Abstract
Objectives: The indications for video-assisted thoracoscopic surgery (VATS) for advanced-stage lung cancer are expanding, but the criteria vary among institutions. This study compared the minimal invasiveness and oncologic validity of VATS lobectomy and thoracotomy lobectomy for the treatment of large-diameter primary lung cancer.
Methods: We retrospectively reviewed clinical features and surgical outcomes of 68 patients who underwent anatomical pulmonary resection for primary lung cancer of >5-cm diameter from July 2006 to March 2013. The patients were divided into a VATS group (Group V, n = 35) and a thoracotomy group (Group T, n = 33).
Results: Group V exhibited less intraoperative bleeding (p = 0.012) and had a shorter length of postoperative hospital stay (p = 0.024). The 1- and 5-year overall survival rates were 91.3% and 39.3% in Group V and 84.8% and 56.9% in Group T, respectively (p = 0.48). Multivariate analysis showed that limited lymph node dissection contributed to local recurrence. The extraction bag lavage cytology in Group V revealed that the positivity rate was 35.7%.
Conclusions: VATS for primary lung cancer of >5-cm diameter is similar to thoracotomy in terms of surgical outcomes. Large tumors must be carefully maneuvered during VATS to prevent cancer cell spillage.
Keywords: primary lung cancer, thoracotomy, video-assisted thoracoscopic surgery, tumor size
Introduction
During the past decade, video-assisted thoracoscopic surgery (VATS) has evolved to become the standard approach in thoracic surgery. VATS lobectomy is an acceptable treatment for early-stage non-small cell lung cancer.1,2) However, the oncological outcome of VATS lobectomy for patients with advanced lung cancer remains controversial3) because VATS has some disadvantages associated with en bloc resection and lymph node dissection.4,5) The indications for VATS for advanced-stage lung cancer are expanding, but the criteria vary among institutions. In particular, large lung tumors make it difficult to establish an operative field via thoracoscopy, and the risk of cancer cell spillage may be higher than that associated with thoracotomy. VATS has the potential to increase the risk of local recurrence and thus negatively affect the long-term prognosis. The present study compared the minimal invasiveness and oncologic validity of VATS lobectomy versus thoracotomy lobectomy for primary lung cancer with a diameter of >5 cm (more than T2b according to the TNM classification).6)
Materials and Methods
In total, 68 patients underwent anatomical pulmonary resection (segmentectomy or lobectomy) without incomplete resection for primary lung cancer of >5-cm diameter (more than T2b) at Jichi Medical University and Jichi Medical University Saitama Medical Center from July 2006 to March 2013. The patients were divided into two groups: those who underwent video-assisted thoracoscopic surgery (Group V, n = 35) and those who underwent thoracotomy (Group T, n = 33). The hospital records of all patients were reviewed. Patients who underwent a median sternotomy approach, pneumonectomy, chest wall resection, pericardiectomy, tracheobronchoplasty, and angioplasty were excluded to match the conditions of the two groups. Four patients were also excluded when the procedure was converted from VATS to thoracotomy. The reasons for conversion were vascular injury (n = 2), bronchial injury and repair (n = 1), and adhesion and calcification of lymph nodes (n = 1). Patient characteristics, preoperative status, surgical procedures, perioperative course, pathological findings, and long-term prognoses were evaluated by review of the hospital records.
Surgical Technique
All patients were placed in the lateral decubitus position under general anesthesia with selective one-lung ventilation. We performed VATS pulmonary resection using a five-port non-rib-spreading technique. A thoracostomy of 1.5 to 3.0 cm at the fifth or sixth intercostal space (ICS) was created along the midaxillary line to allow for the advancement of a 45° thoracoscope through a 10.5-mm trocar or wound retractor (Alexis; Applied Medical, Rancho Santa Margarita, CA). Three or four ports of 1 to 2 cm were placed in the anterior axillary line (at the third or fourth ICS and fifth or sixth ICS) and in the posterior axillary line (at the fifth or sixth ICS and seventh or eighth ICS) and protected with a 5.0- or 10.5-mm trocar. The tumor specimen was extracted in a plastic bag (LiNA Bag; Proseed Co., Ltd., Tokyo, Japan or MemoBag; Teleflex, Athlone, Ireland). The skin incision of one port was extended or the incision of two ports was connected only as long as necessary according to the size of the specimen. Pulmonary resection was performed through the thoracotomy using a 25- to 30-cm posterolateral skin incision with splitting of the anterior serratus muscle, dorsal latissimus muscle, and rib. The fourth, fifth, or sixth ICS was used. The major vascular branches and pulmonary parenchyma were transected with a stapler. The lobar and segmental bronchi were closed with a stapler. The minor vascular branches and small bronchi were ligated with sutures.
