Abstract
Purpose: To identify prognostic factors for pathologic N2 (pN2) non-small cell lung cancer (NSCLC) treated by surgical resection.
Methods: Between 1990 and 2009, 287 patients with pN2 NSCLC underwent curative resection at the Cancer Institute Hospital without preoperative treatment.
Results: The 5-year overall survival (OS), cancer-specific survival (CSS), and recurrence-free survival (RFS) rates were 46%, 55% and 24%, respectively. The median follow-up time was 80 months. Multivariate analysis identified four independent predictors for poor OS: multiple-zone mediastinal lymph node metastasis (hazard ratio [HR], 1.616; p = 0.003); ipsilateral intrapulmonary metastasis (HR, 1.042; p = 0.002); tumor size >30 mm (HR, 1.013; p = 0.002); and clinical stage N1 or N2 (HR, 1.051; p = 0.030). Multivariate analysis identified three independent predictors for poor RFS: multiple-zone mediastinal lymph node metastasis (HR, 1.457; p = 0.011); ipsilateral intrapulmonary metastasis (HR, 1.040; p = 0.002); and tumor size >30 mm (HR, 1.008; p = 0.032).
Conclusion: Multiple-zone mediastinal lymph node metastasis, ipsilateral intrapulmonary metastasis, and tumor size >30 mm were common independent prognostic factors of OS, CSS, and RFS in pN2 NSCLC.
Keywords: Lung cancer surgery, Prognosis, Mediastinal lymph nodes, Outcomes, pathologic N2, survival analysis
Introduction
Stage III non-small cell lung cancer (NSCLC) is a heterogeneous condition with presentation ranging from apparently resectable tumors with occult pathologic N2 (pN2) to unresectable, multistation, bulky, and extranodal pN2 disease; however, the prognoses of these groups are heterogeneous and remain unsatisfactory.1) Unfortunately, a clear and widely accepted characterization of subgroups has not yet emerged.1)
The American College of Chest Physicians has published guidelines for the treatment of Stage III NSCLC.1) In these guidelines, grade 1A recommendations are “In patients with discrete N2 involvement identified preoperatively (IIIA), either definitive chemoradiation therapy or induction therapy followed by surgery is recommended over either surgery or radiation alone (Grade 1A)” and “In patients with resected NSCLC (R0) who were found to have incidental (occult) N2 disease (IIIA) despite thorough preoperative staging and who have good performance status, adjuvant platinum-based chemotherapy is recommended (Grade 1A).” However, the efficacy of adjuvant chemotherapy in Japanese phase III trials has been controversial,2,3) and the significance of surgery and optimal treatment strategies for this population remain unknown.
For all types of stage III NSCLC, the role of surgical resection remains controversial and surgery alone is not clearly indicated for pN2 NSCLC. Although there are relatively few case reports in the literature, radical resection is indicated for curative treatment.
In recent years, the overall survival (OS) and cancer-specific survival (CSS) rates for recurrent disease have improved by treatment regimens of chemotherapy, radiotherapy, and molecular targeting drugs.4) In Japan, the proportion of epidermal growth factor receptor (EGFR) mutation-positive lung cancer is higher than in other regions, and the effect of molecular targeting drugs is more pronounced.5,6)
However, the severity of side effects of anticancer agents used in prophylactic treatments before and after recurrence remains unacceptable. Therefore, it is necessary to consider an adaptations of adjuvant therapy to reduce the relapse rate, accelerate healing after resection, and improve the effect of adjuvant chemotherapy.
The objective of the present study was to identify prognostic factors for OS, CSS, and recurrence-free survival (RFS) in pN2 patients who have undergone complete resection without neoadjuvant treatment, as well as to clarify an optimal therapeutic approach for NSCLC.
Materials and Methods
Between 1990 and 2009, 2439 patients with NSCLC underwent curative surgical resection at the Cancer Institute Hospital (Japanese Foundation for Cancer Research, Tokyo, Japan). Of these, 287 (11.8%) patients were diagnosed with pN2 NSCLC on the basis of systematic mediastinal lymph node dissection and had not received neoadjuvant therapy. Since this was a retrospective study and the individual patients were not identifiable, our Institutional Review Board waived the requirement to obtain patient consent.
