Abstract
PRKD2 plays an important role in tumor cell survival, proliferation, migration, and angiogenesis. We recently reported that cell death and impaired blood vessel formation evoked by inhibition of the HSP90 chaperone in human cancer cells of various tissue origins is mediated by destabilization of PRKD2.
Keywords: heat shock protein 90 (HSP90) chaperone, hypoxia, nuclear factor kappa B (NF-kB), Protein kinase D2, tumor angiogenesis
The protein kinase D (PRKD) family of serine/threonine kinases belongs to the calcium/calmodulin-dependent protein kinase superfamily and comprises 3 isoforms, PRKD1, PRKD2, and PRKD3. Our laboratory recently described PRKD2 as an essential mediator of tumor–endothelial cell communication in gastrointestinal cancers.1 Around the same time, Moulick and colleagues applied a proteomic strategy to identify cancer-specific networks coordinated by heat shock protein 90 (HSP90), a chaperone that stabilizes various proteins and in particular protein kinases,2 and identified PRKD2 as a potential HSP90 “client” in chronic myeloid leukemia.3 Based on the observation that RNA interference-mediated depletion of PRKD2 in pancreatic cancer cells was associated with an increase in apoptosis similar to that invoked by pharmacologic HSP90 inhibition, we hypothesized that PRKD2 may be an important HSP90 client in this context. Following the demonstration that PRKD2 interacts with HSP90, we treated various epithelial cancer and glioblastoma cell lines with 2 HSP90 inhibitors currently in clinical development: PU-H71, an optimized water-soluble member of the purine class of HSP90 inhibitors, and ganetespib (also called STA9090), a resorcinol-containing triazole molecule. HSP90 inhibition led to proteasomal degradation of PRKD2 followed by tumor cell killing, consistent with the previously reported antiapoptotic function of PRKD2.4 Moreover, the fact that ectopic expression of PRKD2 restored cancer cell viability after HSP90 inhibition substantiates the role of PRKD2 in the killing of cancer cells.5 These findings raised 2 questions: Given the established role of PRKD2 in tumor angiogenesis,1 does HSP90 inhibition also affect tumor vascularization? What is the molecular mechanism by which PRKD2 orchestrates HSP90 tumor growth and angiogenic actions?
To investigate the contribution of a HSP90–PRKD2 axis to tumor angiogenesis in colon and breast carcinoma we used 2 in vivo models, the chicken chorioallantoic membrane assay and human tumor xenografts in nude mice. In both systems, forced expression of PRKD2 restored vascularization and tumor cell viability in the presence of a pharmacologic HSP90 inhibitor, indicating that HSP90 does indeed promote tumor angiogenesis via stabilization of PRKD2.5 We next investigated the mechanism through which HSP90-stabilized PRKD2 mediates new blood vessel formation in these systems. Specifically, we evaluated the impact of PRKD2 on secretion of vascular endothelial growth factor A (VEGFA) and accumulation of hypoxia-inducible factor 1α (HIF-1α) in hypoxic tumors in the context of HSP90 inhibition because VEGFA, one of the most potent mediators of angiogenesis, is upregulated via stabilization of HIF-1α6 and HSP90 interacts with both HIF-1α and PRKD2.5 RNA interference-mediated knockdown of PRKD2 resulted in impaired hypoxia-induced HIF-1α accumulation and decreased VEGFA levels, similar to genetic or pharmacologic abrogation of HSP90.5
The most important finding, however, comes from experiments showing that ectopic PRKD2 partially restores hypoxia-induced HIF-1α accumulation and VEGFA secretion following HSP90 inhibition. These data imply that hypoxia-stabilized HIF-1α is regulated by both HSP90 and PRKD2, and that the latter may function as a signaling hub for chaperone and hypoxia-mediated pathways.5 Further investigation is required to elucidate to what extent HSP90 utilizes PRKD2 to regulate HIF-1α/VEGFA/angiogenesis and whether PRKD2 concomitantly shares this function with other HSP90 clients.