Data Collection
Data collected from the medical records included age, sex, lesion location, clinical N (cN) status, operative procedure, pathologic tumor size and findings, pathologic stage, operation findings, perioperative course, survival time, and death or survival (all death or censored). We classified the cN status as positive when the shortest diameter was >10 mm on a computed tomography scan. Each institute’s pathologist performed the postoperative pathologic evaluation and staging by the TNM classification according to General Rule for Clinical and Pathological Record of Lung Cancer (7th edition) by the Japanese Lung Cancer Society.6) The pathologist also evaluated pleural invasion. Visceral pleural invasion was classified into positive and negative groups: positivity was diagnosed when the tumor invaded beyond the external elastic membrane of the lung parenchyma. Local recurrence was defined as recurrence in the ipsilateral lung including the presence of a resection stump, ipsilateral mediastinal lymph node involvement, ipsilateral malignant pleural effusion, and ipsilateral pleural dissemination.
Statistical Analysis
The following variables were compared between Groups V and T in the background analysis: age, sex, smoking history, respiratory function test results, comorbidity, lesion location, cN status, operative procedure, nodal dissection, pathologic tumor size, histological findings, pathologic pleural invasion, pathologic N (pN) status, and pathologic stage (p-Stage). The following variables were compared between the two groups in the outcome analysis: operative duration, intraoperative blood loss, complications, mortality, duration of drainage, length of postoperative stay, recurrence and metastasis, overall survival (OS), and recurrence-free survival (RFS).
