Abstract
Convergence of multiple stromal cell types is required to develop a tumorigenic niche that nurtures the initial development of cancer and its dissemination. Although the immune and vascular systems have been shown to have strong influences on cancer, a growing body of evidence points to a role of the nervous system in promoting cancer development. This review discusses past and current research that shows the intriguing role of autonomic nerves, aided by neurotrophic growth factors and axon cues, in creating a favorable environment for the promotion of tumor formation and metastasis.
Abbreviations
- Ach
acetylcholine
- Adrβ
adrenergic receptor
- ANS
autonomic nervous system
- BDNF
brain-derived eurotrophic factor
- BMDC
bone marrow-derived cells
- Chrm1
type 1 cholinergic muscarinic receptor
- DCC
deleted in colorectal cancer
- EGF
epidermal growth factor
- Eph
Ephrin receptor
- FGF2
fibroblast growth factor 2
- G-CSF
granulocyte colony-stimulating factor
- HPA
hypothalamic-pituitary-adrenal axis
- MSC
mesenchymal stem cells
- MDSC
myeloid cell-derived suppressor cells
- NE
Norepinephrine
- NGF
nerve growth factor
- NT
neurotrophin
- PIN
prostate intraepithelial neoplasia
- PNI
perineural invasion
- PNS
parasympathetic nervous system
- Robo
slits-roundabout receptor
- SAM
sympathetic-adrenal-medullary axis
- SGZ
subgranular zone
- SVZ
subventricular zone
- SNS
sympathetic nervous system
- TAM
tumor-associated macrophages
- TEM
Tie-2-expressing monocytes
- TGF
transforming growth factor
- VEGF
vascular endothelial growth factor
Although the emergence of malignant cancer cells is the result of interplay between genetic and epigenetic alterations of epithelial cells, numerous studies have shown the influence of the tumorigenic microenvironment.1 Indeed, a tumor seems to behave as an organ within which the microenvironment affects the gene expression and phenotype of cancer cells.2,3 For example, teratocarcinoma cells can form tumors when implanted in the flank of 129/SV mice, but are unable to develop cancer when placed in the blastocyst of a pseudopregnant C57BL/6 mouse.1,4 Conversely, implantation of normal mammary epithelial cells in an activated microenvironment induced through overexpression of different cytokines by fibroblasts is sufficient to induce the development of invasive carcinomas.5-7 Cancer cells, in turn, recruit and corrupt normal cell types of the stroma, such as bone marrow-derived or endothelial cells, to support the initial phases of tumor formation and also promote tumor cell dissemination (Fig. 1). Recent studies have revealed that sympathetic and parasympathetic nerve fibers from the autonomic nervous system (ANS) infiltrate prostate or gastric tumors and contribute to the early stages of prostate cancer development, as well as tumor invasion and metastasis.8-10
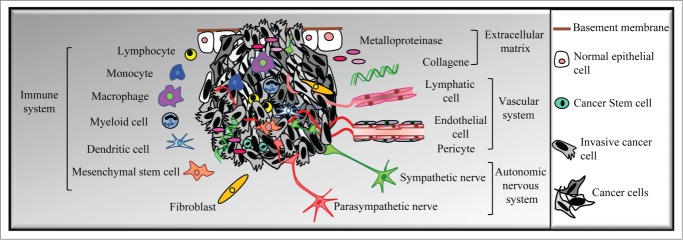
Figure 1.
Tumor heterogeneity. Primary tumors contain different phenotypic profiles of cancer cells as a result of genetic or epigenetic changes. In addition, the tumor microenvironment—including bone marrow-derived cells (BMDCs) such as tumor-associated macrophages (TAM), myeloid cell-derived suppressor cells (MDSC), mesenchymal stem cells (MSC), or Tie2-expressing monocytes (TEM); fibroblasts; endothelial and lymphatic cells; extracellular matrix; and autonomic nerve fibers—further increases tumor heterogeneity by promoting the survival, proliferation, and dissemination of cancer cells. (Adapted from Magnon. Med Sci, 2013).
