Abstract
A new cycloartane-type saponin with unusual hydroxylation at C-17 and a unique side chain, 9 (R), 19, 22 (S), 24 (R) bicyclolanost-3β, 12α, 16β, 17α tetrol-25-one 3-O-β-d-glucopyranosyl-(1→2)-β-d-glucopyranoside (1) and two new monoterpenoid glucoindole alkaloids, 10-methoxy pumiloside (2) and the previously chemically synthesized, 10-methoxy strictosidine (3) along with other five known compounds, 7α-morroniside (4), 7-epi-loganin (5), (7β)-7-O-methylmorroniside (6), 5(S)-5-carboxystrictisidine (7) and apigenin-7-O-neohesperidoside (8) were isolated from the aerial parts of Mussaenda luteola (Rubiaceae). The structural elucidation of the isolates was accomplished by extensive (1D and 2D NMR) spectroscopic data analysis and HR-ESI-MS. Compounds 4–8 were reported for the first time from the genus Mussaenda. Interestingly, this is the first report for the occurrence of the monoterpenoid glucoindole-type alkaloids in the genus which might be useful for the chemotaxonomic evaluation of the genus Mussaenda. All isolates were evaluated for their antiprotozoal activities. Compound 7 showed good antitrypanosomal activity with IC50 and IC90 values of 13.7 and 16.6 µM compared to IC50 and IC90 values of 13.06 and 28.99 µM for the positive control DFMO, difluoromethylornithine.
Keywords: Mussaenda luteola, Rubiaceae, Antitrypanosomal, Cycloartane-type saponin, Monoterpenoid glucoindole alkaloid
1. Introduction
Mussaenda species (Rubiaceae) are native to the old world tropics and have historically been used in Chinese and Fijian traditional medicine [1]. Mussaenda is an important source of medicinal natural products [1]. Several species of Mussaenda have been found to be biologically active and were utilized as diuretic, abortifacient, antiphlogistic, expectorant, antimicrobial, and antipyretic [2,3]. Previous phytochemical studies on Mussaenda were focused on the presence of triterpenoid saponins of cycloartane, ursane, and oleanane types, iridoids, and flavonoids [4–9].
Several triterpenoid cycloartane saponins have been isolated from M. pubescens [3]. In a previous paper, we have reported the isolation and structure elucidation of five new antitrypanosomal saponins from Mussaenda luteola [10]. In continuation of our studies, the aerial plant materials were further investigated. As a result, one new cycloartane-type saponin and two new monoterpenoid glucoindole alkaloids along with other five known compounds were isolated and their antiprotozoal activities were studied.
2. Experimental
2.1. General
Optical rotations were measured with Autopol IV polarimeter. IR spectra were obtained using a Bruker Tensor 27 instrument. UV spectra were recorded on Cary-50 Bio spectrophotometer. The 1H, 13C and 2D NMR spectra were recorded on a Varian Mercury 400 MHz spectrometer at 400 (1H) and 100 (13C), Bruker Avance DRX spectrometer at 600 MHz (1H) and 150 MHz (13C) using TMS as internal standard. The HR-ESI-MS were obtained using a Bruker Bioapex-FTMS with electrospray ionization (ESI). Semi-preparative HPLC (Waters delta prep 4000) was performed using Waters, Econosil C-18 [10 µm [22(ID) × 250 (L) mm]. Column chromatography (CC) was performed on silica gel 60 F254 (0.2 mm, Merck), Diaion HP-20, Sephadex™ LH-20 and MN-polyamide-SC-6.
2.2. Plant material
Aerial parts of M. luteola were collected from El-Zohria research garden, Cairo, Egypt, In May 2012. The plant material was identified by Professor Mo'men Mostafa Mahmoud, Professor of Taxonomy, Faculty of Science, Assiut University, Assiut, Egypt. A voucher specimen (No. 36) has been deposited at the herbarium of Pharmacognosy Department, Faculty of Pharmacy, Assiut University, Egypt.
2.3. Extraction and isolation