Differences were statistically evaluated using a t-test for numerical variables and χ2 test for categorical variables. A p-value of <0.05 was considered statistically significant. OS and RFS curves were generated via the Kaplan–Meier method, and statistical differences between Groups V and T were evaluated by the log-rank test. Univariate and multivariate analyses using a logistic regression model were also performed to evaluate the significance of factors related to local recurrence in both groups of patients. Statistical analyses were performed using the StatMate IV software package (ATMS Co., Ltd., Tokyo, Japan).
Results
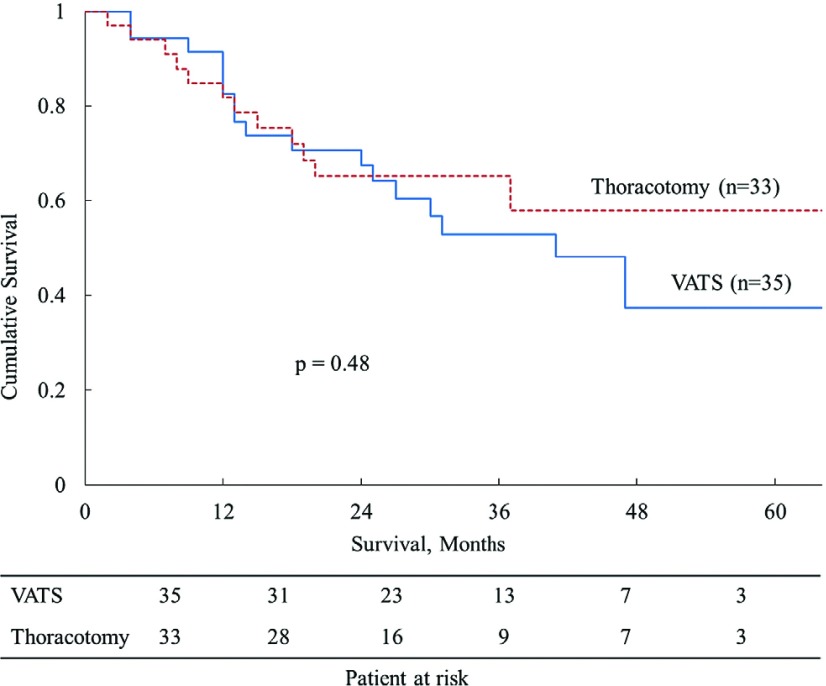
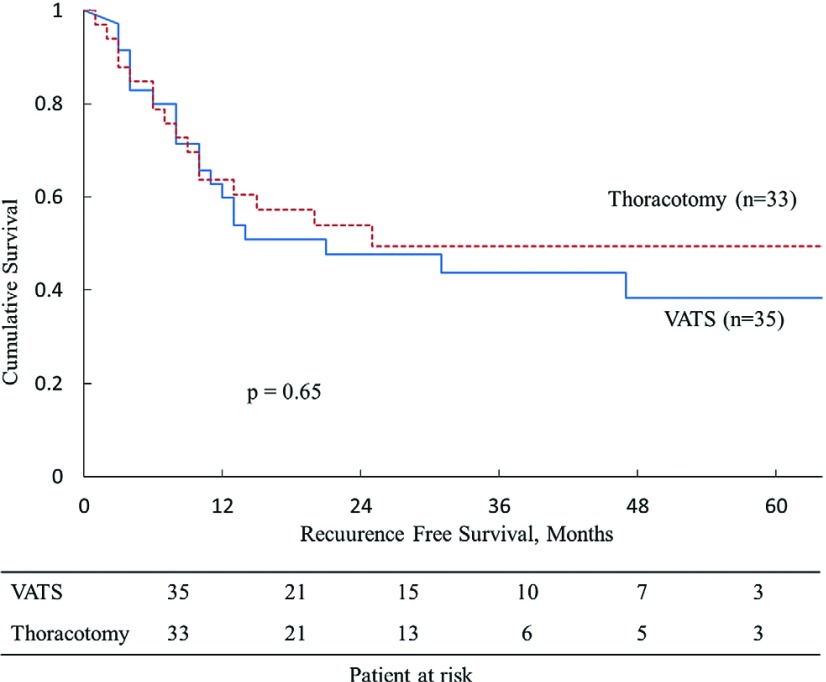
In the background analysis, Groups V and T exhibited statistically significant differences in age (p = 0.0021) pathologic tumor size (p = 0.0050), and histological findings (p = 0.0058) (Table 1). With respect to the histological findings, Group T had a significantly larger number of patients with poorly differentiated carcinoma. Other patient characteristics, preoperative status, surgical procedures, pathological findings, and pathologic stage were similar between the two groups. In the outcome analysis, Group V showed less intraoperative bleeding (p = 0.012), a shorter duration of drainage (p = 0.0039), and a shorter postoperative hospital stay (p = 0.024) (Table 2). The operation duration, complications, and mortality were similar between the two groups. The extraction bag lavage cytology (BLC) for 14 patients in Group V were performed to evaluate cancer cell spillage, the BLC positivity was found in five patients (35.7%). No significant differences were observed in the recurrence and/or metastasis rate (p = 0.62) or local recurrence rate (p = 0.19), but local recurrence showed a slight tendency to develop in Group V. The 1- and 5-year OS rates were 91.3% and 39.3% in Group V and 84.8% and 56.9% in Group T (p = 0.48). The 1- and 5-year RFS rates were 62.3% and 38.7% in Group V and 63.6% and 48.0% in Group T (p = 0.65). No significant differences were seen in the OS or RFS curves between the two groups (Figs. 1 and 2).
Table 1.
