Abstract
The Cancer Genome Atlas undertook a comprehensive genetic analysis of chromophobe renal cell carcinoma, the first of the rare tumor types to be analyzed. This analysis identified the putative region of origin as the distal nephron. Alterations in mitochondrial function, mtDNA mutations, and recurrent structural rearrangements within the TERT promoter region were also identified.
Keywords: cancer metabolism, chromophobe renal cell carcinoma, clear cell renal cell carcinoma, RCC, TERT
Auto-commentary
The Cancer Genome Atlas projects on rare tumor types offer unique insights into the mechanisms of tumorigenesis. The first such project studied chromophobe renal cell carcinoma (ChRCC).1 ChRCC represents 5% of renal cell carcinoma cases,2 and is associated with a striking aneuploidy pattern.3 Our analysis of 66 cases of non-hereditary ChRCC included whole-exome DNA sequencing for all cases, together with whole-genome DNA sequencing in 50 of these cases and mtDNA sequencing by long-range PCR in 61 cases. All cases also were subjected to copy number analysis, mRNA and miRNA sequencing, and CpG DNA methylation.
Our analyses verified large-scale chromosomal loss in ChRCC that has previously been described, with 86% of cases showing loss of one copy of the entire chromosome for most or all of chromosomes 1, 2, 6, 10, 13, and 17. Between 12% and 58% of cases also showed entire chromosomal loss for chromosomes 3, 5, 8, 9, and 21. Chromophobe RCC has also been defined by histological classification as classic or eosinophilic. All of the 47 tumors with classic histology showed the characteristic chromosomal loss, whereas only 10 of the 19 eosinophilic cases showed these losses. Some eosinophilic cases showed no chromosomal loss, suggesting degree of genomic heterogeneity as a distinguishing characteristic of ChRCC histological classification.
Whole exome sequencing showed relatively low numbers of somatic mutations, with only 2 reaching the threshold for designation as frequently mutated genes (i.e., occurring in >5% of cases). TP53 mutations were identified in 21 cases (32%), and PTEN was mutated in 6 cases (9%). With only 2 common mutations, our attention turned to methylation changes, mitochondrial DNA, and structural changes.
By comparing methylation patterns between the most common subtypes of RCC—clear cell renal cell carcinoma (ccRCC) and ChRCC—we were able to identify distinct differences between these 2 subtypes that suggested a potential difference in cell of origin. To further examine this possibility, we compared RNA seq gene expression data from both diseases to an external gene expression data set of normal kidney that had been microdissected from various regions of the nephron.4 ChRCC mRNA expression highly correlated with expression profiles from distal regions of the nephron, whereas ccRCC mRNA expression correlated with expression profiles from the proximal nephron. These results suggest that molecular differences between these subtypes may be defined and influenced by distinct cells of origin.
Bioenergetic features were prominent in both ccRCC and ChRCC, but in highly divergent patterns. In ChRCC, nearly all genes involved in the Krebs cycle showed increased expression when compared to normal kidney, as did at least one gene for each complex involved in the electron transport chain (ETC). ChRCC also exhibited increased mitochondrial genome content. Interestingly, mitochondria-related genes are strongly repressed in ccRCC, highlighting another important distinction between these 2 subtypes.5 These differences suggest alternative strategies to support tumor growth rather than minimizing reliance on oxidative phosphorylation.
Further mitochondrial analysis revealed a high frequency of somatic mutations in mitochondrial genes at various levels of heteroplasmy. Twelve tumors had non-silent mutations, including many frameshift mutations. Mitochondrial gene mutations in ChRCC have been described previously,6 but this analysis confirms both the high frequency of events and a close association of these mutations with the eosinophilic subgroup. Generally, these mutations were predicted to be inactivating. Specific mutations were identified in MT-ND5, an essential member of ETC complex I, including one previously identified cancer-associated mutation.7 Intriguingly, mutations in complex I were not correlated with alterations in expression patterns associated with loss of oxidative phosphorylation. This suggests an alternative role for complex I alteration in cancer metabolism, or a compensatory mechanism of increased gene expression that does not reflect the same change in metabolic activity. Ultimately, measurements of substrate utilization and dynamic metabolic processing will be required to resolve the true metabolic state of these tumors.
Whole genome analysis identified 2 novel findings in ChRCC cases. A subset of samples displayed kataegis, the occurrence of highly localized substitution mutations (C > T or C > G),8,9 that were localized within regions of genomic rearrangement. Among the samples with a strong kataegis pattern, TERT was identified as a differentially expressed gene. To further understand the role of TERT in ChRCC, we examined the sequence of the TERT promoter and identified 3 cases with the previously described C228T mutation.10 In addition, several cases displayed novel genomic rearrangements involving the TERT promoter, which were associated with the highest level of TERT expression. These variants were observed with a very high allelic frequency, suggesting that TERT-associated rearrangements are early, and potentially driver, events. No equivalent findings have been reported in ccRCC.