Activation of Neural Pathways During Tumor Development
Thirty years ago, J.G. Batsakis was the first to describe the presence of large nerves located in the vicinity of human epithelial carcinomas, such as head and neck, gastric, or prostate cancers.11-13 These nerves were described as paths of metastatic spread through a process called perineural invasion (PNI), in which neoplastic cancer cells are able to invade and migrate in, around, and through the nerves. PNI is frequently associated with poor clinical outcomes. A striking illustration of this process was provided in a cohort of prostate cancer patients in whom the presence of PNI was correlated to poor prognosis compared with patients without pathological evidence of PNI.14 Despite increasing clinical recognition of this pathological entity, the molecular and cellular mechanisms of PNI are not yet well understood. Previous studies have established connections between cancer and the nervous system.15,16 In vitro, cancer–nerve interactions were imaged in cultures of sensory neurons from dorsal root ganglia that exhibit directional neurite outgrowth toward prostate cancer cells.17 In vivo, the number of neurons per ganglia is increased in prostate cancer patients, suggesting that nerves play a role in tumor progression.16
The role of the hypothalamic-pituitary-adrenal and sympathetic-adrenal-medullary axes in cancer
The findings described above complement further observations on the influence of social networks on health.18 Social factors or stressors might predict health outcomes among cancer patients,19 and epidemiological data show that psychological and social stress factors may be associated with cancer onset.20 Similarly, studies performed decades ago on animal models revealed that the neural stimulation associated with chronic or prolonged stress promotes tumor growth and cancer cell dissemination and decreases survival.21-25 The authors did not propose any mechanism but invoked that activation of the hypothalamic–pituitary–adrenal (HPA) axis might support the effect of stress on tumor incidence through the secretion of corticosteroids by the adrenal gland.19,26 Glucocorticoids control growth and metabolism, suppress immune responses, and mediate the stress response to a variety of peripheral organs.27 Activation of the HPA axis could play a role in termination of the stress response in cancer by altering the immunological control of cancer cells through loss or inactivation of T cells.19 However, it was recently found that chronically stressed mice had increased numbers of neutrophils, monocytes, and lymphocytes in the blood as a result of activation of proliferation of hematopoietic stem cells in the bone marrow associated with an increased expression of adrenergic neurotransmitters.28 Thus, it seems less likely that the HPA axis might control stress-induced tumor development through the immunosuppressive activity of corticosteroid hormones. Another possible explanation for tumor formation associated with stress might rely on the activation of the sympathetic nervous system (SNS) through the sympathetic–adrenal–medullary (SAM) axis, which controls the release of adrenergic neurotransmitters such as epinephrine or norepinephrine by the adrenals into the bloodstream in support of the fight-or-flight reflex. Involvement of adrenergic neurotransmitters in cancer has recently been revealed from studies monitoring tumor formation through pharmacological manipulations of the β-adrenergic signaling pathway.29-31 Mice subjected to chronic stress conditions display an ovarian tumor burden associated with high blood levels of adrenergic neurotransmitters and increased expression of the vascular endothelial growth factor (VEGF) and metalloproteinases, leading to the development of abundant tumor vascularization.29 Pharmacological blockade of β2 adrenergic receptors expressed on cancer cells leads to regulation of VEGF gene expression and cancer cell apoptosis.29,30 The β-adrenergic signaling pathway is also able to regulate VEGF expression in adipose tissue and different cancer cell lines.32,33 An intriguing study showed that putting animals in an enriched living environment increases expression of the gene encoding brain-derived neurotrophic factor (BDNF) in the hypothalamus of the mouse brain.34 In turn, BDNF downregulates the production of leptin in adipocytes through activation of β-adrenergic receptors in white adipose tissue, leading to delayed tumor growth and increased survival. Taken together, these data suggest a key role for stress-related β-adrenergic signaling in the development of carcinoma, rather than the neuroendocrine regulation of cancer cell behavior via glucocorticoid release. This further raises the intriguing possibility that catecholamines might be able to bind different cell targets, depending on the type of cancer and leading to opposite activities. However, further research is needed to explain the divergent activity of the β-adrenergic signaling pathway in stress-mediated cancer.
The autonomic nervous system as a potential regulator of cancer
Activation of the sympathetic nervous system leads to the release of catecholamine neurotransmitters from the adrenal glands into the bloodstream through the SAM axis. Nevertheless, the uncertain role of the adrenal gland in controlling solid tumors35 led us to consider an alternative hypothesis in which the sympathetic branch of the autonomic nervous system directly modulates cancer development through the local release of adrenergic neurotransmitters in peripheral tissues. Nerves partner with blood and lymphatic vessels throughout the body from development to adulthood.36 Whereas tumor angiogenesis and lymphangiogenesis have been extensively explored,37,38 the presence and spreading of nerves into tumors has remained elusive. Recent studies indicated that autonomic adrenergic nerve fibers may infiltrate prostate adenocarcinoma and control malignant progression through the release of catecholamine neurotransmitters in the tumor microenvironment.8 As summarized in Figure 2, chemical or surgical ablation of sympathetic adrenergic nerves prevents the formation of xenogeneic orthotopic or transgenic prostate tumors. Genetic depletion of β2- and β3-adrenergic receptors in the tumor microenvironment alters the transmission of adrenergic signals involved in the early phases of tumor development. Furthermore, the ANS is divided into 2 subsystems—the SNS and the parasympathetic nervous system (PNS)—that work in tandem in homeostasis. In cancer mouse models, parasympathetic cholinergic signaling has been identified as a key regulator of tumor invasion and metastatic spread by activation of the type 1 cholinergic muscarinic receptor (Chrm1) expressed in the stroma. Pharmacological and genetic approaches recapitulate the mechanism by which parasympathetic nerves in tumor tissues might be able to release acetylcholine that binds to Chrm1-expressing cell targets in the stroma. An additional blinded analysis of nerve densities in treatment-naïve patients with prostate adenocarcinoma revealed that sympathetic and parasympathetic nerve densities are significantly higher in tumors classified as high risk and that nerve density is associated with poor clinical outcome. Based on these data, both branches of the ANS have distinct but complementary functions in prostate tumor development, suggesting that tumor nerves might interact with different partners during the course of cancer.8,9
Figure 2.