PRKD2 itself may employ several mechanisms to relay the angiogenic signals necessary for generation of tumor-derived blood vessels. We recently demonstrated that PRKD2 can regulate hypoxia-induced VEGFA secretion through induction of TR3/Nur77, an orphan member of the steroid/thyroid receptor family that is involved in VEGFA-induced angiogenesis.1,7 Interestingly, Choi and colleagues demonstrated that TR3/Nur77 is activated by HIF-1α8 while in the same year Yoo et al. revealed that TR3/Nur77 stabilizes HIF-1α.9 These findings and our current work suggest that PRKD2 might govern 2 interrelated signaling pathways converging on VEGFA (Fig. 1).
Figure 1.
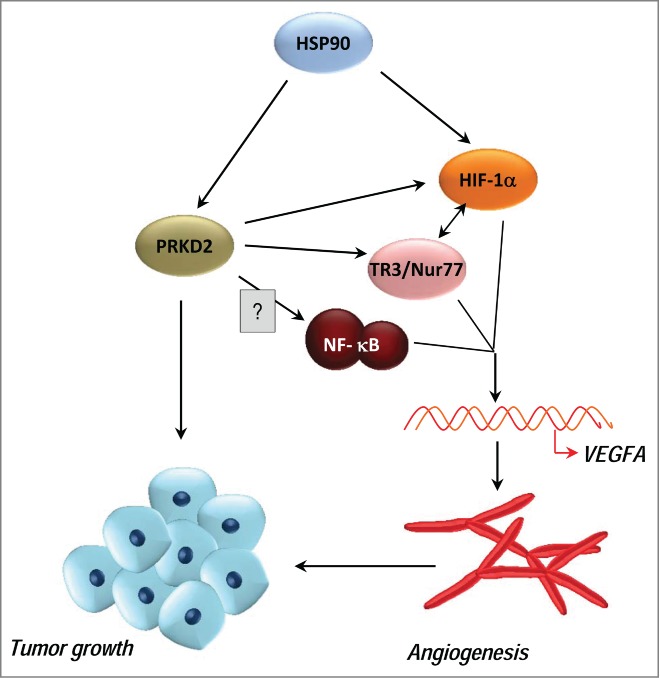
Molecular signals orchestrated by HSP90-stabilized and hypoxia-activated PRKD2 during tumor growth and angiogenesis. Stabilization of PRKD2 by HSP90 contributes to enhanced tumor viability and vascularization. In this scenario, PRKD2 is responsible for augmented HIF-1α accumulation in a low-oxygen environment, resulting in activation of NF-κB and its target VEGFA. The extent to which PRKD2 regulates VEGFA secretion, either directly through HIF-1α and/or TR3/Nur77 or indirectly through NF-κB, remains to be elucidated. PRKD2, protein kinase D2; HSP90, heat shock protein 90; HIF-1α, hypoxia-inducible factor 1-α; VEGFA, vascular endothelial growth factor A. ? unknown molecule.
Finally, we identified yet another signaling route leading to increased vascularization in tumors. Since the transcription factor NF-κB (and its target gene VEGFA) is activated by hypoxia, HSP90, and PRKD2,10 we reasoned that NF-κB signaling might be connected to the hypoxic response regulated by the HSP90–PRKD2 axis. However, PRKD2 only marginally restored hypoxia-induced NF-κB promoter activity in the context of HSP90 inhibition, suggesting that additional factors/clients are required to transmit HSP90's angiogenic signals through the NF-κB pathway5. Whether PRKD2 activates NF-κB/VEGFA via upregulation of HIF-1α—for example, through induction of a TR3 → HIF-1α→ NF-κB/VEGFA cascade—or whether interaction with other molecules, such as inducible kappa B kinase (IKK), is required to activate NF-κB/VEGFA remains to be elucidated (Fig. 1). Lastly, our work may have clinical implications since several HSP90 and PRKD2 inhibitors are currently being developed as anticancer agents.