During the period of 1990–2004, the significance of adjuvant chemotherapy or adjuvant radiotherapy was not established. Therefore, the indications for these therapies were dependent on the characteristics of each patient. Adjuvant chemotherapy was recommended for pN2 patients from 2004 onward.
All patients underwent preoperative assessment, which included chest X-ray, thoracic and upper abdominal computed tomography (CT), bone scan, brain magnetic resonance imaging or CT, basic blood tests, and cardiopulmonary evaluation. Mediastinoscopy was not performed in this series. Preoperative diagnoses of lymph node metastasis were performed using CT. Mediastinal lymph nodes with a short axis (<1 cm) on CT were regarded as clinical N2. Histopathological studies were conducted according to the criteria established by the World Health Organization (WHO).
The absence of cancer cells was based on both surgical and pathological findings. Staging definitions for T (primary tumor), N (regional lymph nodes), and M (distant metastasis) components were made according to the International Staging System for Lung Cancers.7)
All patients were followed up after surgery. Physical examinations and chest radiography were performed every 3 months, and chest and abdominal CT images were taken every 6 months for the first 2 years. Thereafter, patients underwent physical examinations and chest radiography every 6 months, with annual chest CT imaging for 5 years.
Tumor recurrence was defined as evidence of tumors that involved the surgical margin, hilum, or mediastinal lymph nodes (locoregional recurrence), or as evidence of tumors in other lobes or outside the hemithorax (distant recurrence). Time to recurrence was defined as the period between surgery and the discovery of recurrence using either imaging or cytopathological examination. Patients with signs or symptoms that correlated with tumor recurrence or metastasis were assessed using brain or bone CT scans, as needed.
OS was defined as the interval between the date of surgery and death due to any cause over the 5-year postoperative period. The duration of cancer-specific survival was defined as the interval between the date of surgery and death due to lung cancer, and deaths due to other causes were recorded as censored observations. RFS was defined as time to lung cancer recurrence or non-lung cancer death.
To identify prognostic factors, patients were evaluated on the basis of age, gender, largest tumor diameter, clinical (c-) T factor, pathological (p-) T factor, histological findings, c-N factor, number of metastatic mediastinal lymph nodes (single or multiple), distribution of metastatic lymph nodes (skip N2 or non-skip N2), and adjuvant therapy.
Survival rates from the date of surgery were calculated using the Kaplan–Meier life table method and compared between groups using the log-rank test. Dichotomous variables are presented as percentages and were compared between groups using the chi-squared or Fisher’s exact test where appropriate. A probability (p) value of <0.05 was considered statistically significant. Statistical analysis was performed using SPSS analytical software (SPSS Inc., Chicago, Illinois, USA).
Results
Patient characteristics are presented in Table 1. Of the 287 patients included for analysis, 169 were males and 118 females, with a median age of 62 years (range, 16–84 years). CT evaluation revealed cN0 in 156 cases (54.3%), cN1 in 67 cases (23.4%), and cN2 in 64 cases (22.3%). The positive predictive value was 22.3% (64/287). Among the patients who underwent complete resection, lobectomy was performed in 212 (73.8%), bilobectomy in 34 (11.8%), and pneumonectomy in 41 (14.4%) cases, respectively. Surgical resection was performed in 74, 68, 56, and 89 patients during the periods of 1990–1994, 1995–1999, 2000–2004, and 2005–2009, respectively.
Table 1.