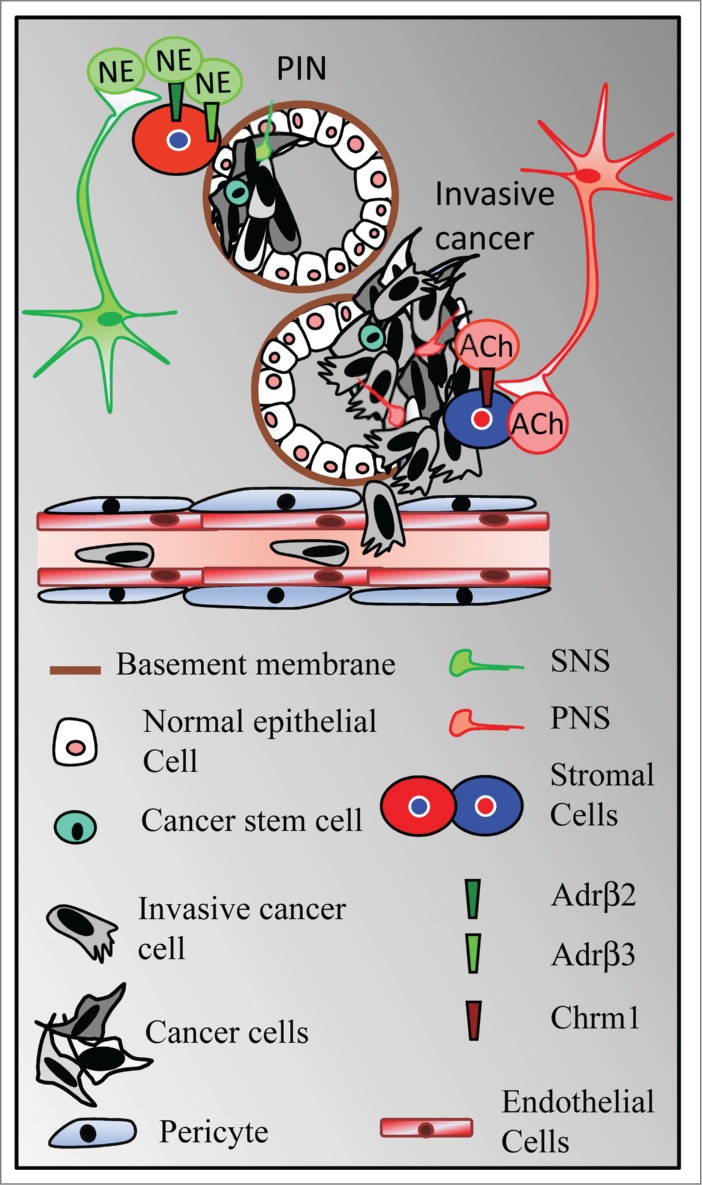
The autonomic nervous system contributes to tumor initiation and progression. Whereas the sympathetic nervous system (SNS) controls the early phases of tumor formation through activation of β2- and β3-adrenergic receptors (Adrβ2 et Adrβ3) expressed in the stroma, the parasympathetic nervous system (PNS) promotes tumor cell dissemination through activation of the type 1 muscarinic receptor (Chrm1) expressed in the tumor microenvironment. NE, Norepinephrine; Ach, Acetylcholine; PIN, prostate intraepithelial neoplasia. (Adapted from Magnon. Med Sci, 2013).