Air-dried powdered plant material (600 g) was exhaustively extracted by maceration with 70% methanol (4 L × 3) at room temperature for 3 days. The combined extracts were evaporated under reduced pressure to afford a dry residue (50 g). Vacuum liquid chromatography (VLC) was used for initial fractionation of the total methanolic extract. Step gradient elution with a nonpolar solvent (n-Hexane, 2 L) and increasing the gradient with a polar solvents: (EtOAc, 2 L), followed by (EtOAc:MeOH 1–1, 2 L) then (MeOH, 2 L) to give three main fractions (F1–F3). F2 (4.5 g) was subjected to silica gel (180 g) CC [3(ID) × 80 (L) cm] which was eluted initially with DCM-MeOH (95:5) then gradient polarity increase to (90:10), (85:15) and (80:20) to afford subfractions (Fr. A–E). Fr. C (316 mg) was subjected to silica gel (12 g) CC [1(ID) × 20 (L) cm] which was eluted initially with DCM-MeOH (95:5) then gradient increase till 80:20 to afford subfractions C-(1–4). Subfraction C-(1) (55 mg) was further purified with HPLC using MeCN-H2O-FA (15:85:0.1) as elution system with a flow rate of 15 to afford compound 4 (5.4 mg) at retention time of 7.5. Fr. E (1.5 g) was subjected to silica gel (60 g) CC [1.5 (ID) × 50 (L) cm] which was eluted initially with DCM-MeOH (95:5) then gradient increase till 80:20 to afford subfractions E-1–4. Subfraction E-1 (60.4 mg) was further purified with HPLC using MeCN-H2O-FA (10:90:0.1) as elution system with a flow rate of 15 mL/min to afford compounds 5 (1.9 mg) and 6 (20 mg) at retention times (13.9, and 14.6, respectively). Subfraction E-2 (491.6 mg) was subjected to silica gel (20 g) CC [1.5 (ID) × 30 (L) cm] which was eluted with EtOAc-DCM-MeOH-H2O (80-40-11-2) to afford subfractions E-2-a to E-2-d. Subfraction E-2-c (43.7 mg) was further purified using Sephadex LH-20 (10 g) CC [1 (ID) × 30 (L) cm] and eluted with MeOH-DCM (3–1) to give compound 3 (18.7 mg). F3 (28 g) was subjected to Diaion-HP20 column and eluted with distilled water then methanol to give two main subfractions. The methanolic fraction (7.0 g) was subjected to MN-polyamide-SC-6 (250 g) which was eluted with water then gradient decreased polarities with water-methanol systems to give 7 subfractions (Fr. 1–7). Fr. 2 was subjected to silica gel (10 g) CC [1(ID) × 20(L) cm] slurried in DCM. The column was eluted initially with DCM followed by DCM-MeOH gradiently to afford compound 7 (68.7 mg). Fr. 4 (719 mg) was subjected to silica gel (25 g) CC [1(ID) × 40(L) cm] which was eluted initially with DCM followed by DCM-MeOH gradiently to afford three subfractions Fr. 4A to Fr. 4C. Subfraction Fr. 4C (50 mg) was subjected to Sephadex LH-20 (50 g) [1(ID) × 100 (L) cm] CC to afford compounds 1 (5.0 mg) and 2 (10.6 mg). Fr. 7 (207 mg) was subjected to Sephadex LH-20 (25 g) CC [1(ID) × 40(L) cm] which was eluted with MeOH to give subfractions A, B and C. Collected subfraction C dried to give compound 8 (2.8 mg).
2.3.1. 9 (R), 19, 22 (S), 24 (R) dicyclolanost-3β, 12α, 16β, 17α tetrol-25-one 3-O-β-d-glucopyranosyl-(1→2)-β-d-glucopyranoside (1)
A faint yellow amorphous powder; (c 0.026, MeOH); IR (KBr) νmax 3350.3, 2928.1, 1681.2, 1075.9 cm−1; For 1H and 13C NMR (CD3OD, 600, 150 MHz) see Table 1; HR-ESI-MS m/z 821.4292 [M+Na]+ (calcd 821.4299) and m/z 833.4093 [M+Cl]− (calcd 833.4090).
Table 1.
1H and 13C NMR spectroscopic data for compound 1 (CD3OD, 600, 150 MHz).
| Position | δH (J in Hz) | δC | Position | δH (J in Hz) | δC |
|---|---|---|---|---|---|
| 1 | Eq.1.32, m ax.1.67, m | 33.2 | 22 | 0.80, m | 30.0 |
| 2 | Eq. 2.10, m ax.1.62, m | 30.4 | 23 | 0.75, m, 0.93, m | 15.9 |
| 3 | 3.31, dd (9.0, 4.2) | 90.7 | 24 | 2.13, m | 32.4 |
| 4 | – | 42.2 | 25 | – | 212.2 |
| 5 | 1.39, dd (3.6, 12.6) | 49.0 | 26 | 2.24, s | 29.9 |
| 6 | 0.84, 1.68, m | 22.1 | 28 | 1.11, s | 25.9 |
| 7 | 0.95, 1.27, m | 27.5 | 29 | 0.90, s | 15.5 |
| 8 | 1.42, m | 51.2 | 30 | 1.46, s | 23.6 |
| 9 | – | 20.1 | G-1 | 4.48, d (6.6) | 105.3 |
| 10 | – | 27.2 | 2 | 3.60, m | 81.4 |
| 11 | β 1.74, dd (15.6, 9.0) α 2.13, dd (15.0, 6.0) |
40.9 | 3 | 3.27, m | 78.5 |
| 12 | 4.20, t (7.8) | 76.6 | 4 | 3.23, m | 71.9 |
| 13 | – | 52.9 | 5 | 3.59, m | 78.3 |
| 14 | – | 48.1 | 6 | 3.65, 3.88, m | 63.1 |
| 15 | β 1.50, dd (3.6, 13.8) α 1.95, dd (9.6, 13.2) |
49.7 | G'-1 | 4.70, d (7.8) | 104.7 |
| 16 | 4.32, br d (8.4) | 77.0 | 2 | 3.25, m | 76.3 |
| 17 | – | 87.2 | 3 | 3.40, m | 77.9 |
| 18 | 1.04, s | 20.9 | 4 | 3.34, m | 71.6 |
| 19 | 0.48, d (4.2) 0.54, d (4.2) | 30.7 | 5 | 3.32, m | 77.7 |
| 20 | 1.28, m | 45.9 | 6 | 3.65, 3.88, m | 62.9 |
| 21 | 1.18, d (6.6) | 15.2 |
2.3.2. 10-Methoxy pumiloside (2)
A yellow amorphous powder; (c 0.05, MeOH); IR (NaCl) νmax 3388.7, 2124.5, 1638.2, and 1077.8, and 1030.9 cm−1; UV λmax nm (log ε) (MeOH): 343.0 (3.5), 329.0 (3.56), 250.0 (4.3), 208.1 (4.4), 206.0 (4.4); For 1H and 13C NMR (DMSO-d6, 400, 100 MHz) see Table 2; HR-ESI-MS m/z 543.1980 [M+H]+ (calcd 543.1977) and m/z 565.1790 [M+Na]+ (calcd 565.1796).
Table 2.