Clinicopathologic profiles of all patients
| Variable | Group V (n = 35) | Group T (n = 33) | p-value |
|---|---|---|---|
| Age, years (mean) | 75.0 ± 6.2 | 69.2 ± 8.5 | 0.0021 |
| Sex (%) | 0.93 | ||
| Male | 31 (88.6) | 30 (90.9) | |
| Female | 4 (11.4) | 3 (9.1) | |
| Never-smokers (%) | 4 (11.4) | 5 (15.2) | 0.92 |
| %VC, % (mean) | 101.4 ± 21.5 | 106.4 ± 20.1a | 0.33 |
| %FEV1, % (mean) | 102.9 ± 21.8 | 96.9 ± 22.4a | 0.27 |
| FEV1%, % (mean) | 72.4 ± 9.2 | 68.8 ± 11.7a | 0.18 |
| Comorbidity (%) | |||
| Diabetes mellitus | 8 (22.9) | 7 (21.2) | 0.90 |
| Heart disease | 7 (20.0) | 5 (15.2) | 0.84 |
| Location of lesions (%) | 0.33 | ||
| Right upper lobe | 4 (11.4) | 9 (27.3) | |
| Right middle lobe | 1 (2.9) | 2 (6.1) | |
| Right lower lobe | 12 (34.3) | 6 (18.2) | |
| Left upper lobe | 9 (25.7) | 6 (18.2) | |
| Left lower lobe | 9 (25.7) | 10 (30.3) | |
| Clinical N status (%) | 0.053 | ||
| N0 | 25 (71.4) | 16 (48.5) | |
| N1-2 | 10 (28.6) | 17 (51.5) | |
| Procedure type (%) | 0.73 | ||
| Lobectomy | 31 (88.6) | 27 (81.8) | |
| Segmentectomy | 1 (2.9) | 2 (6.1) | |
| Complex lobectomy | 3 (8.6) | 4 (12.1) | |
| Nodal dissection (%) | 0.65 | ||
| ND0/1 | 9 (25.7) | 6 (18.2) | |
| ND2 | 26 (74.3) | 27 (81.8) | |
| Pathologic tumor size, mm (mean) | 64.7 ± 11.0 | 73.9 ± 14.8 | 0.0050 |
| Histology (%) | 0.0058 | ||
| Adenocarcinoma | 20 (57.1) | 10 (30.3) | |
| Squamous cell carcinoma | 14 (40.0) | 14 (42.4) | |
| Others | 1 (2.9) | 9 (27.2) | |
| Pathologic pleural invasion (%) | 0.089 | ||
| Negative | 21 (60.0) | 13 (39.4) | |
| Positive | 14 (40.0) | 20 (60.6) | |
| Pathologic N status (%) | 0.73 | ||
| N0 | 23 (65.7) | 23 (69.7) | |
| N1/2 | 12 (34.3) | 10 (30.3) | |
| Pathologic stage (%) | 0.34 | ||
| II | 24 (68.6) | 26 (78.8) | |
| III/IV | 11 (31.4) | 7 (21.2) | |
a The subjects were 32 patients who underwent respiratory function testing. VC: vital capacity; FEV1: forced expiratory volume in 1 second; Complex lobectomy: lobectomy exceeding one lobe; ND: nodal dissection
Table 2.
Perioperative data, complications, death, survival, and recurrence
| Variable | Group V (n = 35) | Group T (n = 33) | p-value |
|---|---|---|---|
| Operation duration, minutes (mean) | 169.4 ± 48.6 | 186.0 ± 58.9 | 0.21 |
| Intraoperative blood loss, ml (mean) | 167.1 ± 163.6 | 335.8 ± 333.3 | 0.012 |
| Complications (%) | 11 (31.4) | 9 (27.3) | 0.71 |
| Mortality (%) | 0 (0.0) | 1 (3.0) | 0.30 |
| Duration of drainage, days (mean) | 4.0 ± 2.4 | 6.2 ± 3.6 | 0.0039 |
| Length of postoperative stay, days (mean) | 11.4 ± 4.2 | 15.9 ± 10.3 | 0.024 |
| Extraction bag lavage cytology (%) | 5 (35.7)a | ||
| Recurrence and/or metastasis (%) | 15 (45.5)b | 13 (39.4) | 0.62 |
| Local recurrence (%) | 0.19 | ||
| Yes | 8 (24.2) | 3 (9.1) | |
| No | 25 (75.7) | 30 (90.9) | |
| Overall survival, % | 0.48 | ||
| 1-year | 91.3 | 84.8 | |
| 5-year | 39.3 | 56.9 | |
| Recurrence-free survival, % | 0.65 | ||
| 1-year | 62.3 | 63.6 | |
| 5-year | 38.7 | 48.0 | |
a The subjects were 14 patients who underwent cytology testing. b The subjects were 33 patients excluding those with pathologic stage IV disease
Fig. 1.