Clinicopathological Characteristics of Patients with pN2 Lung cancer (1990–2009) by time period
| Characteristics | 1990–1994 | 1995–1999 | 2000–2004 | 2005–2009 | Total |
|---|---|---|---|---|---|
| Age (years) | |||||
| <65/≥65 | 49/25 | 38/30 | 36/20 | 46/43 | 169/118 |
| Sex | |||||
| male/female | 50/24 | 40/28 | 39/17 | 51/38 | 180/107 |
| Clinical T factor | |||||
| cT1/2/3/4 | 23/36/14/1 | 28/30/8/2 | 23/23/7/3 | 34/48/7/0 | 108/137/36/6 |
| Clinical N factor | |||||
| cN 0/1/2 | 34/10/30 | 36/15/17 | 33/18/5 | 53/24/12 | 156/67/64 |
| Pulmonary Metastasis | |||||
| pm0/1/2 | 43/27/4 | 56/11/1 | 42/11/3 | 77/10/2 | 218/59/10 |
| Operation | |||||
| lobectomy/pneumonectomy | 57/17 | 61/7 | 50/6 | 78/11 | 246/41 |
| Number of zone | |||||
| multiple/single | 18/56 | 15/53 | 14/42 | 28/61 | 75/212 |
| Skip pN2 | |||||
| Skip/nonskip | 41/33 | 36/32 | 22/34 | 61/28 | 127/160 |
| Pathological T factor | |||||
| pT1a/1b/2a/2b/3/4 | 1/10/19/1/38/5 | 7/10/18/5/24/4 | 5/8/19/2/17/5 | 13/12/37/6/19/2 | 26/40/93/14/98/16 |
| Tumor size | |||||
| >31 mm/≤30 mm | 46/28 | 40/28 | 28/28 | 45/44 | 159/128 |
| Adjuvant therapy | |||||
| no/chemotherapy/radiation/chemoradiation | 33/20/16/5 | 61/0/7/0 | 51/2/3/0 | 38/49/1/1 | 183/71/27/6 |
| Pathological type | |||||
| Adenocarcinoma/others | 53/21 | 44/24 | 42/14 | 75/14 | 214/73 |
| EGFR mutation | |||||
| Yes/no/unknow | 0/0/74 | 0/0/68 | 0/0/56 | 20/21/48 | 20/21/246 |
| Recurrence | |||||
| Recurrence/not | 48/26 | 45/23 | 39/17 | 65/24 | 196/91 |
| Median recurrence time (month) | 11 | 13 | 14 | 14 | 13 |
| Recurrence site | |||||
| Local/distant/distant and local | 12/33/3 | 13/27/5 | 8/28/3 | 22/36/7 | 55/123/18 |
| Survival rate | |||||
| 5 year Recurrence free survival (%) | 22 | 34 | 25 | 20 | 24 |
| 5 year over all survival (%) | 34 | 43 | 52 | 60 | 46 |
| Total | 74 | 68 | 56 | 89 | 287 |
pm: pulmonary metastasis; EGFR = epidermal growth factor receptor
Pathologic examinations revealed pT1a in 26 cases (9.1%), pT1b in 40 cases (13.9%), pT2a in 93 cases (32.4%), pT2b in 14 cases (4.9%), pT3 in 98 cases (34.1%), and pT4 in 16 cases (5.6%). Of the T3 cases, 59 patients had pulmonary metastasis in the same lobe and of the T4 cases, 10 patients had pulmonary metastasis of the other lobe on the same side.
Regarding the number of metastatic lymph nodes per zone, there were 212 cases of single zone pN2 and 75 case of multiple zone pN2. Regarding the distribution of metastatic lymph nodes, there were 127 skip pN2 and 160 non-skip pN2. Using the WHO guidelines to classify the histological types in these patients, 214 were classified as adenocarcinomas, 44 as squamous cell carcinomas, 15 as large cell carcinomas, and 14 as other carcinomas.
Correlations between postoperativeon duration years and clinicopathological variables are summarized in Table 1. Regarding historical background, the recommended treatment regimens and accuracy of diagnostic imaging varied over time, thus there were differences in background factors. The use of adjuvant therapy changed along with the evolution of treatment guidelines. The recurrence rates remained at the same level, 65% (48/74) in 1990–1994, 66% (45/68) in 1995–1999, 70% (39/56) in 2000–2004, and 73% (65/89) in 2005–2009. The median recurrence time from surgery was 11 months in 1990–1994, 13 months in 1995–1999, 14 months in 2000–2004, and 14 months in 2005–2009. However, the Kruskal–Wallis test showed that there were no significant differences in the time to recurrence among these periodseras (p = 0.278).
EGFR mutations were examined at the time of relapse and tyrosine kinase inhibitor (TKI) treatment was considered for EGFR mutation-positive cases. Beginning in 2004, it was possible to detect EGFR mutations. Of 41 patients examined, 20 were EGFR mutation-positive and 16 received TKI therapy.