1H and 13C NMR spectroscopic data for compounds 2 (DMSO-d6, 400, 100 MHz) and 3 in (CD3OD, 600, 150 MHz).
| Position | 2 | 3 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 2 | – | 149.6 | – | 130.8 |
| 3 | 4.70, d (11.2) | 59.3 | 4.59, d (11.4) | 53.2 |
| 5 | a. 4.48, d (14.8) b. 4.32, d (14.0) |
47.4 | 3.37, m 3.69, m | 42.7 |
| 6 | – | 111.9 | 2.98, m, 3.07, m | 19.6 |
| 7 | – | 172.1 | – | 107.0 |
| 8 | – | 126.2 | – | 127.8 |
| 9 | 7.51, d (2.8) | 104.1 | 6.91, d (2.4) | 101.2 |
| 10 | – | 155.5 | – | 155.6 |
| 10-OCH3 | 3.82, s | 55.4 | 3.748, s | 56.2 |
| 11 | 7.30, dd (2.8, 8.8) | 121.7 | 6.74, dd (2.4, 9.0) | 113.6 |
| 12 | 7.58, d (8.8) | 120.5 | 7.15, d (9.0) | 113.0 |
| 13 | – | 135.4 | – | 133.3 |
| 14 | α. 1.98, m β. 2.49, m | 28.3 | α. 2.17, m β. 2.28, m | 34.8 |
| 15 | 3.26, m | 23.7 | 3.03, m | 32.4 |
| 16 | – | 108.9 | – | 109.0 |
| 17 | 7.04, d (2.4) | 145.0 | 7.76, s | 156.8 |
| 18 |
trans. 5.47, d (16.8) cis. 5.34, d (10.8) |
120.7 |
trans. 5.31, d (17.4) cis. 5.23, d (10.2) |
119.8 |
| 19 | 5.79, m | 132.3 | 5.81, m | 135.4 |
| 20 | 2.63, m | 43.6 | 2.70, m | 45.4 |
| 21 | 5.39, s | 94.9 | 5.81, d (9.0) | 97.3 |
| 22 | – | 163.9 | – | 171.2 |
| 22-OCH3 | – | – | 3.753, s | 52.6 |
| 1' | 4.55, d (7.6) | 97.8 | 4.75, d (7.8) | 100.4 |
| 2' | 3.03, m | 73.2 | 3.18, m | 74.7 |
| 3' | 3.26, m | 77.3 | 3.40, m | 78.0 |
| 4' | 3.07, m | 70.1 | 3.26, m | 71.7 |
| 5' | 3.05, m | 76.7 | 3.35, m | 78.8 |
| 6' | 3.45, d (6.4, 12.0) 3.70, d (12.0) |
61.2 | 3.59, dd (6.6, 12.0) 3.93, d (12.0) |
63.0 |
2.3.3. 10-Methoxy strictosidine (3)
A yellow amorphous powder; (c 0.05, MeOH); IR (NaCl) νmax 3283.2, 2360.3, 1627.9, and 1076 cm−1; UV (MeOH) λmax (log ε) nm; 454.1 (2.23), 205.1 (3.6); For 1H and 13C NMR (CD3OD, 600, 150 MHz) see Table 2; HR-ESI-MS m/z 561.2447 [M+Na]+ (calcd 561.2448),m/z 583.2271 [M+Na]+ (calcd 583.2267), and m/z 595.2045 [M+Cl]− (calcd 595.2058).
2.4. Antiprotozoal assay
Compounds 1–8 were tested for their antiprotozoal activities against Leishmania donovani Promastigote, L. donovani Amastigote, L. donovani Amastigote/THP1 cells and Trypanosoma brucei brucei employing the methods described previously [11]. The in vitro antileishmanial and antitrypanosomal assays were done on cell cultures of L. donovani promastigotes, axenic amastigotes, THP1-amastigotes, and Trypanosoma brucei trypomastigotes by Alamar Blue assay as described earlier [11]. The assays have been adapted to 384 well microplate format. In a 384 well micro-plate, the samples with appropriate dilution were added to the L. donovani promastigotes or L. donovani axenic amastigotes or T. brucei trypomastigotes cultures (2 × 106 cell/mL). The compounds were tested at three concentrations ranging from 40 to 1.6 µg/mL or 10–0.25 µg/mL. The plates were incubated at 26 °C for 72 h (37 °C for axenic amastigotes and T. brucei trypomastigotes) and growth of the parasites in cultures were determined by Alamar Blue assay [11]. The compounds were also tested against L. donovani intracellular amastigotes in THP1 cells employing a parasite-rescue and transformation assay [12]. The compounds were simultaneously tested for cytotoxicity against THP1 cell cultures. The conditions for seeding the THP1 cells, exposure to the test compounds and evaluation of cytotoxicity were the same as described in parasite-rescue and transformation assay [12]. IC50 and IC90 values were computed from the dose response curves using XLfit software. DFMO was used as a positive control.
