Abstract
Inverse gene expression profiling was recently shown to help drug repositioning. We showed that this approach works best for cancer and predicted novel drug candidates that may reduce metastasis in colorectal cancer. Antimetastatic activity of our predicted candidate, citalopram, was validated in an orthotopic mouse model of metastatic colorectal cancer.
Keywords: drug-repositioning, citalopram, colorectal cancer, metastasis, systems pharmacology, orthotopic model
With recent advances in high-throughput technologies, data from large-scale studies are now emerging that allow us to characterize the genomic and molecular basis of disease on an unprecedented scale.1 Specifically, gene expression profiling is a powerful tool that summarizes the molecular phenotypes of disease as transcriptional signatures. These signatures provide a common language by which diseases can be functionally linked with drug-induced gene expression profiles2 (Fig. 1). It has been suggested that drugs with an expression response that inversely correlates to the disease profile might actually ameliorate the disease phenotype. A recent study by Sirota et al. applied this concept to 100 diseases and showed overall enrichment of known drug–disease indications among anti-correlated disease–drug profiles.3 The clinical value of this approach was further demonstrated with the experimental validation of several drug repositioning predictions such as the antiulcer agent cimetidine for non-small cell lung cancer,3 and the antidepressant imipramine and the antiemetine promethazine for small cell lung cancer.4
Figure 1.
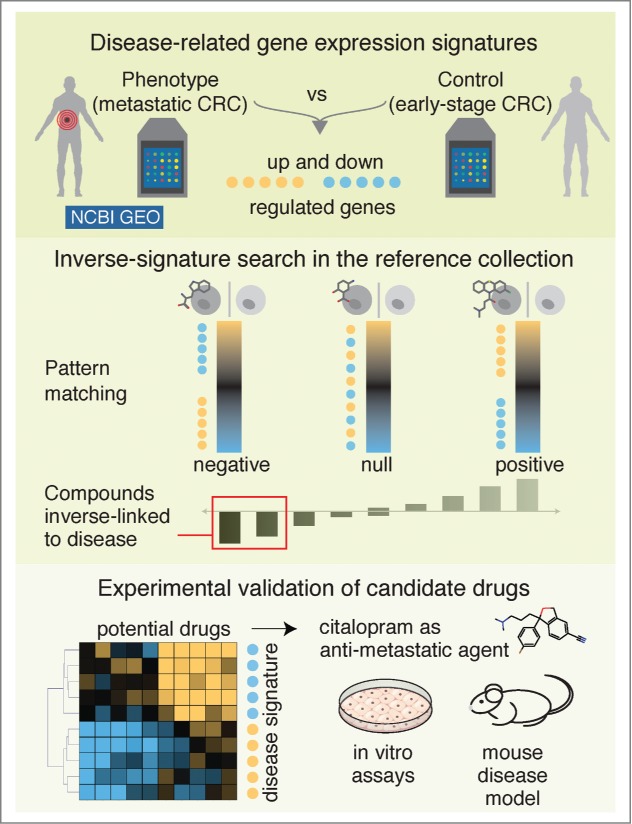
Workflow of the inverse-signature approach to identify potential therapeutic compounds. A disease transcriptional signature was extracted by comparing phenotype versus control (e.g., metastatic vs. primary colorectal cancer) gene expression profiles publicly available from Gene expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) at NCBI (National Center for Biotechnology Information). Next, the disease-related signature was queried against the reference collection of drug-induced gene expression profiles. Drug candidates with anti-correlated profiles were further refined using hierarchical clustering and the efficacy of selected candidates (e.g., citalopram) was experimentally tested using in vitro and in vivo disease models (e.g., colorectal cancer [CRC]).