The 5YOS and 5YRFS rates were 34% and 22% in 1990–1994, 43% and 34% in 1995–1999, 52% and 25% in 2000–2004, and 60% and 20% in 2005–2009, respectively. The recent improvement in OS was remarkable, whereas the RFS rate did not improve, indicating an increasing dissociation between OS and RFS (Table 1).
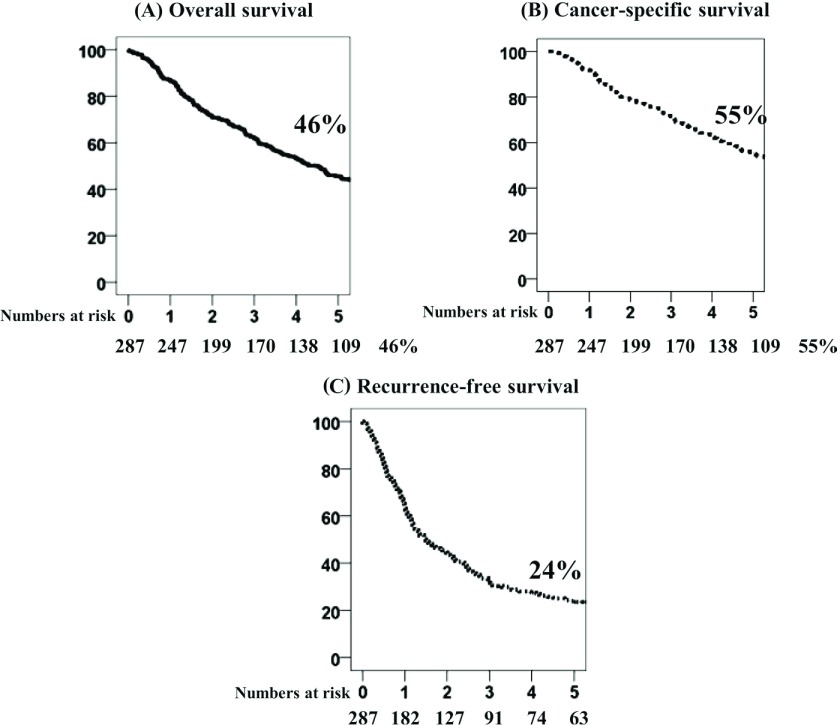
For the entire cohort, the 5-year overall survival (5YOS), 5-year cancer-specific survival (5YCSS), and 5-year recurrence-free survival (5YRFS) rates were 46%, 55%, and 24%, respectively (Fig. 1). The median follow-up period was 80 months.
Fig. 1.
Overall (OS), cancer-specific (CSS), and recurrence-free (RFS) survival curves for p-stage N2 non-small cell lung cancer patients. (A) OS, (B) CSS, and (C) RFS rates are 46%, 55% and 24%, respectively, from 1990 to 2009.
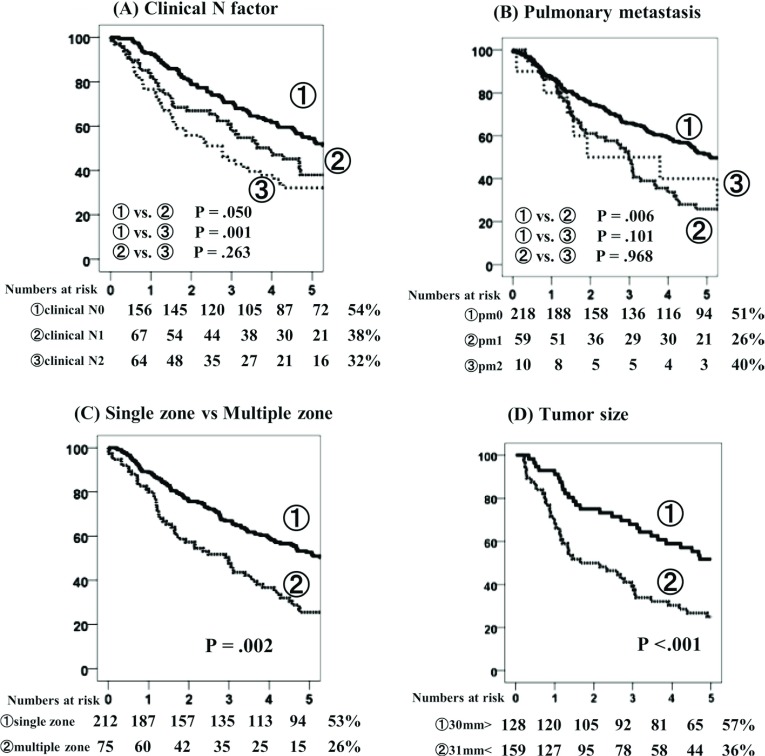
Survival analysis of the 5YOS rates revealed significant differences between cN0 and cN1 (p = 0.050), and cN0 and cN2 (p = 0.001), but not between cN1 and cN2 (p = 0.263) (Fig. 2A). Regarding pm factor, pm0, pm1, and pm2 disease was identified in 51%, 26%, and 40% of cases, respectively, and significant differences were observed between pm0 and pm1 (p = 0.006), (Fig. 2B). Regarding the