Overall survival (OS) of patients with lung cancers of >50 mm after resection (VATS: video-assisted thoracoscopic surgery).
Fig. 2.
Recurrence-free survival (RFS) of patients with lung cancers of >50 mm after resection (VATS: video-assisted thoracoscopic surgery).
Univariate and multivariate analysis of 66 patients who underwent surgery for primary lung tumors of >5-cm diameter, excluding patients with pathologic stage IV cancer, showed that limited lymph node dissection independently contributed to local recurrence (Tables 3 and 4). The VATS approach was not an independent risk factor for local recurrence or long-term prognoses compared with the thoracotomy approach.
Table 3.
Univariate analysis of factors predicting local recurrence in patients who underwent surgery (excluding pathologic Stage IV) (n = 66)
| Odds ratio | 95% CI | p-value | |
|---|---|---|---|
| Age (>70 vs ≤70 years) | 1.35 | (0.36–5.17) | 0.66 |
| Tumor size (>70 vs ≤70 mm) | 0.33 | (0.066–1.690) | 0.18 |
| Surgical site (left vs right) | 2.26 | (0.59–8.62) | 0.23 |
| Surgical approach (VATS vs thoracotomy) | 3.20 | (0.77–13.36) | 0.11 |
| Lymph node dissection (ND0/1 vs ND2) | 4.90 | (1.20–19.93) | 0.027 |
| Histology (Sq vs others) | 1.16 | (0.32–4.26) | 0.82 |
| Pathologic pleural invasion (positive vs negative) | 0.34 | (0.081–1.400) | 0.13 |
| Pathologic N status (pN1/2 vs pN0) | 3.20 | (0.85–12.06) | 0.086 |
CI: confidence interval; Sq: squamous cell carcinoma; VATS: video-assisted thoracoscopic surgery; ND: nodal dissection
Table 4.
Multivariate analysis of factors predicting local recurrence in patients who underwent surgery (excluding pathologic Stage IV) (n = 66)
| Odds ratio | 95% CI | p-value | |
|---|---|---|---|
| Tumor size (>70 vs ≤70 mm) | 0.59 | (0.077–3.040) | 0.57 |
| Surgical site (left vs right) | 2.23 | (0.40–11.51) | 0.32 |
| Surgical approach (VATS vs thoracotomy) | 2.53 | (1.16–32.16) | 0.28 |
| Lymph node dissection (ND0/1 vs ND2) | 5.37 | (0.94–8.19) | 0.047 |
| Pathologic pleural invasion (positive vs negative) | 0.19 | (0.035–1.200) | 0.074 |
| Pathologic N status (N1/2 vs N0) | 4.80 | (0.96–24.76) | 0.060 |
CI: confidence interval; VATS: video-assisted thoracoscopic surgery; ND: nodal dissection
Discussion
Advantages of VATS over thoracotomy have been strongly emphasized in previous studies and include less wound pain, fewer pulmonary complications, and a shorter postoperative hospital stay.7,8) Large-scale clinical studies have confirmed that VATS lobectomy has obvious perioperative advantages and a more favorable prognosis in the treatment of stage I lung cancer than thoracotomy.9–11) VATS has been recently indicated for advanced-stage lung cancer of >3-cm diameter. To date, however, few reports about the safety and efficacy of VATS lobectomy in the treatment of larger lung cancer have been published.12) The present study was designed to compare the minimal invasiveness and oncologic validity of five-port VATS lobectomy with those of thoracotomy lobectomy for the treatment of larger lung cancer (>5 cm in diameter). Five-port VATS, which is usually performed in our institute, enables both the operator and assistant to ensure a wide operative field with multiple surgical devices. Additionally, the surgery is safer and faster, either equaling or surpassing the safety and speed of thoracotomy by the ability of the operator and assistant to share deep and narrow surgical views.