Neurotrophic Factors and Axon Guidance Molecules in Cancer
The formation of nerves, a process called neurogenesis, occurs throughout life in restricted neurogenic regions of the brain—the subgranular zone (SGZ) in the dentate gyrus of the hippocampus and the subventricular zone (SVZ) of the lateral ventricules.39 This process is finely tuned and requires a variety of stimuli including neural growth factors, cytokines, neurotrophins, and axon guidance molecules for the activation of neural precursors within the neurogenic niche as well as for neuronal development and plasticity. For example, epidermal growth factor (EGF) and fibroblast growth factor 2 (FGF2) support the maintenance of adult neural stem cells in vitro and promote proliferation of neural precursors in the SVZ. The brain-derived neurotrophic factor also regulates neurogenesis in the brain.40
Similarly, a process of neurogenesis might occur in cancer as directional outgrowth of neurites from the dorsal root ganglia toward prostate cancer cells has been observed in vitro.17 Indeed, cancer cells produce and release neurotrophic factors and axon guidance molecules that might be able to orchestrate axon outgrowth, pruning, and remodeling similar to the release of angiogenic growth factors to promote blood supply to tumors.41-43 Conversely, the environment of the nerves surrounding tumors, which is particularly enriched in nerve-derived growth factors, promotes the survival and growth of cancer cells.44,45 Additional clinical data support the potential processes of axonogenesis and neurogenesis in prostate tumors.16 Cancer patients possess larger prostate ganglia associated with an increased number of neurons. These data suggest the secretion of soluble factors by cancer cells that might promote nerve sprouting or branching.16 In support of these observations, nerve growth factor (NGF) has been identified in a variety of cancers, such as breast or prostate cancer, as a key regulator of tumor apoptosis, angiogenesis, and bone cancer pain.46-48 Targeting NGF with sequestering antibodies prevents nerve sprouting, angiogenesis, and tumor-induced pain.46,48,49 BDNF has been identified in various carcinomas compared with healthy tissues, suggesting a specific role of BDNF in cancer development.50-52 The neurotrophins (NT) family supports survival and proliferation of multiple cancers through deregulation of the PI3K/Akt and Ras/MEK/MAPK pathways.53-55 Aberrant expression of FGF ligands and their cognate receptors leads to the activation of downstream pathways involved in cancer progression and tumor angiogenesis.56 Although FGF determines neuronal survival and proliferation during development and adulthood, it has been also described as a pivotal regulator of neuronal migration, guidance, and synaptogenesis, suggesting a potential role in controlling nerve development in cancer.57 Similarly, granulocyte colony-stimulating factor (G-CSF), the most commonly used hematopoietic stem cell mobilizer, acts as a bifunctional growth factor: it can stimulate the hematopoietic system while activating the nervous system. In mice, G-CSF elicits autonomic nerve survival, outgrowth, and spreading in prostate tumors, leading to tumor formation and dissemination.58 Multiple functions of transforming growth factors (TGFs)/bone morphogenetic proteins have been described in the regulation of several life processes and in the tumor microenvironment.59 The role of TGFs in neuronal development, such as the regulation of nerve survival or tissue repair, suggests further potential influences on tumor neurogenesis.60
In addition to these growth factors, axon guidance molecules have been revealed as intriguing partners in tumor progression.61-63 The axons of developing neurons actively extend or retract under different circumstances in response to 4 families of guidance cues—netrins, slits, ephrins and semaphorins—that respectively bind UNC5 and deleted in colorectal cancer (DCC) receptors, slits-roundabout receptors (Robos), Ephrin receptors (Eph), and plexins or neuropilins.64 In some instances, netrins determine axon outgrowth and provide guidance in the central nervous system as well as in vascular development, as nerves and vessels share cellular and molecular mechanisms to orchestrate the development of their reciprocal networks.65-67 Also, netrins have been identified in peripheral organs and play a role in tumorigenesis by preventing cancer cell apoptosis. Controversial data preclude any definite conclusions on a potential direct effect of netrins on cancer cell migration;61 however, their function in guidance of axons or vessels makes them attractive possible targets for inhibiting tumor angiogenesis and neurogenesis. In addition to their role in axon guidance that plays a role in vessel development, major vascular growth factors such as the vascular endothelial growth factor (VEGF) regulate development of the nervous system and increase neuronal plasticity during development and in the adult.67 In the periphery, autonomic sympathetic axons follow arteries that release neurotrophic factors such as artemin and endothelin, which are necessary for axon outgrowth.68,69 Conversely, the SNS regulates angiogenesis and arteriogenesis in hindlimb ischemic models.70,71 This suggests an intricate interface between nerves and vessels that might support the development of both networks in cancer.
Perspectives: The Autonomic Nervous System as a Therapeutic Target
The finding that the autonomic nervous system promotes the development and progression of prostate or gastric cancer may open up neurogenesis as a frontier for cancer drug development.8-10 Sympathetic nerve fibers play an intriguing role in helping tumors to grow and develop by interacting with β2- and β3-adrenergic receptors on stromal cells. In mice, a deficiency in β-adrenergic receptors impairs prostate cancer formation. These data are consistent with recent epidemiological reports that men with prostate adenocarcinoma who take non-selective β blockers have lower prostate cancer-specific mortality rates.72,73 Additional clinical studies describe a similar activity of β blockers in melanoma or breast cancer patients,74,75 indicating that adrenergic signaling might be involved in various types of cancer. Whereas existing β blockers primarily bind the β1-adrenergic receptor, future drug development would aim to selectively target β2- and β3-adrenergic receptors.
In addition, inhibition of NGF or BDNF has been shown to impair cancer cell proliferation as well as tumor angiogenesis and growth.46,49,76 Based on an intriguing parallel between neural and vascular development, VEGF and VEGFR have emerged as potent regulators of neurogenesis and neural plasticity, and may represent therapeutic targets for cancer. However, further research is needed to identify the mechanism by which tumor progression overcomes VEGF therapy.77,78 Several FGFR tyrosine kinase inhibitors are in the early phases of clinical trials.56 The high similarity between the kinase domains of FGFR, PDGFR, and VEGFR provides the advantage of possibly targeting both vessel and nerve development and overriding resistance to antiangiogenic drugs. Intervening in the TGFβ signaling pathway may have relevant effects in the stroma, leading to inhibition or reversal of changes in the microenvironment during tumor progression, and clinical trials have assessed the use of inhibitory antibodies or small molecule inhibitors targeting TGFs.79
In addition to the compelling evidence that autonomic nerves contribute to cancer progression through adrenergic or cholinergic signaling, this research raises questions over which mechanisms control cancer-related neurogenesis and, conversely, which biological systems or pathways might be regulated by the nervous system. Understanding the specific mechanisms of cancer–nerve interactions is pivotal to the development of novel cancer therapies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Magnon laboratory is supported by an Atomic Energy Commission (CEA) starting grant for research leaders and an ATIP-AVENIR group leader grant (INSERM-CNRS).