prevalence of metastatic mediastinal lymph node zone, single zone pN2 and multiple zone pN2 disease were observed in 53% and 26% of cases, respectively, indicating a significant difference (p = 0.002) (Fig. 2C). Tumor size of >30 mm and ≤30 mm accounted for 57% and 36% of cases, respectively, indicating a statistically significant difference (p <0.001) (Fig. 2D).
Fig. 2.
Overall survival (OS) curves according to clinical N factor (A), pulmonary metastasis (B), number of zones (C), and tumor size (D).
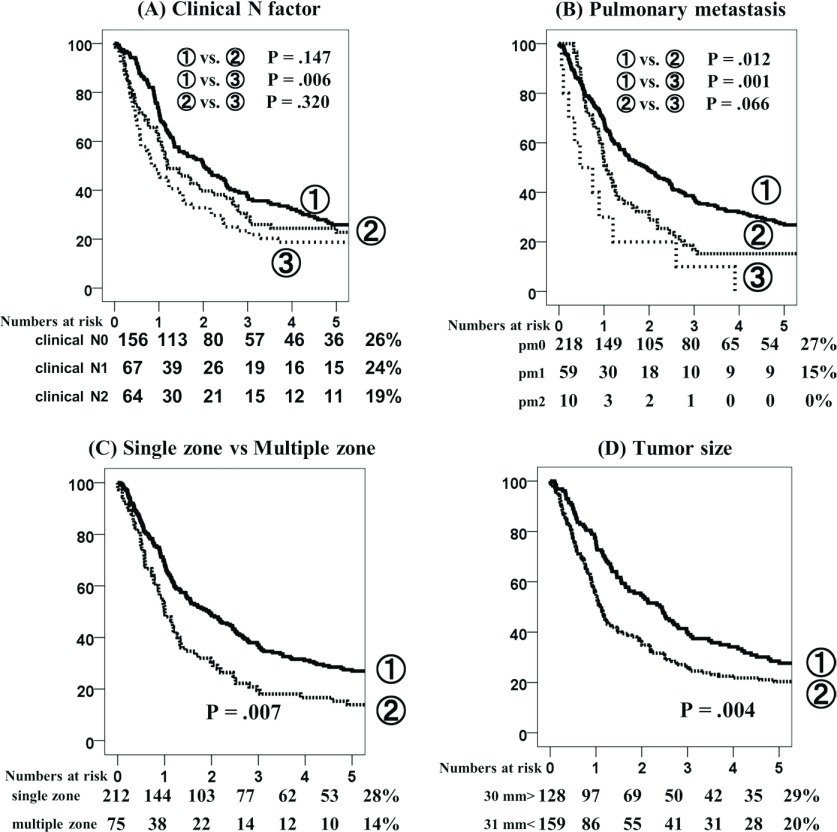
Survival analysis of the 5YRFS rate for patients with c-N0, c-N1, and c-N2 disease were 26%, 24%, and 19%, respectively (Fig. 3A) with significant differences observed between cN0 and cN2 (p = 0.006), but not between cN0 and cN1, or cN1 and cN2. Regarding pm factor, pm0, pm1, and pm2 disease was observed in 27%, 15%, and 0% of cases, respectively, with a significant difference between pm0 and pm1 (p = 0.012) (Fig. 3B). Regarding the metastatic mediastinal lymph node zone, single zone pN2 and multiple zone pN2 disease were observed in 28% and 14% of cases, respectively (p = 0.007) (Fig. 3C). A tumor size of >30 mm and ≤30 mm was observed in 29% and 20% of cases, respectively (p = 0.004) (Fig. 3D).
