Abstract
Objective: Early and mid-term result of transapical aortic (TAA) cannulation technique was evaluated compared with femoral artery (FA) cannulation in Acute Type A Aortic Dissection(AAAD).
Methods: From January 2000 to October 2013, 80 consecutive patients with AAAD were underwent the ascending aortic replacement at Nagasaki Kouseikai Hospital. These patients were divided into two groups according to the cannulation site, FA cannulation (n = 34) and TAA cannulation (n = 46). Early and mid-term outcomes were compared between two groups.
Result: Preoperative patient characteristics were almost comparable between groups. The time from skin incision to starting cardiopulmonary bypass (CPB) was significantly shorter in the TAA group (45 ± 16 vs 23 ± 5.1 min; P <0.001). There were no significant differences in post-operative cerebral infarction in two groups (17% versus 11%; P = NS). The operative mortality rate was 8.8% in FA group and 4.3% in TAA group (P = NS). During follow up (mean, 6.8 years), survival at 3 years and 5 years was 77.4% and 71.9% in TAA group and 76.3% and 73.8% in FA group, respectively.
Conclusion: The postoperative morbidity and mortality between the two groups were almost the same. TAA cannulation for acute Type A aortic dissection is faster, easy and safe with acceptable early and mid-term outcome.
Keywords: acute aortic dissection, transapical, femoral, cannulation site, cardiopulmonary bypass (CPB)
Introduction
Recently, the surgical outcomes acute type A aortic dissection (AAAD) have been improved.1–5) However, it is still difficult to rescue patients who have fallen into hypotensive hypotensive shock and critical branch ischemia, such as cerebral infarction, myocardial infarction and intestinal infarction. In the case of critical organ ischemia, the rapid establishment of the cardiopulmonary bypass (CPB) for organ perfusion and systemic cooling is essential to save the patient. The femoral artery has been the standard cannulation site for CPB in treating AAAD. However, the retrograde blood stream sometime causes serious complications, such as cerebral embolism and organ malferfusion.6,7) On the other hand, axillary artery has been used for cannulation site by many surgeons. However, this technique is time consuming and not always safe or reliable. If the axillary is small, CPB flow might be insufficient and there is a risk of injury.8–10) In the emergency cases like aortic dissection, adequate evaluation was not performed for peripheral arteries, the small and atherosclerotic peripheral arteries were not suitable for enough CPB flow. Since October 2004, transapical aortic (TAA) cannulation technique was introduced and has been performed for AAAD in our institute for ten years.
We reviewed our early and mid-term result of TAA cannulation compared with femoral artery (FA) cannulation in AAAD.
Methods
Between January 2000 and October 2013, a total of 112 consecutive patients with AAAD were treated with artificial graft replacement at Nagasaki Kouseikai Hospital. Patients who underwent total arch replacement, aortic root replacement, concomitant operation and re-do cases were excluded from this study. A total of 80 patients underwent the ascending aortic replacement were evaluated. Thirty-four patients with FA cannulation and 46 patients with TAA cannulation were evaluated in this study. Preoperative patient characteristic, operative parameter, operative mortality, morbidity and mid-term results were compared. After approval of the institutional review board, follow-up was obtained through contact with home doctor, the patient or the family directly. All patients were followed up completely.
Monitoring Methods
Intraoperative monitoring was performed to detect the development of organ malperfusion. The arterial pressure of both right radial artery and left femoral artery were routinely monitored. Bilateral brain oxygen saturation was monitored with the INVOSTM cerebral somatic oximetry system (COVIDIEN. INC., Boston, Massachusetts, USA). Transesophageal echocardiography (TEE) was also need to evaluate blood flow in the true lumen. We never clamp the ascending aorta, until the circulatory arrest was obtained, because which sometimes causes severe mulperfusion.