References
- 1. Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 2011; 17:320-9; PMID:21383745; http://dx.doi.org/ 10.1038/nm.2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21:309-22; PMID:22439926; http://dx.doi.org/ 10.1016/j.ccr.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 3. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646-74; PMID:21376230; http://dx.doi.org/ 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 4. Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Nat Acad Sci U S A 1975; 72:3585-9; PMID:1059147; http://dx.doi.org/ 10.1073/pnas.72.9.3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, Richardson A, Weinberg RA. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Nat Acad Sci U S A 2004; 101:4966-71; PMID:15051869; http://dx.doi.org/ 10.1073/pnas.0401064101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 2004; 303:848-51; PMID:14764882; http://dx.doi.org/ 10.1126/science.1090922 [DOI] [PubMed] [Google Scholar]
- 7. Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005; 121:335-48; PMID:15882617; http://dx.doi.org/ 10.1016/j.cell.2005.02.034 [DOI] [PubMed] [Google Scholar]
- 8. Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS. Autonomic nerve development contributes to prostate cancer progression. Science 2013; 341:1236361; PMID:23846904; http://dx.doi.org/ 10.1126/science.1236361 [DOI] [PubMed] [Google Scholar]
- 9. Isaacs JT. Cancer. Prostate cancer takes nerve. Science 2013; 341:134-5; PMID:23846894; http://dx.doi.org/ 10.1126/science.1241776 [DOI] [PubMed] [Google Scholar]
- 10. Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, Flatberg A, Johannessen H, Friedman RA, Renz BW, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med 2014; 6:250ra115; PMID:25143365; http://dx.doi.org/ 10.1126/scitranslmed.3009569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Batsakis JG. Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol 1985; 94:426-7; PMID:4026129 [PubMed] [Google Scholar]
- 12. Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev 2010; 21:77-82; PMID:20060768; http://dx.doi.org/ 10.1016/j.cytogfr.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 13. Rodin AE, Larson DL, Roberts DK. Nature of the perineural space invaded by prostatic carcinoma. Cancer 1967; 20:1772-9; PMID:6058181; http://dx.doi.org/ 10.1002/1097-0142(196710)20:10%3c1772::AID-CNCR2820201028%3e3.0.CO;2- [DOI] [PubMed] [Google Scholar]
- 14. Anderson PR, Hanlon AL, Patchefsky A, Al-Saleem T, Hanks GE. Perineural invasion and Gleason 7-10 tumors predict increased failure in prostate cancer patients with pretreatment PSA . Int J Radiat Oncol Biol Phys 1998; 41:1087-92; PMID:9719119; http://dx.doi.org/ 10.1016/S0360-3016(98)00167-9 [DOI] [PubMed] [Google Scholar]
- 15. Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer 2009; 115:3379-91; PMID:19484787; http://dx.doi.org/ 10.1002/cncr.24396 [DOI] [PubMed] [Google Scholar]
- 16. Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, Shine D, Kadmon D, Thompson T, Miles BJ, et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res 2008; 14:7593-603; PMID:19047084; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-1164 [DOI] [PubMed] [Google Scholar]
- 17. Ayala GE, Wheeler TM, Shine HD, Schmelz M, Frolov A, Chakraborty S, Rowley D. In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate 2001; 49:213-23; PMID:11746267; http://dx.doi.org/ 10.1002/pros.1137 [DOI] [PubMed] [Google Scholar]
- 18. Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol 1979; 109:186-204; PMID:425958 [DOI] [PubMed] [Google Scholar]
- 19. Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 2005; 5:243-51; PMID:15738954 [DOI] [PubMed] [Google Scholar]
- 20. Penninx BW, Guralnik JM, Pahor M, Ferrucci L, Cerhan JR, Wallace RB, Havlik RJ. Chronically depressed mood and cancer risk in older persons. J Nat Cancer Inst 1998; 90:1888-93; PMID:9862626; http://dx.doi.org/ 10.1093/jnci/90.24.1888 [DOI] [PubMed] [Google Scholar]
- 21. Riley V. Mouse mammary tumors: alteration of incidence as apparent function of stress. Science 1975; 189:465-7; PMID:168638; http://dx.doi.org/ 10.1126/science.168638 [DOI] [PubMed] [Google Scholar]
- 22. Sklar LS, Anisman H. Stress and coping factors influence tumor growth. Science 1979; 205:513-5; PMID:109924; http://dx.doi.org/ 10.1126/science.109924 [DOI] [PubMed] [Google Scholar]