In conclusion, in this study we have addressed the role of PRKD2 as a novel HSP90 client, thereby providing a link between aberrant chaperone activity and increased angiogenesis in various cancers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the German Research Foundation (grant AZ.96/1–1 to N. Azoitei, grant SE.676/10–1 to T. Seufferlein), the German Cancer Aid (grant 109373 to T. Seufferlein). C. Scholl was supported by an Emmy Noether Fellowship from the DFG.
References
- 1. Azoitei N, Pusapati GV, Kleger A, Möller P, Küfer R, Genze F, Wagner M, van Lint J, Carmeliet P, Adler G, et al. Protein kinase D2 is a crucial regulator of tumor cell-endothelial cell communication in gastrointestinal tumors. Gut 2010. Oct; 59(10):1316-30; http://dx.doi.org/ 10.1136/gut.2009.206813 [DOI] [PubMed] [Google Scholar]
- 2. Azoitei N, Hoffmann CM, Ellegast JM, Ball CR, Obermayer K, Gößele U, Koch B, Faber K, Genze F, Schrader M, et al. Targeting of KRAS mutant tumors by HSP90 inhibitors involves degradation of STK33. J Exp Med 2012. Apr 9; 209(4):697-11; http://dx.doi.org/ 10.1084/jem.20111910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moulick K, Ahn JH, Zong H, Rodina A, Cerchietti L, Gomes DaGama EM, Caldas-Lopes E, Beebe K, Perna F, et al. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol. 2011. Sep 25; 7(11):818-26; http://dx.doi.org/ 10.1038/nchembio.670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem 2005. Apr 8; 280(14):13205-8; http://dx.doi.org/ 10.1074/jbc.R500002200 [DOI] [PubMed] [Google Scholar]
- 5. Azoitei N, Diepold K, Brunner C, Rouhi A, Genze F, Becher A, Kestler H, Van Lint J, Chiosis G, Koren 3rd John, Fröhling S, Scholl C, Seufferlein T. HSP90 supports tumor growth and angiogenesis through PRKD2 protein stabilization. Cancer Res 2014; 74(23):7125-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Minet E, Mottet D, Michel G, Roland I, Raes M, Remacle J, Michiels C. Hypoxia-induced activation of HIF-1: role of HIF-1a-Hsp90 interaction. FEBS Lett 1999. Oct 29; 460(2):251-6; http://dx.doi.org/ 10.1016/S0014-5793(99)01359-9 [DOI] [PubMed] [Google Scholar]
- 7. Zeng H, Qin L, Zhao D, Tan X, Manseau EJ, Van Hoang M, Senger DR, Brown LF, Nagy JA, Dvorak HF. Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J Exp Med 2006; 203:719-29; PMID:16520388; http://dx.doi.org/ 10.1084/jem.20051523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi JW, Park SC, Kang GH, Liu JO, Youn HD. Nur77 activated by hypoxia-inducible factor 1 alpha overproduces proopiomelanocortin in von Hippel-Lindau mutated renal cell carcinoma. Cancer Res 2004; 64:35-39; PMID:14729605; http://dx.doi.org/ 10.1158/0008-5472.CAN-03-0145 [DOI] [PubMed] [Google Scholar]
- 9. Yoo YG, Yeo MG. Kim DK, Park H, Lee MO. Novel function of orphan nuclear receptor Nur77 in stabilizing HIF-1a. J Biol Chem 2004. Jul 28; 279(51):53365-73; http://dx.doi.org/ 10.1074/jbc.M408554200 [DOI] [PubMed] [Google Scholar]
- 10. Culver C, Sundqvist A, Mudie S, Melvin A, Xirodimas D, Rocha S. Mechanism of hypoxia-induced NF-kappaB. Mol Cell Biol 2010. Oct; 30(20):4901-21; http://dx.doi.org/ 10.1128/MCB.00409-10 [DOI] [PMC free article] [PubMed] [Google Scholar]