As the first comprehensive molecular analysis of ChRCC, our studies identified several unique characteristics of this tumor type that further reinforce its position as a distinct tumor entity from ccRCC. The combination of methylation pattern differences and expression correlations suggest a distal nephron cell of origin, while indicating a proximal nephron origin of ccRCC. The finding of mtDNA alterations raises intriguing questions about the divergent strategies by which kidney cells induce altered metabolism in cancer, as our data suggest a complex metabolic phenotype reflecting more than the loss of oxidative phosphorylation. Finally, the finding of genomic rearrangements of the TERT promoter suggests alteration of TERT expression as a potential driver of this cancer (Fig. 1). These findings were only possible due to the integrated analysis of several data types and the extension of sequencing beyond the confines of the exome. The comparison to ccRCC further reinforces the distinct origins and tumorigenic features of these cancers, which has important implications for future therapeutic innovations.
Figure 1.
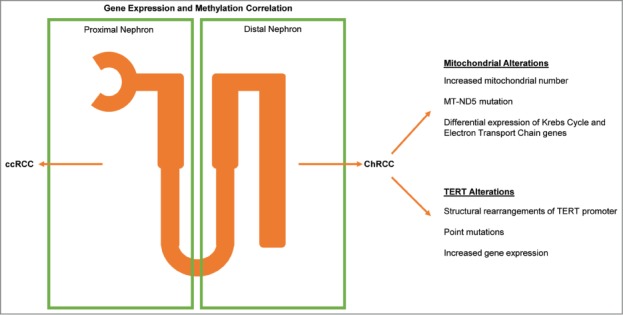
Integrated analysis identifies key characteristics of chromophobe renal cell carcinoma (ChRCC). ChRCC and clear cell renal cell carcinoma (ccRCC) originate from different regions of the kidney nephron. ChRCC is characterized by mitochondrial and TERT alterations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Funding to support this work came from 5T32GM008719-12 and 8TL1TR000085-05.
References
- 1. Davis CF, Ricketts CJ, Wang M, Yang L, Cherniack AD, Shen H, Buhay C, Kang H, Kim SC, Fahey CC, et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell 2014; 26:319-30; PMID:25155756; http://dx.doi.org/ 10.1016/j.ccr.2014.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Störkel S, Eble JN, Adlakha K, Amin M, Blute ML, Bostwick DG, Darson M, Delahunt B, Iczkowski K. Classification of renal cell carcinoma: workgroup no. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer 1997; 80:987-9; PMID:9307203; http://dx.doi.org/ 10.1002/(SICI)1097-0142(19970901)80:5%3c987::AID-CNCR24%3e3.0.CO;2-R [DOI] [PubMed] [Google Scholar]
- 3. Speicher MR, Schoell B, du Manoir S, Schröck E, Ried T, Cremer T, Störkel S, Kovacs A, Kovacs G. Specific loss of chromosomes 1, 2, 6, 10, 13, 17, and 21 in chromophobe renal cell carcinomas revealed by comparative genomic hybridization. Am J Pathol 1994; 145:356-64; PMID:7519827 [PMC free article] [PubMed] [Google Scholar]
- 4. Cheval L, Pierrat F, Rajerison R, Piquemal D, Doucet A. Of mice and men: divergence of gene expression patterns in kidney. PLoS One 2012; 7:e46876; PMID:23056504; http://dx.doi.org/ 10.1371/journal.pone.0046876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The Cancer Genome Atlas Research Network . Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013; 499:43-9; PMID:23792563; http://dx.doi.org/ 10.1038/nature12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagy A, Wilhelm M, Sükösd F, Ljungberg B, Kovacs G. Somatic mitochondrial DNA mutations in human chromophobe renal cell carcinomas. Genes Chromosomes Cancer 2002; 35:256-60; PMID:12353267; http://dx.doi.org/ 10.1002/gcc.10118 [DOI] [PubMed] [Google Scholar]
- 7. Larman TC, DePalma SR, Hadjipanayis AG, Protopopov A, Zhang J, Gabriel SB, Chin L, Seidman CE, Kucherlapati R, Seidman JG. Spectrum of somatic mitochondrial mutations in five cancers. Proc Natl Acad Sci U S A 2012; 109:14087-91; PMID:22891333; http://dx.doi.org/ 10.1073/pnas.1211502109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin A V, Bignell GR, Bolli N, Borg A, Børresen-Dale A-L, et al. Signatures of mutational processes in human cancer. Nature 2013; 500:415-21; PMID:23945592; http://dx.doi.org/ 10.1038/nature12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nik-Zainal S, Alexandrov LB, Wedge DC, Van Loo P, Greenman CD, Raine K, Jones D, Hinton J, Marshall J, Stebbings LA, et al. Mutational processes molding the genomes of 21 breast cancers. Cell 2012; 149:979-93; PMID:22608084; http://dx.doi.org/ 10.1016/j.cell.2012.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang FW, Hodis E, Xu MJ, Kryukov G V, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science 2013; 339:957-9; PMID:23348506; http://dx.doi.org/ 10.1126/science.1229259 [DOI] [PMC free article] [PubMed] [Google Scholar]