3. Results and discussion
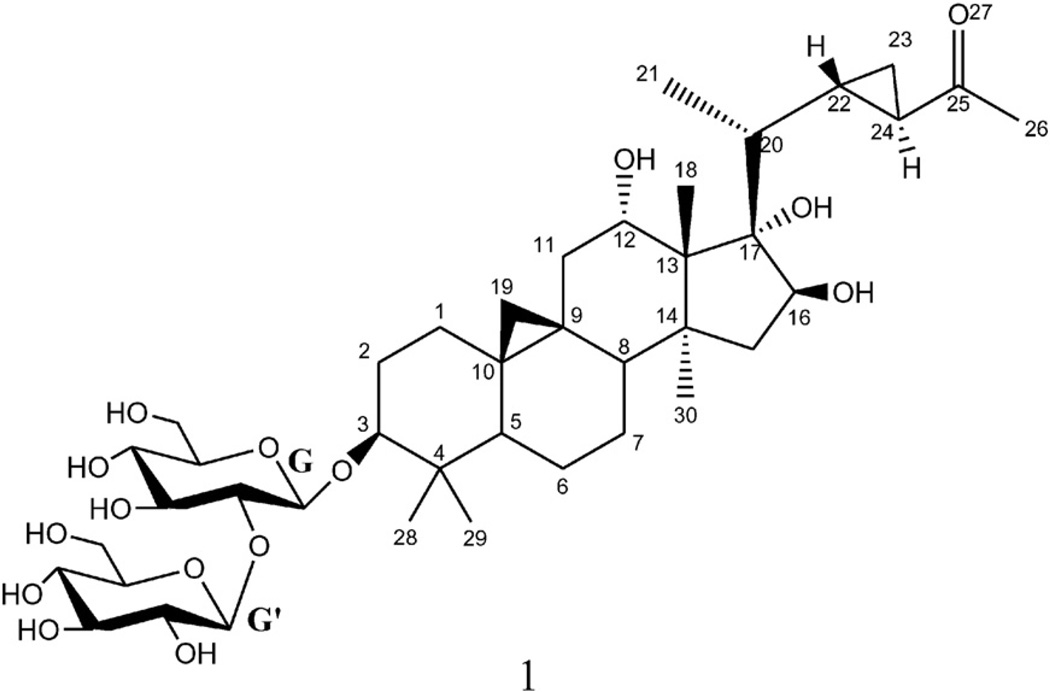
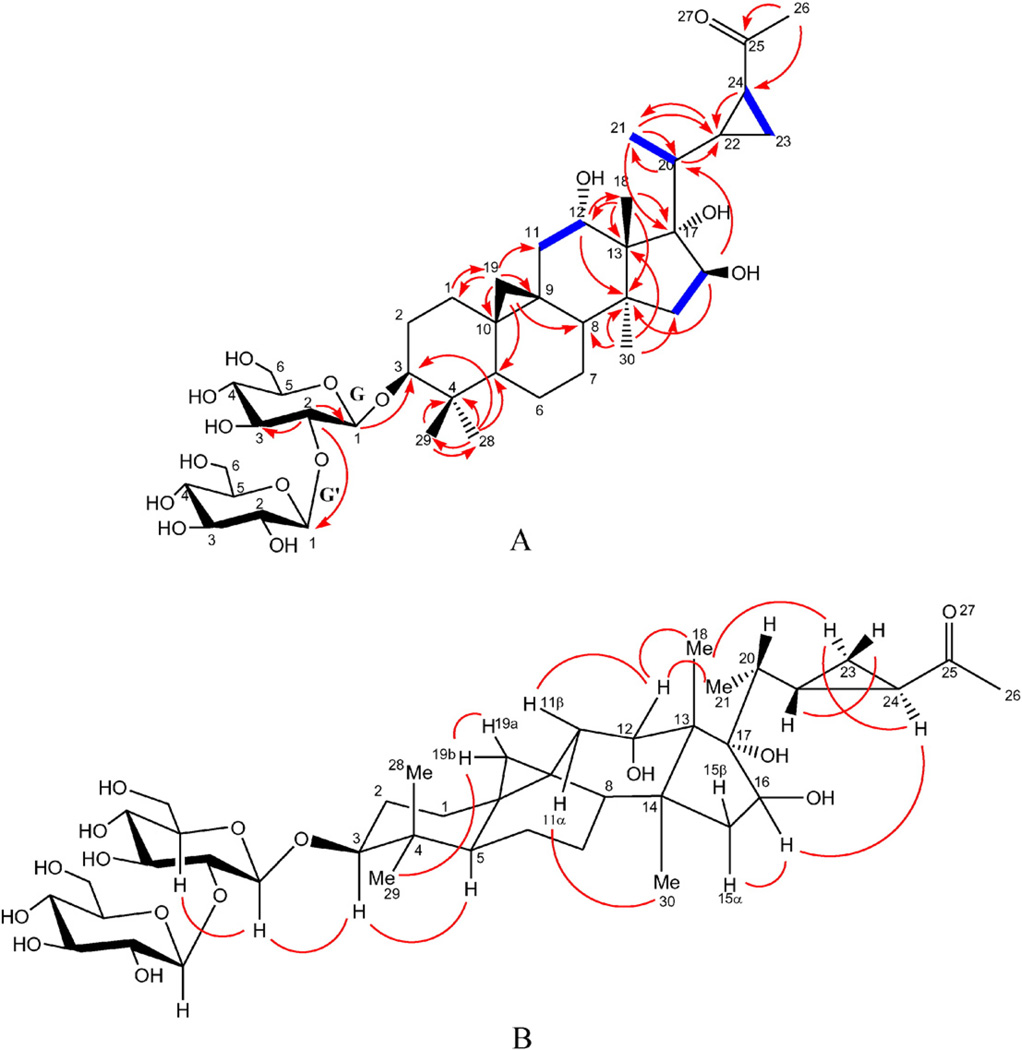
Compound 1 (Fig. 1) was obtained as a faint yellow amorphous powder with a molecular formula C41H66O15 as established from the positive HR-ESI-MS by a molecular ion peak at m/z 821.4292 [M+Na]+ (calcd 821.4299) and confirmed by the negative HR-ESI-MS by a molecular ion peak at m/z 833.4093 [M+Cl]− (calcd 833.4090). The 1H NMR spectrum showed the presence of two cyclopropane methylene proton signals. One of them appeared as a pair of upfield doublets at δH 0.48 and 0.54 (J = 4.2 Hz) indicating the presence of the usual 9,19-cycloartane ring junction, which is an important triterpenoid class in Mussaenda. The other appeared as two multiplets at δH 0.75 and 0.93 which correlated in the 1H–1H COSY with a deshielded methine at δH 2.13 (δC 32.4) which in turn showed an HMBC correlation to another methine at δC 30.0 indicating the presence of a disubstituted cyclopropane fragment in the side chain of the cycloartane. Five singlets for tertiary methyl groups were also observed at δH 1.04 (Me-18), 1.11 (Me-28), 0.91 (Me-29), 1.46 (Me-30), and a highly deshielded one at δH 2.24 (Me-26). The later methyl singlet showed an HMBC correlation with a methine resonating at δC 32.4 and a carbonyl carbon at δC 212.2 which clearly indicated the presence of an acetyl moiety attached to the cyclopropane fragment in the side chain terminal. A doublet for secondary methyl group at δH 1.18 (J = 6.6 Hz, Me-21) was also observed in 1H NMR spectrum. Three oxygenated methines at δH 3.31 (dd, J = 9.0, 4.2Hz), 4.20 (t, J = 7.8 Hz), and 4.32 (br d, J = 8.4 Hz), indicative for secondary alcoholic functions, which were assigned to H-3, H-12 and H-16, respectively, two anomeric proton signals at δH 4.48 (d, J = 6.6 Hz) and 4.70 (d, J = 7.8 Hz) of two β-d-glucopyranosyl units were also detected. The assignments of all the 1H and 13C NMR signals of compound 1 were successfully carried out with the analysis of 1H–1H COSY, HMQC, and HMBC experiments. The 13C NMR spectrum of compound 1 confirmed the presence of these functionalities and showed in addition, a quaternary carbon, which was oxygen-bearing at δC 87.2, was readily assigned for C-17 based on the analysis of the HMBC correlations which showed cross peaks from both of Me-18 and Me-21 to this interesting quaternary carbon with unusual hydroxylation (Fig. 2A). The HMBC analysis showed the presence of long-range correlations between H-12/C-18, and H-12/C-14, which confirmed the presence of a hydroxy group at C-12 (Fig. 2A). Also, long range correlations observed between H-16/C-14, and H-16/C-20 confirmed the presence of a hydroxy group at C-16. It has been reported that H-3, Me-30 and Me-21 protons have invariably