- 23. Simon RH, Lovett EJ, 3rd, Tomaszek D, Lundy J. Electrical stimulation of the midbrain mediates metastatic tumor growth. Science 1980; 209:1132-3; PMID:6250220; http://dx.doi.org/ 10.1126/science.6250220 [DOI] [PubMed] [Google Scholar]
- 24. Visintainer MA, Volpicelli JR, Seligman ME. Tumor rejection in rats after inescapable or escapable shock. Science 1982; 216:437-9; PMID:7200261; http://dx.doi.org/ 10.1126/science.7200261 [DOI] [PubMed] [Google Scholar]
- 25. Hermes GL, Delgado B, Tretiakova M, Cavigelli SA, Krausz T, Conzen SD, McClintock MK. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc Nat Acad Sci U S A 2009; 106:22393-8; PMID:20018726; http://dx.doi.org/ 10.1073/pnas.0910753106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol 2004; 5:617-25; PMID:15465465; http://dx.doi.org/ 10.1016/S1470-2045(04)01597-9 [DOI] [PubMed] [Google Scholar]
- 27. Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol 2005; 67:259-84; PMID:15709959; http://dx.doi.org/ 10.1146/annurev.physiol.67.040403.120816 [DOI] [PubMed] [Google Scholar]
- 28. Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med 2014; 20:754-8; PMID:24952646; http://dx.doi.org/ 10.1038/nm.3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 2006; 12:939-44; PMID:16862152; http://dx.doi.org/ 10.1038/nm1447 [DOI] [PubMed] [Google Scholar]
- 30. Hassan S, Karpova Y, Baiz D, Yancey D, Pullikuth A, Flores A, Register T, Cline JM, D'Agostino R, Jr, Danial N, et al. Behavioral stress accelerates prostate cancer development in mice. J Clin Invest 2013; 123:874-86; PMID:23348742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shahzad MM, Arevalo JM, Armaiz-Pena GN, Lu C, Stone RL, Moreno-Smith M, Nishimura M, Lee JW, Jennings NB, Bottsford-Miller J, et al. Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J Biol Chem 2010; 285:35462-70; PMID:20826776; http://dx.doi.org/ 10.1074/jbc.M110.109579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fredriksson JM, Lindquist JM, Bronnikov GE, Nedergaard J. Norepinephrine induces vascular endothelial growth factor gene expression in brown adipocytes through a beta -adrenoreceptor/cAMP/protein kinase A pathway involving Src but independently of Erk1/2. J Biol Chem 2000; 275:13802-11; PMID:10788502; http://dx.doi.org/ 10.1074/jbc.275.18.13802 [DOI] [PubMed] [Google Scholar]
- 33. Lutgendorf SK, Cole S, Costanzo E, Bradley S, Coffin J, Jabbari S, Rainwater K, Ritchie JM, Yang M, Sood AK. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res: An Off J Am Assoc Cancer Res 2003; 9:4514-21; PMID:14555525 [PubMed] [Google Scholar]
- 34. Cao L, Liu X, Lin EJ, Wang C, Choi EY, Riban V, Lin B, During MJ. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell 2010; 142:52-64; PMID:20603014; http://dx.doi.org/ 10.1016/j.cell.2010.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peters LJ, Kelly H. The influence of stress and stress hormones on the transplantability of a non-immunogenic syngeneic murine tumor. Cancer 1977; 39:1482-8; PMID:856441; http://dx.doi.org/ 10.1002/1097-0142(197704)39:4%3c1482::AID-CNCR2820390420%3e3.0.CO;2-P [DOI] [PubMed] [Google Scholar]
- 36. Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature 2005; 436:193-200; PMID:16015319; http://dx.doi.org/ 10.1038/nature03875 [DOI] [PubMed] [Google Scholar]
- 37. Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Disc 2007; 6:273-86; PMID:17396134; http://dx.doi.org/ 10.1038/nrd2115 [DOI] [PubMed] [Google Scholar]
- 38. Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Investigat 2014; 124:878-87; PMID:24590272; http://dx.doi.org/ 10.1172/JCI71603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 2011; 70:687-702; PMID:21609825; http://dx.doi.org/ 10.1016/j.neuron.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell 2008; 132:645-60; PMID:18295581; http://dx.doi.org/ 10.1016/j.cell.2008.01.033 [DOI] [PubMed] [Google Scholar]
- 41. Arakawa H. Netrin-1 and its receptors in tumorigenesis. Nat Rev Cancer 2004; 4:978-87; PMID:15573119 [DOI] [PubMed] [Google Scholar]
- 42. Dolle L, El Yazidi-Belkoura I, Adriaenssens E, Nurcombe V, Hondermarck H. Nerve growth factor overexpression and autocrine loop in breast cancer cells. Oncogene 2003; 22:5592-601; PMID:12944907; http://dx.doi.org/ 10.1038/sj.onc.1206805 [DOI] [PubMed] [Google Scholar]
- 43. Hondermarck H. Neurotrophins and their receptors in breast cancer. Cytokine Growth Factor Rev 2012; 23:357-65; PMID:22749855; http://dx.doi.org/ 10.1016/j.cytogfr.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 44. Ayala GE, Dai H, Ittmann M, Li R, Powell M, Frolov A, Wheeler TM, Thompson TC, Rowley D. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res 2004; 64:6082-90; PMID:15342391; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-0838 [DOI] [PubMed] [Google Scholar]
