immunosuppressed mice. Mycoses. 1991;34:205–215. doi: 10.1111/j.1439-0507.1991.tb00645.x. [DOI] [PubMed] [Google Scholar]
- Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- Rivera A, Hohl TM, Collins N, Leiner I, Gallegos A, Saijo S, Coward JW, Iwakura Y, Pamer EG. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. The Journal of experimental medicine. 2011;208:369–381. doi: 10.1084/jem.20100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzetto L, Giovannini G, Bromley M, Bowyer P, Romani L, Cavalieri D. Strain dependent variation of immune responses to A. fumigatus: definition of pathogenic species. PloS one. 2013;8:e56651. doi: 10.1371/journal.pone.0056651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, Reis e Sousa C. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Said-Sadier N, Padilla E, Langsley G, Ojcius DM. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PloS one. 2010;5:e10008. doi: 10.1371/journal.pone.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz J, Perez E, Gomez-Lopera S, Jurado M. IL1 gene cluster polymorphisms and its haplotypes may predict the risk to develop invasive pulmonary aspergillosis and modulate C-reactive protein level. J Clin Immunol. 2008;28:473–485. doi: 10.1007/s10875-008-9197-0. [DOI] [PubMed] [Google Scholar]
- Sato K, Yang XL, Yudate T, Chung JS, Wu J, Luby-Phelps K, Kimberly RP, Underhill D, Cruz PD, Jr, Ariizumi K. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. The Journal of biological chemistry. 2006;281:38854–38866. doi: 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- Savers A, Rasid O, Parlato M, Brock M, Jouvion G, Ryffel B, Cavaillon JM, Eberl G, Ibrahim-Granet O. Infection-Mediated Priming of Phagocytes Protects against Lethal Secondary Aspergillus fumigatus Challenge. PloS one. 2016;11:e0153829. doi: 10.1371/journal.pone.0153829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelenz S, Smith DA, Bancroft GJ. Cytokine and chemokine responses following pulmonary challenge with Aspergillus fumigatus: obligatory role of TNF-alpha and GM-CSF in neutrophil recruitment. Medical mycology. 1999;37:183–194. doi: 10.1046/j.1365-280x.1999.00219.x. [DOI] [PubMed] [Google Scholar]
- Serrano-Gomez D, Dominguez-Soto A, Ancochea J, Jimenez-Heffernan JA, Leal JA, Corbi AL. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin mediates binding and internalization of Aspergillus fumigatus conidia by dendritic cells and macrophages. Journal of immunology. 2004;173:5635–5643. doi: 10.4049/jimmunol.173.9.5635. [DOI] [PubMed] [Google Scholar]
- Shepardson KM, Jhingran A, Caffrey A, Obar JJ, Suratt BT, Berwin BL, Hohl TM, Cramer RA. Myeloid derived hypoxia inducible factor 1-alpha is required for protection against pulmonary Aspergillus fumigatus infection. PLoS pathogens. 2014;10:e1004378. doi: 10.1371/journal.ppat.1004378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepardson KM, Ngo LY, Aimanianda V, Latge JP, Barker BM, Blosser SJ, Iwakura Y, Hohl TM, Cramer RA. Hypoxia enhances innate immune activation to Aspergillus fumigatus through cell wall modulation. Microbes and infection / Institut Pasteur. 2013;15:259–269. doi: 10.1016/j.micinf.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, Brown GD. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS pathogens. 2005;1:e42. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, Apewokin S, Horn DL. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. The Journal of infection. 2012;65:453–464. doi: 10.1016/j.jinf.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, Hopfner KP, Brombacher F, Urlaub H, Baier G, Brown GD, Leitges M, Ruland J. Syk kinase-coupled C-type lectin receptors engage protein kinase C-sigma to elicit Card9 adaptor-mediated innate immunity. Immunity. 2012;36:32–42. doi: 10.1016/j.immuni.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SD, Rohn TT, Quinn MT. Neutrophil priming in host defense: role of oxidants as priming agents. Antioxid Redox Signal. 2002;4:69–83. doi: 10.1089/152308602753625870. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Roy S, Leal SM, Jr, Sun Y, Howell SJ, Cobb BA, Li X, Pearlman E. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nature immunology. 2014;15:143–151. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres IM, Patankar YR, Shabaneh TB, Dolben E, Hogan DA, Leib DA, Berwin BL. Acidosis potentiates the host proinflammatory interleukin-1beta response to Pseudomonas aeruginosa infection. Infection and immunity. 2014;82:4689–4697. doi: 10.1128/IAI.02024-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Yamamoto Y, Klein TW, Friedman H, Yamaguchi H. Granulocyte-colony stimulating factor facilitates the restoration of resistance to opportunistic fungi in leukopenic mice. J Med Vet Mycol. 1992;30:293–300. doi: 10.1080/02681219280000381. [DOI] [PubMed] [Google Scholar]
- VandenBergh MF, Verweij PE, Voss A. Epidemiology of nosocomial fungal infections: invasive aspergillosis and the environment. Diagn Microbiol Infect Dis. 1999;34:221–227. doi: 10.1016/s0732-8893(99)00026-7. [DOI] [PubMed] [Google Scholar]
- Vinh DC, Sugui JA, Hsu AP, Freeman AF, Holland SM. Invasive fungal disease in autosomal-dominant hyper-IgE syndrome. J Allergy Clin Immunol. 2010;125:1389–1390. doi: 10.1016/j.jaci.2010.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. Journal of immunology. 2009;182:4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willger SD, Puttikamonkul S, Kim KH, Burritt JB, Grahl N, Metzler LJ, Barbuch R, Bard M, Lawrence CB, Cramer RA., Jr A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog. 2008;4:e1000200. doi: 10.1371/journal.ppat.1000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz A, Gresnigt MS, Lecompte T, Bibert S, Manuel O, Joosten LA, Rueger S, Berger C, Boggian K, Cusini A, Garzoni C, Hirsch HH, Weisser M, Mueller NJ, Meylan PR, Steiger J, Kutalik Z, Pascual M, van Delden C, van de Veerdonk FL, Bochud PY Swiss Transplant Cohort S, Swiss Transplant Cohort Study S. IL1B and DEFB1 Polymorphisms Increase Susceptibility to Invasive Mold Infection After Solid-Organ Transplantation. The Journal of infectious diseases. 2015;211:1646–1657. doi: 10.1093/infdis/jiu636. [DOI] [PubMed] [Google Scholar]
- Xia Y, Vetvicka V, Yan J, Hanikyrova M, Mayadas T, Ross GD. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. Journal of immunology. 1999;162:2281–2290. [PubMed] [Google Scholar]
- Yamasaki S, Matsumoto M, Takeuchi O, Matsuzawa T, Ishikawa E, Sakuma M, Tateno H, Uno J, Hirabayashi J, Mikami Y, Takeda K, Akira S, Saito T. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci U S A. 2009;106:1897–1902. doi: 10.1073/pnas.0805177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CS, Lee JS, Rodgers M, Min CK, Lee JY, Kim HJ, Lee KH, Kim CJ, Oh B, Zandi E, Yue Z, Kramnik I, Liang C, Jung JU. Autophagy protein Rubicon mediates phagocytic NADPH oxidase activation in response to microbial infection or TLR stimulation. Cell host & microbe. 2012a;11:264–276. doi: 10.1016/j.chom.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CS, Rodgers M, Min CK, Lee JS, Kingeter L, Lee JY, Jong A, Kramnik I, Lin X, Jung JU. The autophagy regulator Rubicon is a feedback inhibitor of CARD9-mediated host innate immunity. Cell host & microbe. 2012b;11:277–289. doi: 10.1016/j.chom.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarember KA, Sugui JA, Chang YC, Kwon-Chung KJ, Gallin JI. Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. Journal of immunology. 2007;178:6367–6373. doi: 10.4049/jimmunol.178.10.6367. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Humphry M, Maguire JJ, Bennett MR, Clarke MC. Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1alpha, controlling necrosis-induced sterile inflammation. Immunity. 2013;38:285–295. doi: 10.1016/j.immuni.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Zhao XQ, Jiang C, You Y, Chen XP, Jiang YY, Jia XM, Lin X. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity. 2013;39:324–334. doi: 10.1016/j.immuni.2013.05.017. [DOI] [PubMed] [Google Scholar]