The success of this approach is strongly dependent on the reference expression compendia profiling a wide variety of drug-like molecules (Fig. 1). The largest public resource available for this purpose is the Connectivity Map (CMap), which contains gene expression profiles from 4 human cancer cell lines treated with more than 1,300 drugs.2 In order to analyze this large-scale heterogeneous data set we previously developed a batch correction procedure that improved the comparability of expression measurements across batches, thereby enhancing data quality for further analysis.5
In a more recent study, we set out to evaluate the validity of the inverse signature approach for individual diseases using known drug–disease indications.6 Our analysis was able to recapitulate antineoplastic agents for multiple cancer types, but showed poor performance for other diseases. Thus, although there is an overall enrichment of drug–disease indications among anti-correlated profiles, these indications are not evenly distributed among diseases. Among 40 diseases included in the analysis, the inverse signature approach was most successful for colorectal, brain, small-cell lung, and ovarian cancers and soft tissue sarcoma. This predilection for cancer might be partially explained by the fact that the CMap reference profiles were generated in a limited set of cancer cell lines.
Encouraged by the promising results for cancer, we decided to focus our analysis on colorectal cancer (CRC), which is the fourth leading cause of cancer death globally.7 The main clinical concern in CRC is the development of metastasis, which occurs in 50–60% of patients after the initial diagnosis.8 Although surgical intervention improves the outcome of patients with non-metastatic disease, the survival rate of CRC patients decreases dramatically with metastatic spread to distant organs. Therefore, there is a clinical need for new antimigratory agents to decrease the incidence of tumor dissemination during the course of CRC.
To address this need, we sought to apply the inverse signature approach to the prediction of antimigratory drugs against colorectal cancer. For this purpose, we first selected a transcriptional signature9 derived from a comparison between metastatic and early-stage non-metastatic colorectal cancers. This signature represents the molecular phenotype of metastatic colorectal cancer rather than general processes related to cancer such as proliferation. We next queried the metastasis signature against the reference CMap collection to predict antimetastatic compounds. Our top predictions were already reported in the literature to have anti-migratory and/or anti-metastatic activity in cancer cells, thus validating this approach. Detailed investigation of expression profiles for the top drug candidates revealed heterogeneity in the regulation of the metastasis signature. For example, we observed that expression of fibronectin 1 and its receptor integrin β 5 was strongly downregulated only by a subset of drug candidates. Therefore, we performed hierarchical clustering to define robust expression patterns that were coherently regulated by drug candidates (Fig. 1). This clustering guided us in the selection of more relevant drug candidates that exert similar expression responses to known antimetastatic compounds. Finally, we chose 3 novel drug repositioning candidates for further experimental investigation. The selected candidates—citalopram (antidepressant), enilconazole (antifungal), and troglitazone (antidiabetic)—not only have similar profiles to those of known antimetastatic drugs, but also share the same mode of action through inhibition of TGF-β signaling.6
In the last part of our study, we experimentally assessed the antimetastatic activity of drug candidates. First, the inhibitory effects of citalopram, enilconazole, and troglitazone on migration were demonstrated with an in vitro assay conducted in HCT 116 and HT-29 human CRC cells. We next checked whether drug treatment has any undesirable stimulatory effect on tumor growth and found that all 3 compounds had antiproliferative effects in cell culture and a mouse xenograft tumor model. Last, we examined the effect of citalopram in an orthotopic mouse model, a clinically relevant model that recapitulates the metastatic cascade of colorectal cancer.10 In accordance with our prediction, the number of circulating tumor cells and the number of metastases in distant organs (e.g., liver) were significantly lower in citalopram-treated mice compared to control mice. This illustrates the therapeutic potential of citalopram and proposes citalopram (antidepressant) as a preventive measure against the metastatic progression of colorectal cancer. Beyond the examples presented here, this study contributes to the establishment of inverse-correlation of transcriptional profiles as a promising approach for drug discovery and repositioning.6
Recent efforts have dramatically increased the number of drugs and cell types used in expression profile screening. As the successor of the Connectivity Map approach, the ‘Library of Integrated Network-Based Cellular Signatures’ (LINCS, http://www.lincscloud.org) program will make one of the largest collections of gene expression data accessible. LINCS collected over 1 million gene expression profiles from 77 distinct cell types perturbed with more than 20,000 chemical and genetic reagents. We believe that as data on more cell types and drugs become available, inverse gene expression profiling will prove useful in many other diseases and their subtypes. Moreover, gene expression profiling of individual patients, in combination with this enhanced collection of drug-induced profiles, should pave the way for personalized cancer therapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Georg Zeller for critical reading of the manuscript.