α orientations while H-8, Me-18 have β orientation in this class of natural products [13]. The cross-peak observed between H-5 and H-3 in the ROESY spectrum confirmed the β-hydroxylation at C-3. The α configuration of the C-12 hydroxy group was established based on the analysis of the coupling constant between H-12 and H2-11. In the 1H NMR spectrum, the H-12 proton appeared as a t-like (J = 7.8 Hz) [14]. Moreover, in the ROESY spectrum, no cross peak was observed between H-12 and Me-30 while cross peaks were observed between H-12/Me-18, H-12/Me-21, and H-12/H-11β which further confirmed the α orientation of C-12 hydroxy group. Comparison with previously reported data H-15α and H-15β were differentiated from each other [14]. Analysis of coupling constants between H-15β at δH 1.50 (dd, 2J15β,15α = 13.8 Hz, 3J15β,16 = 3.6 Hz), H-15α at δH 1.95 (dd, 2J15α,15β = 13.2 Hz, 3J15α,16 = 9.6 Hz), and H-16 (br d, 3J16,15α = 8.4 Hz), this large coupling constant between H-16 and H-15α suggested their cis-relationship and confirmed by the observed cross peak in the ROESY and 1H–1H-COSYspectra between H-16 and H-15α and absence of any correlations between H-16 and H-15β in both spectra. The α configuration of C-17 hydroxy group was deduced from the biogenetic pathway and comparison of its 13C NMR value with previously reported compounds having similar ring D [15]. The relative configurations of C-22 and C-24 were established as S and R, respectively based on the ROESY correlations between H-22/H-23β, H-24/H-23α, and H-24/Me-21 (Fig. 2B). The structure of the sugar moiety was established on the basis of HMBC correlations between HG-1/C-3, and HG'-1/CG-2. It should be noted that Δ22 unsaturated triterpenes are very common in Mussaenda saponins [10]. Therefore, the cyclopropane function in the side chain through bioalkylation of this nucleophilic site (Δ22 double bond precursor) is possible [16]. Previous studies dealing with triterpenoid biosynthesis in plants suggested the requirement of different enzymes (lanostane synthase and cycloartane synthase) to catalyze its synthesis. Plants can polyhydroxylate and oxidize terpenoid cores with hydroxylase and oxidase, respectively [17]. Therefore, compound 1 was identified as 9 (R), 19, 22 (S), 24 (R) bicyclolanost-3β, 12α, 16β, 17α tetraol-25-one 3-O-β-d-glucopyranosyl-(1→2)-β-d-glucopyranoside. To the best of our knowledge, compound 1 is a newly reported natural cycloartane saponin with unusual hydroxylation at C-17 and a unique side chain.
Fig. 1.
Chemical structure of compound 1.
Fig. 2.
A. Important HMBC (H C) and 1H–1H COSY (
C) and 1H–1H COSY ( ) correlations of compound 1. B. Important ROESY correlations of compound 1.
) correlations of compound 1. B. Important ROESY correlations of compound 1.