Surgical procedure
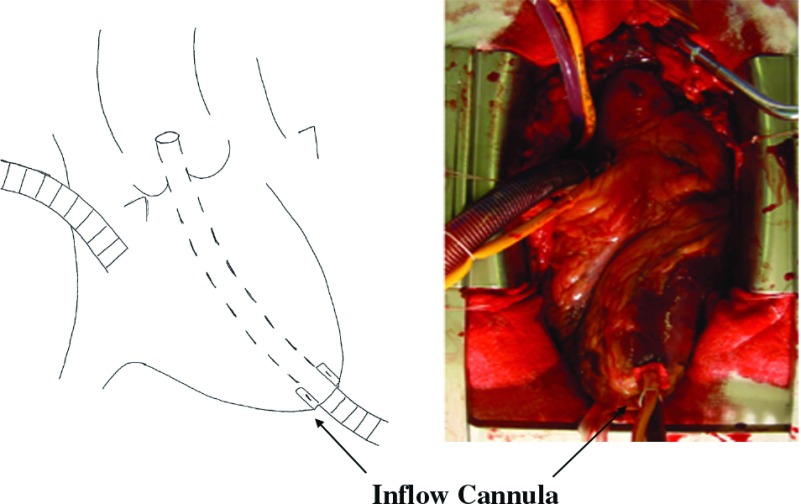
All patients were operated through a median sternotomy. Initially, all patients were operated using FA. After TAA cannulation was introduced in October 2004, arterial cannulation site was decided according to the patient’s status. In these days, almost all patients were operated using TAA cannulation, except contra indication case, such as severe aortic stenosis or re-do case. Our TAA cannularion technique is described below (Figure 1). After right atrium cannulation is performed for venous drainage, a 1-cm incision is made in the apex of the left ventricle with felt pledget suture, and a 7-mm cannula (Terumo Soft-flow Extended Aortic cannula) with stylet is passed through the apex and across the aortic valve until positioned in the ascending aorta at the level of sinotublar junction under transesophageal echocardiographic guidance. When the tip of the cannula is confirmed to be accurately situated in the true lumen under transesophageal echocardiographic guidance, CPB is established and systemic cooling is initiated. The position of aortic cannula is also confirmed by epiaortic echography. The left ventricular vent is inserted through the right superior pulmonary vein. For cerebral protection, we use moderate hypothermic circulatory arrest simply at the bladder temperatures 25°C without any cerebral perfusion. After circulatory arrest is achieved, the apical cannula is removed and the incision in the left ventricular apex is closed with interrupted sutures with felt pledgets. After ascending aorta is incised, cardioplegic solution is given selectively through the coronary ostium. Open distal anastomosis is performed and cardiopulmonary bypass is re-started through a 10-mm-diameter branch graft. A proximal anastomosis is performed during rewarming. Then ascending aortic replacement is accomplished.
Fig. 1.
Transapical aortic cannulation: Aortic cannla is passed through the left ventricular apex.
Statistical Analysis
Results were expressed as mean ± standard deviation. Statistical analysis was performed using Student t test for continuous variables or χ2 tests (Fisher exact tests if n ≦ 5) for categorical variables. Kaplan–Meier analysis was used to estimate late mortality in subjected groups. A probability value of <0.05 was considered significant. All statistical analyses were performed using SPSS 16.0 software (Dr. SPSS, Inc, Chicago, Illinois, USA).
Results
Patient Characteristics
Clinical background and preoperative clinical conditions of the patients were shown in Table 1. The two groups were similar with regard to preoperative patient characteristics except for the ratio of patent pseudo lumen (P = 0.016). There were more shock status patients in TAA group (23% vs 39%) without significances. Preoperative conscious disturbance was already occurred in 2 patients in FA group and 5 patients in TAA group. Merperfusion was observed 20% in FA group and 33% in TAA group. Therefore, there was some trend to select TAA cannulation for the patients with severe situation.
Table 1.
Patient’s characteristics
| Total (80) | FA (34) | TAA (46) | P value | |
|---|---|---|---|---|
| Age | 73.0 ± 10.5 | 74.5 ± 8.7 | 71.9 ± 11.7 | 0.26 |
| Male | 24 (30%) | 8 (24%) | 16 (35%) | 0.27 |
| DeBakey I | 40 (50%) | 16 (47%) | 24 (52%) | 0.65 |
| Hypertension | 54 (47%) | 20 (59%) | 34 (74%) | 0.15 |
| Conscious disturbance | 7 (12%) | 2 (5.8) | 5 (11%) | 0.43 |
| Shock status | 26 (33%) | 8 (23%) | 18 (39%) | 0.14 |
| Pericardial effusion | 36 (45%) | 15 (44%) | 21 (46%) | 0.89 |
| Malperfusion | 22 (28%) | 7 (20%) | 15 (33%) | 0.23 |
| Aortic regurgitation | 40 (50%) | 15 (44%) | 25 (54%) | 0.36 |
| Patent pseudo lumen | 43 (54%) | 13 (38%) | 30 (65%) | 0.016 |
FA: Femoral artery group; TAA: Transapical aortic group
Intraoperative Parameter
The Patients’ intraoperative parameter were listed in Table 2. The time from skin incision to starting CPB was significant shorter in the TAA group (45 ± 16 vs 23 ± 5.1 min; P <0.001). Operation time was also shorter in TAA group (249 ± 46 vs 222 ± 28 min; P <0.005). There were no differences between groups in terms of CPB time, circulatory arrest time, CPB flow, cooling time and rewarming time.