Fig. 3.
Recurrence-free survival (RFS) curves according to clinical N factor (A), pulmonary metastasis (B), number of zone (C), and tumor size (D).
Univariate analysis of 5YOS rates showed that clinical T factor, clinical N factor, ipsilateral intrapulmonary metastasis, multiple-zone mediastinal lymph node metastasis, and tumor size were associated with poor prognosis, whereas age, sex, type of pathological typey, lobectomy, and pneumonectomy were not significantly correlated with survival. Multivariate analysis using the stepwise Cox proportional hazard model confirmed that clinical N factor (hazard ratio [HR], 1.051; p = 0.030), ipsilateral intrapulmonary metastasis (HR, 1.042; p = 0.002), multiple-zone mediastinal lymph node metastasis (HR, 1.616; p = 0.003), and tumor size (HR, 1.013; p = 0.002) were independent prognostic factors (Table 2).
Table 2.
Univariate and multivariate analyses of survival for pN2 NSCLC
| Variable | Overall survival | Cancer specific survival | Recurrence-free survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uni | Multivariate | Uni | Multivariate | Uni | Multivariate | ||||||||
| p | HR | 95% CI | p | p | HR | 95% CI | p | p | HR | 95% CI | p | ||
| Age | |||||||||||||
| <65/≥65 | 0.051 | 0.496 | 0.097 | ||||||||||
| Sex | |||||||||||||
| male/female | 0.775 | 0.971 | 0.414 | ||||||||||
| Clinical T factor | |||||||||||||
| T3-4/T1-2 | 0.022 | 1.002 | 0.983–1.021 | 0.823 | 0.033 | 1.004 | 0.982–1.027 | 0.696 | 0.107 | ||||
| Clinical N factor | |||||||||||||
| cN1-2/cN0 | 0.001 | 1.051 | 1.005–1.099 | 0.030 | 0.021 | 1.292 | 0.893–1.870 | 0.174 | 0.017 | 1.035 | 0.996–1.076 | 0.078 | |
| pm factor | |||||||||||||
| pm1-2/pm0 | 0.003 | 1.042 | 1.015–1.070 | 0.002 | 0.000 | 2.071 | 1.439–2.982 | 0.000 | 0.001 | 1.040 | 1.014–1.065 | 0.002 | |
| Operation | |||||||||||||
| lob/pn | 0.194 | 0.882 | 0.271 | ||||||||||
| Number of zones | |||||||||||||
| multiple/single | 0.002 | 1.616 | 1.175–2.221 | 0.003 | 0.018 | 1.537 | 1.051–2.248 | 0.027 | 0.007 | 1.457 | 1.089–1.950 | 0.011 | |
| Tumor size | |||||||||||||
| >31 mm/≤30 mm | 0.000 | 1.013 | 1.005–1.021 | 0.002 | 0.001 | 1.740 | 1.192–2.538 | 0.004 | 0.004 | 1.008 | 1.001–1.014 | 0.032 | |
| Skip | |||||||||||||
| Skip/nonskip | 0.341 | 0.862 | 0.719 | ||||||||||
| Adjuvant therapy | |||||||||||||
| yes/no | 0.076 | 0.052 | 0.194 | ||||||||||
| Pathology | |||||||||||||
| AD/non AD | 0.496 | 0.772 | 0.276 | ||||||||||
pN2: pathologic N2; NSCLC: non-small cell lung cancer; Uni: Univariate; HR: hazard ratio; CI: confidence interval; pm: pulmonary metastatis; AD: adenocarcinoma; lob: lobectomy; pn: pneumonectomy
On univariate analysis for 5YCSS, clinical T factor, clinical N factor, ipsilateral intrapulmonary metastasis, multiple-zone mediastinal lymph node metastasis, and tumor size were associated with poor prognosis. Multivariate analysis confirmed that ipsilateral intrapulmonary metastasis (HR, 2.071; p = 0.00), multiple-zone mediastinal lymph node metastasis (HR, 1.537; p = 0.027), and tumor size (HR, 1.740; p = 0.004) were identified as independent prognostic factors (Table 2).