The disadvantage of VATS for large lung cancer, however, is less mobilization of the tumor and a reduced operating space under thoracoscopic surgery. Moreover, repeated turning and mobilization is likely to cause tumor fragmentation and dissemination as well as bronchial or vascular injury. Another disadvantage of VATS is its predisposition to the development of obstructive pneumonia and consequent reactive nodal hyperplasia, increasing surgical difficulty. Conversion from VATS to thoracotomy was required in 4 of 39 patients because of injury to a bronchus and pulmonary vessel during resection of large tumors. The reported conversion rates for thoracoscopic lobectomy to thoracotomy range from 2.5% to 7.0%;13–16) the conversion rates in the present study were slightly higher. Surgical manipulations such as a prior bronchial transaction technique, the intralobar nontouch access technique (INTACT), and intrapericardial transaction of pulmonary veins may be necessary depending on the tumor location to ensure a wider surgical view.
The first goal of this study was to clarify the minimal invasiveness of VATS, evidenced by factors such as intraoperative bleeding, duration of drainage, and length of postoperative hospital stay, when VATS anatomical pulmonary resection could be completed. This minimal invasiveness was confirmed despite the fact that Group V had significantly older patients than did Group T because of bias associated with patient selection.
The second goal of this study was to clarify the oncologic validity of VATS. Watanabe et al.17) and Carbone et al.18) considered a 5-cm diameter to be a prognostic threshold for tumors larger than 3 cm. The oncological outcomes of VATS lobectomy for such large lung tumors remain controversial3) because VATS is associated with certain disadvantages associated with en bloc resection and lymph node dissection.4,5) The results of this study showed no significant difference in the OS or RFS between the two groups. These rates obtained in the present study are equivalent to those in a previous study reporting the 5-year survival rates of patients undergoing thoracotomy resection for lung tumors of >5 cm (31.4% to 35.5%).17,19) Group V, however, showed a slightly higher local recurrence rate than did Group T, but the difference was not statistically significant. Local recurrence after VATS for large lung cancer may be associated with the risk of cancer cell spillage during specimen extraction. Specimen extraction from the thoracic cavity through an ICS during VATS can result in cancer cell contamination by tumor crushing and tumor cell extravasation. The BLC results reported in our previous study showed a 13.6% rate of cancer cell spillage within the bag during the VATS procedure, and this rate increased with the tumor size.20) In the present study, the incidence of BLC positivity for lung cancer of >5-cm diameter was 35.7% among 14 patients who underwent the extraction BLC test. Large tumors must be carefully maneuvered during VATS to prevent cancer cell spillage.
We also found that local recurrence was independently subject to limited nodal dissection. Local recurrence after VATS for a large lung tumor may also be associated with remnant metastatic lymph nodes. Mediastinal nodal dissection (ND2) was saved during the VATS procedure, especially in older patients. Furthermore, the number of residual lymph nodes after the VATS procedure is reportedly larger than that after open procedures.21) Some patients with pN0 disease in Group V might have been nodal-positive due to this limitation. Nodal dissection during VATS should be performed sharply, even in the oldest patients.
Conclusion
The oncologic validity and perioperative course were similar between VATS and thoracotomy for lung cancer of >5-cm diameter. Large tumors, however, must be carefully maneuvered during VATS to prevent cancer cell spillage, and lymph node dissection should be encouraged.
Disclosure Statement
The authors declare that they have no conflict of interest.