Table 2.
Operative result
| 列 1 | Total (80) | FA (34) | TAA (46) | P value |
|---|---|---|---|---|
| Skin incision ~ CPB start (min) | 33 ± 16 | 45 ± 16 | 23 ± 5.1 | <0.001 |
| Operation time (min) | 233 ± 40 | 249 ± 47 | 221 ± 29 | 0.01 |
| CPB time (min) | 148 ± 20 | 152 ± 30 | 141 ± 17 | 0.068 |
| Cross-clamp time (min) | 32 ± 7.1 | 75 ± 17 | 66 ± 15 | 0.016 |
| Circulatory arrest time (min) | 32 ± 7.1 | 32 ± 5.5 | 32 ± 7.8 | 0.89 |
| Mean CPB flow (L/min) | 3.6 ± 0.5 | 3.6 ± 0.5 | 3.5 ± 0.6 | 0.56 |
| Cooling time (min) | 29 ± 5.0 | 29 ± 6.0 | 29 ± 4.1 | 0.93 |
| Warming time (min) | 72 ± 15 | 72 ± 14 | 74 ± 15 | 0.89 |
| Re-exploration for bleeding | 7 (12%) | 0 (0%) | 7 (15%) | 0.017 |
| Respiratory failure | 11 (14%) | 5 (15%) | 6 (13%) | 0.83 |
| Seizure | 8 (10%) | 5 (15%) | 3 (7%) | 0.23 |
| Stroke | 11 (14%) | 6 (17%) | 5 (11%) | 0.38 |
| Renal failure | 4 (5%) | 3 (9%) | 1 (2%) | 0.21 |
| CK-MB (U/L) | 58 ± 61 | 50 ± 69 | 65 ± 54 | 0.44 |
| LVEF (%) | 67 ± 6.7 | 66 ± 5.6 | 68 ± 4.6 | 0.23 |
| 30-Day Mortality | 5 (6%) | 3 (8.8%) | 2 (4.3%) | 0.41 |
CPB: cardiopulmonary bypass; LVEF: Left Ventricular Ejection Fraction; FA: Femoral artery group; TAA: Transapical aortic group
Postoperative Mortality and Morbidities
Operative mortality and morbidities were listed in Table 2. We did not find any differences in postoperative mortality and morbidity between the groups except for re-exploration for bleeding. There was no cases of bleeding from left ventricular apex related to TAA cannulation.
Totally 5 operative mortality was occurred, 3 patients in FA group (8.8%), 2 patients in TAA group (4.3%) without significances (P = 0.41). Postoperative stroke was occurred 6 patients in FA group and 5 patients in TAA group. However, preoperative conscious disturbance was already occurred in two patients in FA group and five patients in TAA group. Postoperative left ventricular ejection fraction was normal in each group.
Mid-Term Survival
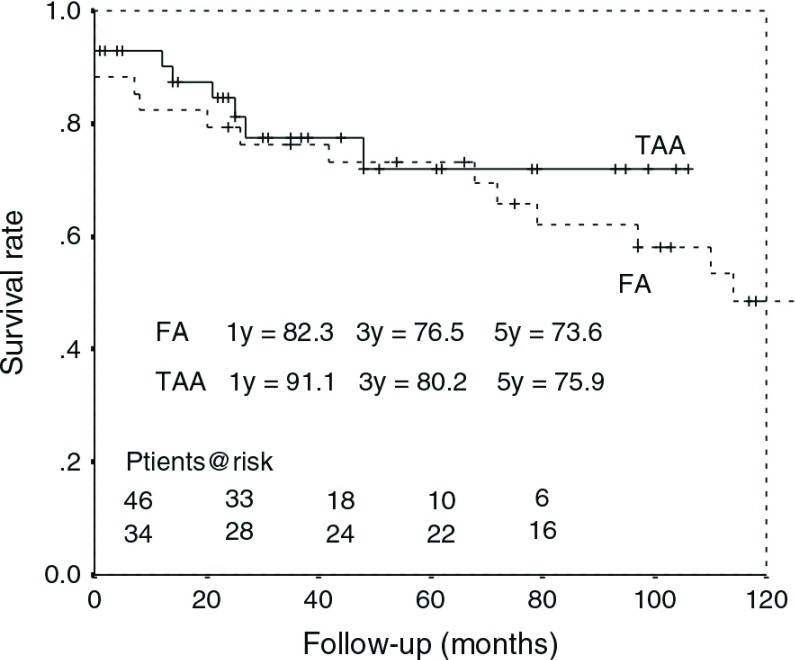
Survival rate was 88 ± 4%, 78 ± 5% and, 75 ± 5%, at 1, 3 and 5 years, respectively, in the entire cohort; 82 ± 6%, 77 ± 7% and 73 ± 8% at 1, 3 and 5 years, respectively, in the FA group; and 91 ± 4%, 80 ± 6% and 76 ± 7% at 1, 3 and 5 years, respectively, in the TAA group (Figure 2). There was no significant difference between groups (P = 0.26). Within 5 years postoperatively, there were six death in FA group and seven death in TAA group. Based on a multivariate analysis (Table 3), postoperative cerebral infarction and postoperative renal failure were significant risk factors for late death (odds ratio 4.15 and 3.76). The cannulation site was not a significant risk factor for late death in this analysis (P = 0.98).