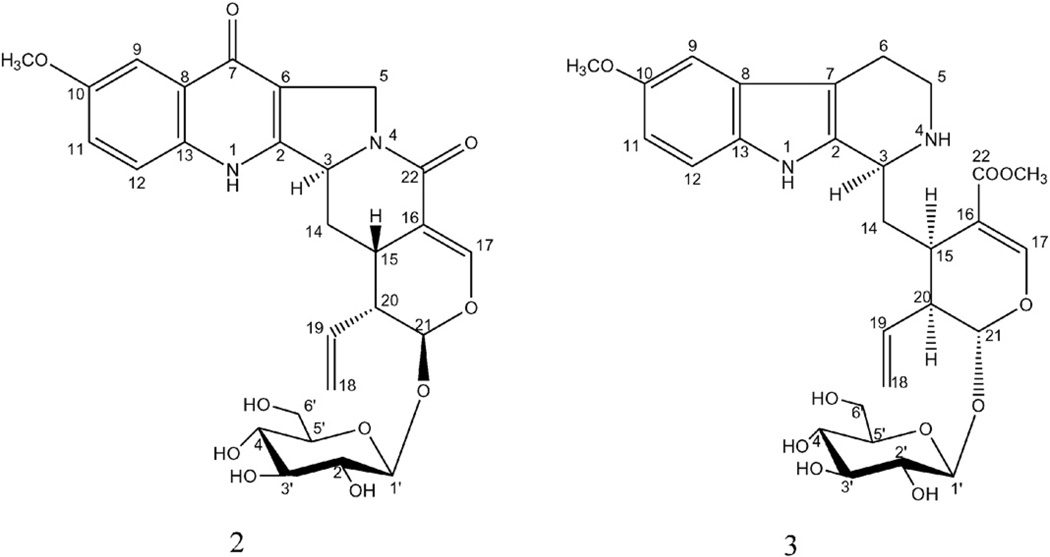
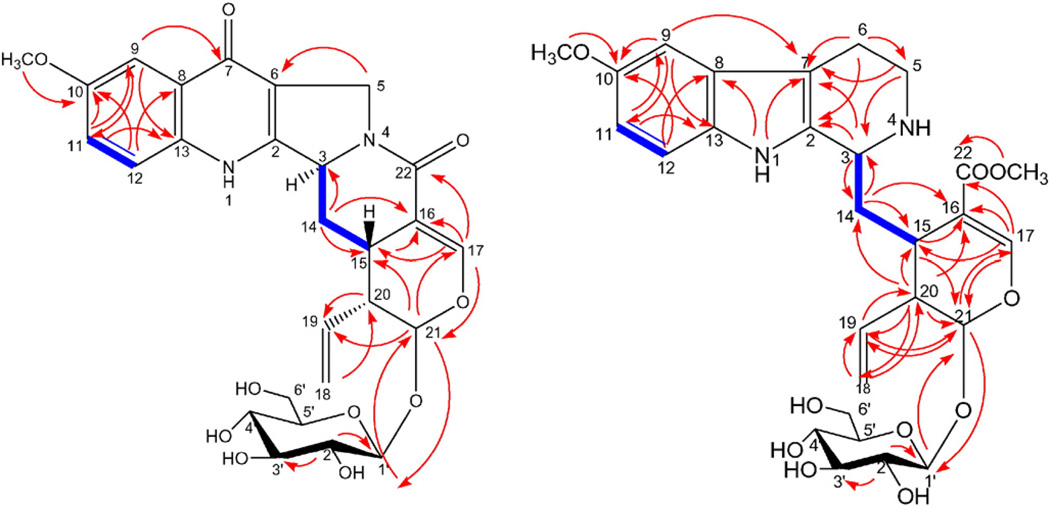
Compound 2 (Fig. 3) was isolated as a yellow amorphous powder. Its positive HR-ESI-MS data suggested a molecular formula of C27H30N2O10 by a molecular ion peak at m/z 543.1980 [M+H]+ (calcd 543.1977) and m/z 565.1790 [M+Na]+ (calcd 565.1796). The 1H NMR spectrum showed three aromatic proton signals that appeared as an ABX system at δH 7.51 (d, J = 2.8 Hz, H-9), 7.30 (dd, J = 2.8, 8.8 Hz, H-11) and 7.58 (d, J = 8.8 Hz, H-12). A characteristic highly deshielded olefinic proton appeared at δH 7.04 (d, J = 2.4 Hz) and by HMQC corresponding to δC 145.0 which indicated the presence of a secoiridoid moiety in this compound; it is assigned to H-17 and confirmed by HMBC correlations with other surrounding carbons in secoiridoid skeleton (Fig. 4). A singlet at δH 3.82 (3H) was observed which showed an HMBC correlation with δC 155.5 indicating methoxy substitution of the aromatic ring. Also, an anomeric proton at δH 4.55 (1H, d, J = 7.6 Hz) suggested the presence of β-d-glucosyl moiety from the large coupling constant of the anomeric proton. Three methylenes were observed in the spectrum; one appeared as two doublet proton signals at δH 5.34 (J = 10.8 Hz) and δH 5.47 (J = 16.8 Hz) which together with a characteristic multiplet at δH 5.79 indicating the existence of terminal vinyl group. Another methylene appeared as two doublets at δH 4.48 (J = 14.8 Hz) and δH 4.32 (J = 14.0 Hz) which in turn coupled only with each other in 1H–1H COSY spectrum as deduced from the coupling constant of them. These later protons were assigned to H-5 protons and their assignment was confirmed through HMBC correlation between them and C-6 (Fig. 4). The third methylene appeared as two multiplets at δH 2.49 and 1.98 which were assigned to H-14 protons. Its assignment is confirmed through HMBC cross peaks between it and C-15, C-16 and C-3. The analysis of 1H–1H COSY spectrum indicated the presence of a cross peak between H-14 and H-3 which is important for the confirmation of the connection between the secoiridoid and alkaloidal parts. The 13C NMR shift values for C-3, C-14 and C-15 matched those reported for pumiloside, previously isolated from the Rubiaceous plants Nauclea officinalis [18,19] and Ophiorrhiza pumila [20], and so, compound 2 has the same relative configuration as that of pumiloside. Therefore, 2 was identified as 10-methoxy pumiloside which is a new natural compound.