Funding
This work was supported by the CancerBiome project (European Research Council project reference 268985).
References
- 1. Weinstein JN, Collisson EA, Mills GB, Shaw KRM, Ozenberger BA, Ellrott K, Shmulevich I, Sander C, Stuart JM. The cancer genome atlas pan-cancer analysis project. Nat Genet 2013; 45:1113–20; PMID:24071849; http://dx.doi.org/ 10.1038/ng.2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, Lerner J, Brunet J-P, Subramanian A, Ross KN, et al. The connectivity map: using gene-expression signatures to connect small molecules, genes, and disease. Science 2006; 313:1929-35; PMID:17008526; http://dx.doi.org/ 10.1126/science.1132939 [DOI] [PubMed] [Google Scholar]
- 3. Sirota M, Dudley JT, Kim J, Chiang AP, Morgan AA, Sweet-Cordero A, Sage J, Butte AJ. Discovery and preclinical validation of drug indications using compendia of public gene expression data. Sci Transl Med 2011; 3:96ra77-96ra77; PMID:21849665; http://dx.doi.org/ 10.1126/scitranslmed.3001318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jahchan NS, Dudley JT, Mazur PK, Flores N, Yang D, Palmerton A, Zmoos A-F, Vaka D, Tran KQT, Zhou M, et al. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discov 2013; 3:1364-77; PMID:24078773; http://dx.doi.org/ 10.1158/2159-8290.CD-13-0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iskar M, Campillos M, Kuhn M, Jensen LJ, van Noort V, Bork P. Drug-induced regulation of target expression. PLoS Comput Biol 2010; 6:e1000925; PMID:20838579; http://dx.doi.org/ 10.1371/journal.pcbi.1000925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Noort V, Schölch S, Iskar M, Zeller G, Ostertag K, Schweitzer C, Werner K, Weitz J, Koch M, Bork P. Novel drug candidates for the treatment of metastatic colorectal cancer through global inverse gene expression profiling. Cancer Res 2014; 74:5690-9; canres.3540.2013; PMID:25038229; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3540 [DOI] [PubMed] [Google Scholar]
- 7. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69-90; PMID:21296855; http://dx.doi.org/ 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 8. Yoo PS, Lopez-Soler RI, Longo WE, Cha CH. Liver resection for metastatic colorectal cancer in the age of neoadjuvant chemotherapy and bevacizumab. Clin Colorectal Cancer 2006; 6:202-7; PMID:17026789; http://dx.doi.org/ 10.3816/CCC.2006.n.036 [DOI] [PubMed] [Google Scholar]
- 9. Jorissen RN, Gibbs P, Christie M, Prakash S, Lipton L, Desai J, Kerr D, Aaltonen LA, Arango D, Kruhøffer M, et al. Metastasis-associated gene expression changes predict poor outcomes in patients with dukes stage B and C colorectal cancer. Clin Cancer Res 2009; 15:7642-51; PMID:19996206; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guilbaud N, Kraus-Berthier L, Meyer-Losic F, Malivet V, Chacun C, Jan M, Tillequin F, Michel S, Koch M, Pfeiffer B, et al. Marked antitumor activity of a new potent acronycine derivative in orthotopic models of human solid tumors. Clin Cancer Res 2001; 7:2573-80; PMID:11489841 [PubMed] [Google Scholar]