Fig. 2.
Kaplan–Meier survival curve for the femoral versus the transapical cannulation group. FA: Femoral artery group, TAA: Transapical aortic group.
Table 3.
Multivariate analysis of risk factors for late death
| P-value | Odds Ratio | |
|---|---|---|
| Patent pseudo lumen | 0.2 | 0.43 |
| Malperfusion | 0.14 | 2.67 |
| Apical cannulation | 0.98 | 0.99 |
| P/O Stroke | 0.076 | 4.15 |
| P/O Respiratory failure | 0.88 | 0.89 |
| P/O Renal failure | 0.081 | 3.76 |
P/O: postoperative
Discussion
Acute aortic dissection is still a disease associated with a high mortality rate and various complications. Especially for AAAD, emergency surgery is required, and should be performed as soon as possible. Recently, the surgical outcomes for AAAD have been improved.1–5) Endovascular repair has also been reported to lead to acceptable results.11,12) However, it is still difficult to rescue some patients who have fallen into hypotensive shock and critical branch ischemia, such as cerebral infarction, myocardial infarction and intestinal infarction. In the case of critical organ ischemia, the rapid establishment of CPB for organ perfusion and cooling is essential to save the patient.
Our present results showed that TAA cannulation was safe and useful for AAAD repair. The time from skin incision to starting CPB was significantly shorter in the TAA group, and the total length of the operation was also shorter in the TAA group. The advantages of this technique are that it is simple, quick, and provides sufficient antegrade aortic flow and secured true lumen perfusion, which decrease the risk of embolization and malperfusion.
The femoral artery has been the standard cannulation site for cardiopulmonary bypass in treating acute type A aortic dissection. However, the retrograde blood stream sometime causes serious complications.6,7) On the other hand, the axillary artery is favored by many surgeons as an alternative cannulation site with the aim of avoiding cerebral atheroembolization, in order to prevent an extension of dissection, which can result in malperfusion, and to facilitate antegrade cerebral perfusion. However, this technique is time consuming and not always safe or reliable.8-10) Using a peripheral artery requires another skin incision in addition to median sternotomy, and requires a longer time to perform due to the more complicated procedures. Using a small artery might cause local complications, including dissections of another artery and insufficient flow, and often requires a side graft sewn to the vessel.13,14) Recently, direct cannulation into the dissected ascending aorta has been reported by several surgeons.15–18) This technique can be performed rapidly without injury to the peripheral arteries, but there are concerns that it may increase the risks of rupture, extension of the dissection and malperfusion.
TAA cannulation is an old technique that was initially described in the early 1970s.19) In 2006, Wada and colleagues reported the safety and usefulness of transapical aortic cannulation for establishing CPB for repairing an AAAD.20) In their report, the surgical mortality rate was high (18.8%) due to the severe preoperative condition of the patients. Almost all of the deaths were caused by organ ischemia from malperfusion that had been present before the operation.21–23) In this study, the surgical mortality rate was acceptable, at 8.8% in the FA group and 4.3% in the TAA group. Regarding the risk of postoperative stroke, six cases in the FA group (17%) and five cases (11%) in the TAA group had some neurological dysfunction. However, one of the six and three of the five had preoperative disturbance of consciousness. Therefore, these cases might have already had a cerebral infarction preoperatively. Immediate repair of the aorta from the onset of symptoms showed satisfactory recovery of consciousness and neurological function in patients with AAAD.24) TAA cannulation might reduce the postoperative organ dysfunction by preventing malperfusion and embolization due to the immediate repair of the aorta soon after the onset of symptoms. The postoperative re-exploration for the bleeding rate was higher in TAA group. However, there were no cases with bleeding from the left ventricular apex cannulation site. Postoperative echocardiography showed good left ventricular function in each group, without any effect on the TAA cannulation. The contraindications for TAA cannulation are considered to include two situations. The first is if the patient has severe aortic stenosis, where the aortic cannula cannot pass the aortic valve. The second is if the patient has severe pericardial adhesions due to a previous cardiac operation or pericarditis, because this makes it difficult to establish TAA cannulation.