References
- 1).Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010; 139: 366-78. [DOI] [PubMed] [Google Scholar]
- 2).McKenna RJ, jr, Wolf RK, Brennner M, et al. Is lobectomy by video-assisted thoracic surgery an adequate cancer operation? Ann Thorac Surg 1998; 66: 1903-8. [DOI] [PubMed] [Google Scholar]
- 3).Flores RM, Alam N. Video-assisted thoracic surgery lobectomy (VATS), open thoracotomy, and the robot for lung cancer. Ann Thorac Surg 2008; 85: S710-5. [DOI] [PubMed] [Google Scholar]
- 4).Denlinger CE, Fernandez F, Meyers BF, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg 2010; 89: 1730-5; discussion 1736. [DOI] [PubMed] [Google Scholar]
- 5).Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007; 83: 1965-70. [DOI] [PubMed] [Google Scholar]
- 6).The Japanese Lung Cancer Society. General rule for clinical and pathological records of lung cancer. Tokyo: Kanehara & Co. Ltd; 2010; 87-94. [Google Scholar]
- 7).Li WW, Lee TW, Lam SS, et al. Quality of life following lung cancer resection: video-assisted thoracic surgery vs thoracotomy. Chest 2002; 122: 584-9. [DOI] [PubMed] [Google Scholar]
- 8).Kaseda S, Aoki T, Hangai N, et al. Better pulmonary function and prognosis with video-assisted thoracic surgery than with thoracotomy. Ann Thorac Surg 2000; 70: 1644-6. [DOI] [PubMed] [Google Scholar]
- 9).Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010; 139: 976-83. [DOI] [PubMed] [Google Scholar]
- 10).West D, Rashid S, Dunning J. Does video-assisted thoracoscopic lobectomy produce equal cancer clearance compared to open lobectomy for non-small cell carcinoma of the lung? Interact Cardiovasc Thorac Surg 2007; 6: 110-6. [DOI] [PubMed] [Google Scholar]
- 11).Kim K, Kim HK, Park JS, et al. Video-assisted thoracic surgery lobectomy: single institutional experience with 704 cases. Ann Thorac Surg 2010; 89: S2118-22. [DOI] [PubMed] [Google Scholar]
- 12).Bu L, Li Y, Yang F, et al. Completely video-assisted thoracoscopic lobectomy versus open lobectomy for non-small cell lung cancer greater than 5 cm: a retrospective study. Chin Med J 2012; 125: 434-9. [PubMed] [Google Scholar]
- 13).McKenna RJ, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006; 81: 421-5; discussion 425-6. [DOI] [PubMed] [Google Scholar]
- 14).Park JS, Kim HK, Choi YS, et al. Unplanned conversion to thoracotomy during video-assisted thoracic surgery lobectomy does not compromise the surgical outcome. World J Surg 2011; 35: 590-5. [DOI] [PubMed] [Google Scholar]
- 15).Marty-Ané CH, Canaud L, Solovei L, et al. Video-assisted thoracoscopic lobectomy: an unavoidable trend? A retrospective single-institution series of 410 cases. Interact Cardiovasc Thorac Surg 2013; 17: 36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Stephens N, Rice D, Correa A, Hoffstetter W, Mehran R, Roth J, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical Stage I non-small-cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg 2014; 46: 607-13. [DOI] [PubMed] [Google Scholar]
- 17).Watanabe Y, Shimizu J, Oda M, et al. Proposals regarding some deficiencies in the new international staging system for non-small cell lung cancer. Jpn J Clin Oncol 1991; 21: 160-8. [PubMed] [Google Scholar]
- 18).Carbone E, Asamura H, Takei H, et al. T2 tumors larger than five centimeters in diameter can be upgraded to T3 in non-small cell lung cancer. J Thorac Cardiovasc Surg 2001; 122: 907-12. [DOI] [PubMed] [Google Scholar]
- 19).Cangir AK, Kutlay H, Akal M, et al. Prognostic value of tumor size in non-small cell lung cancer larger than five centimeters in diameter. Lung Cancer 2004; 46: 325-31. [DOI] [PubMed] [Google Scholar]
- 20).Nakano T, Tetsuka K, Endo T, et al. Extraction bag lavage cytology during video-assisted thoracoscopic surgery for primary lung cancer. Interact Cardiovasc Thorac Surg 2014; 18: 770-4. [DOI] [PubMed] [Google Scholar]
- 21).Sagawa M, Sato M, Sakurada A, et al. A prospective trial of systematic nodal dissection for lung cancer by video-assisted thoracic surgery: can it be perfect? Ann Thorac Surg 2002; 73: 900-4. [DOI] [PubMed] [Google Scholar]