Fig. 3.
Chemical structures of compounds 2 and 3.
Fig. 4.
Important HMBC (H C) and 1H–1H COSY (
C) and 1H–1H COSY ( ) correlations of compounds 2 and 3.
) correlations of compounds 2 and 3.
Compound 3 (Fig. 3) was isolated as a yellow amorphous powder. Its positive HR-ESI-MS data suggested a molecular formula of C28H36N2O10 by a molecular ion peak at m/z 561.2447 [M+H]+ (calcd 561.2448) and m/z 583.2271 [M+Na]+ (calcd 583.2267) and confirmed by the negative HR-ESI-MS by the presence of molecular ion peak at m/z 595.2045 [M+Cl]− (calcd 595.2058). The 1H NMR spectrum of the compound showed three aromatic proton signals that appeared as an ABX system at δH 6.91 (d, J = 2.4 Hz), 6.74 (dd, J = 2.4, 9.0 Hz) and 7.15 (d, J = 9.0 Hz) and one highly shielded olefinic proton signal at δH (7.76, s) which is a characteristic feature of secologanin structure. Also, an anomeric proton at δH 4.75 (d, J = 7.8 Hz) suggested the presence of β-d-glucosyl unit in the compound from the large coupling constant of the anomeric proton. Four methylenes were observed in the spectrum; one appeared as two doublet proton signals at δH 5.23 (d, J = 10.2) and δH 5.31 (d, J = 17.4 Hz) which together with characteristic multiplet at δH 5.81 indicating the existence of terminal vinyl group which confirm the presence of a secologanin unit. Another methylene appeared at δH 2.17 and 2.28 assigned to H-14 protons and its position confirmed by HMBC correlation with C-15, C-3 and C-16. The third one appeared also as two multiplets at δH 2.98 and 3.07 which was assigned to H-6. The fourth methylene appeared at δH 3.37 and 3.69 which was assigned to H-5. In addition to two methoxyl signals at δH 3.748 and 3.753 which showed HMBC correlations with C-10 and C-22, respectively. The 13C NMR chemical shifts together with the 2D experiments suggested that the compound is a conjugate of a 10-methoxy tetrahydro-β-carboline and a secologanin unit. 1H–1H COSY analysis indicated the presence of a cross peak between H-14 and H-3 which confirm the connection between the units. The stereochemistry of the compound was suggested to be as that of strictosidine with the C-3 chiral center in the S configuration [21]. Moreover, the 13C NMR shift values for C-3, secologanin unit's stereocenters and neighboring carbon resonances were close to that of strictosidine derivatives which confirmed that compound 3 is a methoxy derivative of strictosidine [22]. Therefore, compound 3 was identified as 10-methoxystrictosidine. This compound had not been previously reported from a natural source. However, its enzyme catalyzed synthesis was previously reported [23].
Other isolated compounds were identified as 7α-morroniside (4) [24], 7-epi-loganin (5) [25], (7β)-7-O-methylmorroniside (6) [26], 5(S)-5-carboxystrictisidine (7) [27] and apigenin-7-O-neohesperidoside (8) [28].
All isolates were evaluated for their antiprotozoal activities. Compound 7 showed good antitrypanosomal activity with IC50 and IC90 values of 13.7 and 16.6 µM compared to IC50 and IC90 values of 13.06 and 28.99 µM for the positive control DFMO.
To the best of our knowledge, this is the first report for the occurrence of the monoterpenoid glucoindole-type alkaloids in the genus which might be useful for the chemotaxonomic evaluation of the genus Mussaenda. These alkaloids are mostly limited to few families including Rubiaceae, Apocynaceae and Loganiaceae [29].
4. Conclusion
A new cycloartane-type saponin 1, two new monoterpenoid glucoindole alkaloids 2 and 3 along with other 5 known secondary metabolites were isolated from M. luteola. Compound 7 showed a good trypanocidal activity.
Supplementary Material
Acknowledgments
We are grateful to the Egyptian Government, the National Center for Natural Products Research, The University of Mississippi, United States and NIH COBRE grant P20GM104932 for financial support. We are also thankful to Dr. Baharthi Avula for HR-ESI-MS and Dr B. Tekwani for antiprotozoal assay.
Footnotes
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.fitote.2016.03.009.