- 45. Dai H, Li R, Wheeler T, Ozen M, Ittmann M, Anderson M, Wang Y, Rowley D, Younes M, Ayala GE. Enhanced survival in perineural invasion of pancreatic cancer: an in vitro approach. Hum Pathol 2007; 38:299-307; PMID:17097719; http://dx.doi.org/ 10.1016/j.humpath.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 46. Adriaenssens E, Vanhecke E, Saule P, Mougel A, Page A, Romon R, Nurcombe V, Le Bourhis X, Hondermarck H. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res 2008; 68:346-51; PMID:18199526; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-1183 [DOI] [PubMed] [Google Scholar]
- 47. Kruttgen A, Schneider I, Weis J. The dark side of the NGF family: neurotrophins in neoplasias. Brain Pathol 2006; 16:304-10; PMID:17107600; http://dx.doi.org/ 10.1111/j.1750-3639.2006.00037.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jimenez-Andrade JM, Bloom AP, Stake JI, Mantyh WG, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA, Mantyh PW. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci: Off J Soc Neurosci 2010; 30:14649-56; PMID:21048122; http://dx.doi.org/ 10.1523/JNEUROSCI.3300-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mantyh WG, Jimenez-Andrade JM, Stake JI, Bloom AP, Kaczmarska MJ, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA, Mantyh PW. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience 2010; 171:588-98; PMID:20851743; http://dx.doi.org/ 10.1016/j.neuroscience.2010.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bronzetti E, Artico M, Forte F, Pagliarella G, Felici LM, D'Ambrosio A, Vespasiani G, Bronzetti B. A possible role of BDNF in prostate cancer detection. Oncol Rep 2008; 19:969-74; PMID:18357383 [DOI] [PubMed] [Google Scholar]
- 51. Yang ZF, Ho DW, Lam CT, Luk JM, Lum CT, Yu WC, Poon RT, Fan ST. Identification of brain-derived neurotrophic factor as a novel functional protein in hepatocellular carcinoma. Cancer Res 2005; 65:219-25; PMID:15665298 [PubMed] [Google Scholar]
- 52. Lai PC, Chiu TH, Huang YT. Overexpression of BDNF and TrkB in human bladder cancer specimens. Oncol Rep 2010; 24:1265-70; PMID:20878119 [DOI] [PubMed] [Google Scholar]
- 53. Burger R, Bakker F, Guenther A, Baum W, Schmidt-Arras D, Hideshima T, Tai YT, Shringarpure R, Catley L, Senaldi G, et al. Functional significance of novel neurotrophin-1/B cell-stimulating factor-3 (cardiotrophin-like cytokine) for human myeloma cell growth and survival. Brit J Haematol 2003; 123:869-78; PMID:14632778; http://dx.doi.org/ 10.1046/j.1365-2141.2003.04686.x [DOI] [PubMed] [Google Scholar]
- 54. McGregor LM, McCune BK, Graff JR, McDowell PR, Romans KE, Yancopoulos GD, Ball DW, Baylin SB, Nelkin BD. Roles of trk family neurotrophin receptors in medullary thyroid carcinoma development and progression. Proc Nat Acad Sci U S A 1999; 96:4540-5; PMID:10200298; http://dx.doi.org/ 10.1073/pnas.96.8.4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ohta T, Numata M, Tsukioka Y, Futagami F, Kayahara M, Kitagawa H, Nagakawa T, Yamamoto M, Wakayama T, Kitamura Y, et al. Neurotrophin-3 expression in human pancreatic cancers. J Pathol 1997; 181:405-12; PMID:9196438; http://dx.doi.org/ 10.1002/(SICI)1096-9896(199704)181:4%3c405::AID-PATH786%3e3.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- 56. Brooks AN, Kilgour E, Smith PD. Molecular pathways: fibroblast growth factor signaling: a new therapeutic opportunity in cancer. Clin Cancer Res: Off J Am Assoc Cancer Res 2012; 18:1855-62; PMID:22388515; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-0699 [DOI] [PubMed] [Google Scholar]
- 57. Guillemot F, Zimmer C. From cradle to grave: the multiple roles of fibroblast growth factors in neural development. Neuron 2011; 71:574-88; PMID:21867876; http://dx.doi.org/ 10.1016/j.neuron.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 58. Dobrenis K, Gauthier LR, Barroca V, Magnon C. G-CSF off-target effect on nerve outgrowth promotes prostate cancer development. Int J Cancer 2014); PMID:24975135 [DOI] [PubMed] [Google Scholar]
- 59. Pickup M, Novitskiy S, Moses HL. The roles of TGFbeta in the tumour microenvironment. Nat Rev Cancer 2013; 13:788-99; PMID:24132110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci 2005; 6:945-54; PMID:16340955 [DOI] [PubMed] [Google Scholar]