On univariate analysis for 5YRFS, clinical N factor, ipsilateral intrapulmonary metastasis, multiple-zone mediastinal lymph node metastasis, and tumor size were associated with poor prognosis. Multivariate analysis identified three independent predictors for poor RFS: multiple-zone mediastinal lymph node metastasis (HR, 1.457; p = 0.0011), ipsilateral intrapulmonary metastasis (HR, 1.040; p = 0.002), and tumor size >30 mm (HR, 1.008; p = 0.032) (Table 2).
Discussion
The 5-year OS rate of 46% in this study was very close to that in recent reported by Sonobe et al.8) published in 2013 (44.8%) and substantially higher than that reported inof Goldstraw et al.7) in 2007 (of 24%) with a median follow-up period of 76 months. An improvement in the 5-year OS rate was also observed in the present single-institution study: 34% in 1990–1994, 43% in 1995–1999, 52% in 2000–2004, and 60% in 2005–2009 (Table 1). The most recent improvement in OS was remarkable. We believe that improvements in clinical staging by advances in diagnostic imaging led to the improvement in OS by allowing optimal selection of surgical strategies. It is now possible to detect small distant metastases (ipsilateral intrapulmonary metastasis, small liver metastasis, and adrenal metastasis) that were previously difficult to detect before surgery. Another reason for improved survival is the increasingly successful treatment options for recurrent disease, including chemotherapy, radiotherapy, and/or molecular targeting drugs.4) In Japan, for EGFR mutation-positive status lung cancer patients, which may be a better prognostic factor after recurrence; In Japan, the effect of molecular targeting drugs is more pronounced for EGFR mutation-positive lung cancer patients.5,6) In the present study, 65 patients developed recurrence after 2005, 20 patients were EGFR mutation-positive, and 16 patients received TKI treatment.
There were some potential limitations to this study, as the observational period was relatively long and patient backgrounds varied. Initially, we expected that RFS would also improve with time. However, even though the patient backgrounds varied among the periods under observation, RFS did not improve. We suspect that the treatment period was a primary reason for the lack of improvement in RFS. In future cases, we may detect recurrence in asymptomatic cases earlier times because of advances in diagnostic imaging, which was not possible in the 1990s, resulting in shorter RFS durations. RFS may increase because of improvements in imaging precision, although no improvement in RFS was observed in this study.
The use of adjuvant and neoadjuvant therapy continues to change along with the evolution of treatment guidelines. We began adjuvant chemotherapy after surgery in 2004. Although it is difficult to arrive at generalizations because the limited number of target cases in this study, adjuvant chemotherapy was not found to prevent recurrence in pN2 patients. The recurrence rate and median time to relapse did not improve. But adjuvant chemotherapy might improve the OS.
In this study, the positive predictive value of pN2 by CT evaluation findings for pN2 was only 22.3% (64/287). Preoperative diagnoses of lymph node metastasis were performed using only CT. Mediastinal lymph nodes with a short axis (<1 cm) on CT were regarded as clinical N2. A lymph node diagnosis based on only CT has limitations, thus improvements in diagnostic imaging are needed.