In this study, there was a trend toward a lower mortality rate in the patients in the TAA group. Our early outcomes in the TAA group, 4.3% mortality and 11% stroke, are acceptable compared with other reports not only about FA cannulation,25) and ascending cannulation,15–18) but also with axillary cannulation, 8-10) in the treatment of AAAD. As described above, there are advantages and disadvantages to each cannulation technique. However, our results suggest that TAA cannulation is a safe alternative method for the treatment of AAAD.
This study also investigated the mid-term survival after surgical treatment for AAAD, comparing the different cannulation techniques. The calculated five-year survival rate was 73.3% in the FA group and 75.9% in the TAA group. Based on a multivariate analysis, postoperative cerebral infarction and postoperative renal failure were significant risk factors for late death. The cannulation technique had no impact on the mid-term survival. However, the cumulative survival of the entire cohort of patients was comparable to that of other reports.18)
Limitations
This was a single-institution retrospective study, not a randomized trial. The cannulation site was not chosen at random, but was selected individually according to the patient’s status and surgeon’s preference. Thus, a patient selection bias and surgeon preference bias were inevitable in this study. This study cohort was relatively small, and a larger number of patients need to be evaluated to confirm whether there is a significant difference in the 30-day mortality. In addition, this series is a report of a single institutional experience, and the results may not be generalized to other settings. Nevertheless, we consider that the aim of this study was accomplished, and we demonstrated the safety of the TAA cannulation technique in comparison with the FA cannulation technique.
Conclusion
The cannulation site should be chosen according to the patient’s pathology and status, and the present study suggests that TAA cannulation in patients with AAAD can be a safe alternative, offering acceptable early and midterm outcomes.
Disclosure Statement
None.
References
- 1).Comas GM, Leshnower BG, Halkos ME, et al. Acute type a dissection: impact of antegrade cerebral perfusion under moderate hypothermia. Ann Thorac Surg 2013; 96: 2135-41. [DOI] [PubMed] [Google Scholar]
- 2).Rylski B, Hoffmann I, Beyersdorf F, et al. Acute aortic dissection type A: age-related management and outcomes reported in the German Registry for Acute Aortic Dissection Type A (GERAADA) of over 2000 patients. Ann Surg. 2014; 259: 598-604. [DOI] [PubMed] [Google Scholar]
- 3).Tanaka M, Kimura N, Yamaguchi A, et al. In-hospital and long-term results of surgery for acute type A aortic dissection: 243 consecutive patients. Ann Thorac Cardiovasc Surg 2012; 18: 18-23. [DOI] [PubMed] [Google Scholar]
- 4).Olsson C, Hillebrant CG, Liska J, et al. Mortality in acute type A aortic dissection: validation of the Penn classification. Ann Thorac Surg 2011; 92: 1376-82. [DOI] [PubMed] [Google Scholar]
- 5).Sun L, Qi R, Zhu J, et al. Repair of acute type A dissection: our experiences and results. Ann Thorac Surg. 2011; 91: 114-52. [DOI] [PubMed] [Google Scholar]
- 6).Bobicsek F, Guarino RL. Compressing of the true lumen by retrograde perfusion during repair of aortic dissection. J Cardiovasc Surg. 1985; 26: 36-40. [PubMed] [Google Scholar]
- 7).Goldstein LJ, Davies RR, Rizzo JA, et al. Stroke in surgery of the thoracic aorta: incidence, impact, etiology, and prevention. J Thorac Cardiovasc Surg 2001; 122: 935-45. [DOI] [PubMed] [Google Scholar]