References
- 1.Vidyalakshmi KS, Nagarajan S, Vasanthi HR, Rajamanickam V. Hepatoprotective and antioxidant activity of two iridoids from Mussaenda ‘dona aurora’. Z. Naturforsch., C: Biosci. 2009;64:329–334. doi: 10.1515/znc-2009-5-604. [DOI] [PubMed] [Google Scholar]
- 2.Ramakrishnan S, Mohammed S. Evaluation of diuretic activity of Mussaenda frondosa. Asian. J. Pharm. Clin. Res. 2015;8:117–118. [Google Scholar]
- 3.Zhao W, Xu R, Qin G, Tang X, Li X. Chemical constituents from Mussaenda pubescens. Nat. Prod. Sci. 1995;1:61–65. [Google Scholar]
- 4.Xu R, Zhao W, Xu J, Shao B, Qin G. Studies on bioactive saponins from Chinese medicinal plants. Adv. Exp. Med. Biol. 1996;404:371–382. doi: 10.1007/978-1-4899-1367-8_30. [DOI] [PubMed] [Google Scholar]
- 5.Harborne JB, Girija AR, Devi HM, Lakshmi NKM. Anthochlor pigments from the petals of Mussaenda hirsutissima and Zinnia linearis. Phytochemistry. 1983;22:2741–2742. [Google Scholar]
- 6.Dinda B, Debnath S, Majumder S, Arima S, Sato N, Harigaya Y. Chemical constituents of Mussaenda incana. Indian J. Chem., Sect B. 2005;44:2362. [Google Scholar]
- 7.Vidyalakshmi KS, Rajamanickam GV. An iridoid with anticancer activity from the sepals of Mussaenda dona aurora. Indian J. Chem., Sect B. 2009;48:1019–1022. [Google Scholar]
- 8.Li YX, Qiao WT, Yuan K. Isolation and structural elucidation of chemical constituents of Mussaenda hainanensis Merr. J. Med. Plant Res. 2011;5:1459–1465. [Google Scholar]
- 9.De Utpal C, Ghosh R, Chowdhury S, Dinda B. New iridoid from aerial parts of Mussaenda roxburghii. Nat. Prod. Commun. 2012;7:1–2. [PubMed] [Google Scholar]
- 10.Mohamed SM, Bachkeet EY, Bayoumi SA, Jain S, Cutler SJ, Tekwani BL, Ross SA. Potent antitrypanosomal triterpenoid saponins from Mussaenda luteola. Fitoterapia. 2015;107:114–121. doi: 10.1016/j.fitote.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manda S, Khan SI, Jain SK, Mohammed S, Tekwani BL, Khan IA, et al. Synthesis, antileishmanial and antitrypanosomal activities of N-substituted tetrahydro-β-carbolines. Bioorg. Med. Chem. Lett. 2014;24:3247–3250. doi: 10.1016/j.bmcl.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Jain SK, Sahu R, Walker LA, Tekwani BL. A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J. Vis. Exp. 2012;70:4054. doi: 10.3791/4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corey EJ, Matsuda SP, Bartel B. Molecular cloning, characterization, and overexpression of ERG7, the Saccharomyces cerevisiae gene encoding lanosterol synthase. PNAS. 1994;91:2211–2215. doi: 10.1073/pnas.91.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ju JH, Liu D, Lin G, Xu XD, Han B, Yang JS, Ma LB, Beesiosides AF. six new cycloartane triterpene glycosides from Beesia calthaefolia. J. Nat. Prod. 2002;65:42–47. doi: 10.1021/np010293p. [DOI] [PubMed] [Google Scholar]
- 15.Akbay P, Çalis I, Heilmann J, Sticher O. New stigmastane sterols from Ajuga salicifolia. J. Nat. Prod. 2003;66:461–465. doi: 10.1021/np020427e. [DOI] [PubMed] [Google Scholar]
- 16.Goad J, Akihisa T. Analysis of sterols. Springer Science and Business Media. 2012 [Google Scholar]
- 17.Kuete V, editor. Medicinal Plant Research in Africa: Pharmacology and chemistry. Newnes; 2013. [Google Scholar]
- 18.Fan L, Fan CL, Wang Y, Zhang XQ, Zhang QW, Zhang JQ, Ye WC. Alkaloids from the leaves of Nauclea officinalis. Acta Pharm. Sin. 2010;45:747–751. [PubMed] [Google Scholar]
- 19.Zhu FX, Wang JJ, Song J, Ding SM, Jia XB. Chemical constituents of Nauclea officinalis. Acta Pharm. Sin. 2013;48:276–280. [PubMed] [Google Scholar]
- 20.Aimi N, Nishimura M, Miwa A, Hoshino H, Sakai SI, Haginiwa J. Pumiloside and deoxypumiloside; plausible intermediates of camptothecin biosynthesis. Tetrahedron Lett. 1989;30:4991–4994. [Google Scholar]
- 21.Patthy-Lukáts Á, Károlyházy L, Szabó LF, Podányi B. First direct and detailed stereochemical analysis of strictosidine. J. Nat. Prod. 1997;60:69–75. [Google Scholar]
- 22.Arbain D, Putra DP, Sargent MV. The alkaloids of Ophiorrhiza filistipula. Aust. J. Chem. 1993;46:977–985. [Google Scholar]
- 23.Loris EA, Panjikar S, Ruppert M, Barleben L, Unger M, Schübel H, Stöckigt J. Structure-based engineering of strictosidine synthase: auxiliary for alkaloid libraries. Chem. Biol. 2007;14:979–985. doi: 10.1016/j.chembiol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Dinda B, Debnath S, Banik R. Naturally occurring iridoids and secoiridoids. An updated review, part 4. Chem. Pharm. Bull. 2011;59:803–833. doi: 10.1248/cpb.59.803. [DOI] [PubMed] [Google Scholar]
- 25.Dinda B, Debnath S, Harigaya Y. Naturally occurring iridoids. A review, part 1. Chem. Pharm. Bull. 2007;55:159–222. doi: 10.1248/cpb.55.159. [DOI] [PubMed] [Google Scholar]
- 26.Hu JF, Starks CM, Williams RB, Rice SM, Norman VL, Olson KM, Eldridge GR. Secoiridoid glycosides from the pitcher plant Sarracenia alata. Helv. Chim. Acta. 2009;92:273–280. [Google Scholar]
- 27.Aimi N, Seki H, Sakai S. Synthesis of lyaloside, a prototypal β carboline gluco indole alkaloid in rubiaceous plants. Chem. Pharm. Bull. 1992;40(9):2588–2590. [Google Scholar]
- 28.Stein W, Zinsmister HD. New flavonoids fromthemoss Bryum pseudotriquetrum. Z. Naturforsch C. 1990;45:25–31. [Google Scholar]
- 29.Şöhretoğlu D, Masullo M, Piacente S, Kirmizibekmez H. Iridoids, monoterpenoid glucoindole alkaloids and flavonoids from Vinca major. Biochem. Syst. Ecol. 2013;49:69–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.