Various reports have identified prognostic factors for pN2 NSCLC, which included pT and cN classifications, tumor size, and N2 regions.8–11) Sonobe, et al. reported that 5-year OS and RFS rates of completely resected pN2 non-small cell lung cancer were 44.8% and 24.2%, respectively.8) Independent prognostic factors for RFS were pT classification, single or multiple N2 metastases, and skip or non-skip N2 metastasis. In the present multivariate analysis, there were three independent predictors for poor RFS: multiple-zone mediastinal lymph node metastasis, ipsilateral intrapulmonary metastasis, and tumor size >30 mm (Table 2). Ipsilateral intrapulmonary metastasis and tumor size >30 mm are predictive factors for recurrence in T3 and T2 disease respectively and tumor size >30 mm is a factor of T2 disease, indicating that the prognostic factors were the same. However, the factor of skip LN metastasis was not identified as a prognostic factor by univariate analysis in the present study. There are various opinions regarding skip LN metastasis factor as a prognostic factor.12,13) In respect to N2 regions, significance of metastatic lymph node station varies among lobes. In our previous study,14) we also reported that metastasis to the upper mediastinal lymph nodes from lower lobe tumors was indicative of a poor prognosis. In respect to N2 regions, Sonobe, et al. defined N2 metastasis as localized and extended and reported similar results to our findings.8)
In conclusion, OS tended to improve in recent years, although RFS remains poor in pN2 NSCLC. Significant or nearly significant common prognostic factors of OS, CSS, and RFS included multiple-zone mediastinal lymph node metastasis, ipsilateral intrapulmonary metastasis, tumor size >30 mm, and cN factors. Thus, close observation and individualized adjuvant therapy may be helpful for these patients.
Funding Support
The authors received no specific funding for this study.
Disclosure Statement
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
References
- 1).Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e314S-40S.. [DOI] [PubMed] [Google Scholar]
- 2).Wada H, Hitomi S, Teramatsu T. Adjuvant chemotherapy after complete resection in non-small-cell lung cancer. West Japan Study Group for Lung Cancer Surgery. J Clin Oncol 1996; 14: 1048-54. [DOI] [PubMed] [Google Scholar]
- 3).Tada H, Tsuchiya R, Ichinose Y, et al. A randomized trial comparing adjuvant chemotherapy versus surgery alone for completely resected pN2 non-small cell lung cancer (JCOG9304). Lung Cancer 2004; 43: 167-73. [DOI] [PubMed] [Google Scholar]
- 4).Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst Rev 2010; 12: CD007309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Kosaka T, Yatabe Y, Endoh H, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res 2004; 64: 8919-23. [DOI] [PubMed] [Google Scholar]
- 6).Sonobe M, Kobayashi M, Ishikawa M, et al. Impact of KRAS and EGFR gene mutations on recurrence and survival in patients with surgically resected lung adenocarcinomas. Ann Surg Oncol 2012; 19: 347-54. [DOI] [PubMed] [Google Scholar]
- 7).Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. (2007) The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumors. J Throac Oncol 2: 706-714. [DOI] [PubMed] [Google Scholar]
- 8).Sonobe M, Date H, Wada H, et al. Prognostic factors after complete resection of pN2 non-small cell lung cancer. J Thorac Cardiovasc Surg 2013; 146: 788-95. [DOI] [PubMed] [Google Scholar]
- 9).Morgensztern D, Waqar S, Subramanian J, et al. Improving survival for stage IV non-small cell lung cancer: a surveillance, epidemiology, and end results survey from 1990 to 2005. J Thorac Oncol 2009; 4: 1524-1529. [DOI] [PubMed] [Google Scholar]
- 10).Nakagiri T, Sawabata N, Funaki S, et al. Validation of pN2 sub-classifications in patients with pathological stage IIIA N2 non-small cell lung cancer. Interact Cardiovasc Thorac Surg 2011; 12: 733-8. [DOI] [PubMed] [Google Scholar]
- 11).Maximus S, Nguyen DV, Mu Y, et al. Size of Stage IIIA primary lung cancers and survival: a surveillance, epidemiology and end results database analysis. Am Surg 2012; 78: 1232-7. [PubMed] [Google Scholar]
- 12).Riquet M, Assouad J, Bagan P, et al. Skip mediastinal lymph node metastasis and lung cancer: a particular N2 subgroup with a better prognosis. Ann Thorac Surg 2005; 79: 225-33. [DOI] [PubMed] [Google Scholar]
- 13).Misthos P, Sepsas E, Kokotsakis J, et al. The significance of one-station N2 disease in the prognosis of patients with nonsmall-cell lung cancer. Ann Thorac Surg 2008; 86: 1626-30. [DOI] [PubMed] [Google Scholar]
- 14).Uehara H, Sakao Y, Mun M, et al. Prognostic value and significance of subcarinal and superior mediastinal lymph node metastasis in lower lobe tumours. Eur J Cardiothorac Surg 2010; 38: 498-502. [DOI] [PubMed] [Google Scholar]