- 8).Schachner T, Nagiller J, Zimmer A, et al. Technical problems and complications of axillary artery cannulation. Eur J Cardiothorac Surg 2005; 27: 634-7. [DOI] [PubMed] [Google Scholar]
- 9).Sinclair MC, Singer RL, Manley NJ, et al. Cannulation of the axillary artery for cardiopulmonary bypass: safeguards and pitfalls. Ann Thorac Surg 2003; 75: 931-4. [DOI] [PubMed] [Google Scholar]
- 10).Imanaka K, Kyo S, Tanabe H, et al. Fatal intraoperative dissection of the innominate artery due to perfusion through the right axillary artery. J Thorac Cardiovasc Surg 2000; 120: 405-6. [DOI] [PubMed] [Google Scholar]
- 11).Chang Q, Tian C, Wei Y, et al. Hybrid total arch repair without deep hypothermic circulatory arrest for acute type A aortic dissection (R1). J Thorac Cardiovasc Surg 2013; 146: 1393-8. [DOI] [PubMed] [Google Scholar]
- 12).Lu Q1, Feng J, Zhou J, et al. Endovascular repair of ascending aortic dissection: a novel treatment option for patients judged unfit for direct surgical repair. J Am Coll Cardiol. 2013; 61: 1917-24. [DOI] [PubMed] [Google Scholar]
- 13).Schachner T, Vertacnik K, Laufer G, et al. Axillary artery cannulation in surgery of the ascending aorta and the aortic arch. Eur J Cardiothorac Surg 2002; 22: 445-7. [DOI] [PubMed] [Google Scholar]
- 14).Sinclair MC, Singer RL, Manley NJ, et al. Cannulation of the axillary artery for cardiopulmonary bypass: safeguards and pitfalls. Ann Thorac Surg 2003; 75: 931-4. [DOI] [PubMed] [Google Scholar]
- 15).Minatoya K, Karck M, Szpakowski E, et al. Ascending aortic cannulation for Stanford type A acute aortic dissection: another option. J Thorac Cardiovasc Surg 2003; 125: 952-3. [DOI] [PubMed] [Google Scholar]
- 16).Reece TB, Tribble CG, Smith RL, et al. Central cannulation is safe in acute aortic dissection repair. J Thorac Cardiovasc Surg 2007; 133: 428-34. [DOI] [PubMed] [Google Scholar]
- 17).Khaladj N, Shrestha M, Peterss S, et al. Ascending aortic cannulation in acute aortic dissection type A: the Hannover experience. Eur J Cardiothorac Surg 2008; 34: 792-6; disussion 796. [DOI] [PubMed] [Google Scholar]
- 18).Kamiya H, Kallenbach K, Halmer D, et al. Comparison of ascending aorta versus femoral artery cannulation for acute aortic dissection type A. Circulation. 2009; 120: (11 Suppl); S282-6. [DOI] [PubMed] [Google Scholar]
- 19).Zwart HH, Kralios A, Kwan-Gett CS, et al. First clinical application of transarterial closed-chest left ventricular (TaCLV) bypass. Trans Am Soc Artif Intern Organs 1970; 16: 386-91. [PubMed] [Google Scholar]
- 20).Wada S, Yamamoto S, Honda J, et al. Transapical aortic cannulation for cardiopulmonary bypass in type A aortic dissection operations. J Thorac Cardiovasc Surg. 2006; 132: 369-72. [DOI] [PubMed] [Google Scholar]
- 21).Bossone E, Corteville DC, Harris KM, et al. Stroke and outcomes in patients with acute type A aortic dissection. Circulation. 2013; 128: (11 Suppl 1); S175-9. [DOI] [PubMed] [Google Scholar]
- 22).Orihashi K. Malperfusion in acute type a aortic dissection: unsolved problem. Ann Thorac Surg 2013; 95: 1570-6. [DOI] [PubMed] [Google Scholar]
- 23).Pacini D, Leone A, Belotti LM, et al. Acute type A aortic dissection: significance of multiorgan malperfusion. Eur J Cardiothorac Surg 2013; 43: 820-6. [DOI] [PubMed] [Google Scholar]
- 24).Tsukube T, Hayashi T, Kawahira T, et al. Neurological outcomes after immediate aortic repair for acute type A aortic dissection complicated by coma. Circulation. 2011; 124: (11 Suppl); S163-7. [DOI] [PubMed] [Google Scholar]
- 25).Fusco DS, Shaw RK, Tranquilli M, et al. Femoral cannulation is safe for type A dissection repair. Ann Thorac Surg 2004; 78: 1285-9; discussion 1285-9. [DOI] [PubMed] [Google Scholar]