- 61. Mehlen P, Delloye-Bourgeois C, Chedotal A. Novel roles for Slits and netrins: axon guidance cues as anticancer targets? Nat Rev Cancer 2011; 11:188-97; PMID:21326323 [DOI] [PubMed] [Google Scholar]
- 62. Capparuccia L, Tamagnone L. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment-two sides of a coin. J Cell Sci 2009; 122:1723-36; PMID:19461072; http://dx.doi.org/ 10.1242/jcs.030197 [DOI] [PubMed] [Google Scholar]
- 63. Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer 2010; 10:165-80; PMID:20179713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Larrivee B, Freitas C, Suchting S, Brunet I, Eichmann A. Guidance of vascular development: lessons from the nervous system. Circulat Res 2009; 104:428-41; PMID:19246687; http://dx.doi.org/ 10.1161/CIRCRESAHA.108.188144 [DOI] [PubMed] [Google Scholar]
- 65. Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 1996; 87:175-85; PMID:8861902; http://dx.doi.org/ 10.1016/S0092-8674(00)81336-7 [DOI] [PubMed] [Google Scholar]
- 66. Leonardo ED, Hinck L, Masu M, Keino-Masu K, Ackerman SL, Tessier-Lavigne M. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature 1997; 386:833-8; PMID:9126742; http://dx.doi.org/ 10.1038/386833a0 [DOI] [PubMed] [Google Scholar]
- 67. Eichmann A, Thomas JL. Molecular parallels between neural and vascular development. Cold Spring Harb Perspect Med 2013; 3:a006551; PMID:23024177; http://dx.doi.org/ 10.1101/cshperspect.a006551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Damon DH, Teriele JA, Marko SB. Vascular-derived artemin: a determinant of vascular sympathetic innervation? American journal of physiology. Heart Circulat Physiol 2007; 293:H266-73; PMID:17337595; http://dx.doi.org/ 10.1152/ajpheart.00859.2006 [DOI] [PubMed] [Google Scholar]
- 69. Makita T, Sucov HM, Gariepy CE, Yanagisawa M, Ginty DD. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature 2008; 452:759-63; PMID:18401410; http://dx.doi.org/ 10.1038/nature06859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chalothorn D, Zhang H, Clayton JA, Thomas SA, Faber JE. Catecholamines augment collateral vessel growth and angiogenesis in hindlimb ischemia. Am J Physiol Heart Circulat Physiol 2005; 289:H947-59; PMID:15833801; http://dx.doi.org/ 10.1152/ajpheart.00952.2004 [DOI] [PubMed] [Google Scholar]
- 71. Luo MY, Yang BL, Ye F, Wu X, Peng S, Yi B, Wang W, Zhu W, Luo H, Wen JG, et al. Collateral vessel growth induced by femoral artery ligature is impaired by denervation. Mol Cell Biochem 2011; 354:219-29; PMID:21509579; http://dx.doi.org/ 10.1007/s11010-011-0821-6 [DOI] [PubMed] [Google Scholar]
- 72. Grytli HH, Fagerland MW, Fossa SD, Tasken KA, Haheim LL. Use of beta-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapy. Prostate 2013; 73:250-60; PMID:22821802; http://dx.doi.org/ 10.1002/pros.22564 [DOI] [PubMed] [Google Scholar]
- 73. Grytli HH, Fagerland MW, Fossa SD, Tasken KA. Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol 2013; 64:e11-2; PMID:23602404; http://dx.doi.org/ 10.1016/j.eururo.2013.03.045 [DOI] [PubMed] [Google Scholar]
- 74. Lemeshow S, Sørensen HT, Phillips G, Yang EV, Antonsen S, Riis AH, Lesinski GB, Jackson R, Glaser R. beta-Blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomarkers Prev 2011; 20:2273-79; PMID:21933972; http://dx.doi.org/ 10.1158/1055-9965.EPI-11-0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, Sood AK, Conzen SD, Hortobagyi GN, Gonzalez-Angulo AM. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 2011; 29:2645-52; PMID:21632501; http://dx.doi.org/ 10.1200/JCO.2010.33.4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zage PE, Graham TC, Zeng L, Fang W, Pien C, Thress K, Omer C, Brown JL, Zweidler-McKay PA. The selective Trk inhibitor AZ623 inhibits brain-derived neurotrophic factor-mediated neuroblastoma cell proliferation and signaling and is synergistic with topotecan. Cancer 2011; 117:1321-91; PMID:20960503; http://dx.doi.org/ 10.1002/cncr.25674 [DOI] [PubMed] [Google Scholar]
- 77. Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 2009; 15:220-31; PMID:19249680; http://dx.doi.org/ 10.1016/j.ccr.2009.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 2009; 15:232-9; PMID:19249681; http://dx.doi.org/ 10.1016/j.ccr.2009.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Akhurst RJ, Hata A. Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Disc 2012; 11:790-811; PMID:23000686; http://dx.doi.org/ 10.1038/nrd3810 [DOI] [PMC free article] [PubMed] [Google Scholar]