Abstract
Introduction: Extracorporeal membrane oxygenation (ECMO) is widely used to treat respiratory distress during cardiac or respiratory arrest; moreover, its use is being extended to a wide variety of clinical fields. In this study we assess the utility of ECMO in the management of airway obstruction.
Patients and Methods: 15 patients underwent ECMO for airway obstruction. We retrospectively analyzed and evaluated the feasibility of ECMO in the treatment of airway problems.
Results: Seven patients received ECMO to facilitate respiration and promote stability during trachea surgery. In six cases ECMO ceased immediately following the operation; in the remaining case ECMO cessation was delayed due to post-operative ARDS. In three cases emergency ECMO was used in response to respiratory arrest; two patients died. In five cases ECMO was emergently inserted to prevent death, following airway blockade by massive hemoptysis. One patient was not discharged from the intensive care unit. Another patient was transferred to a general ward but died from other causes.
Conclusion: ECMO is useful during anesthesia in patients at high risk of airway blockade, for example due to endobronchial bleeding, and during complex thoracic surgery. ECMO confers a safer environment during airway surgery, and its complication rate is acceptable.
Keywords: extracorporeal membrane oxygenation, air way disease, trachea, hemoptysis
Introduction
Common causes of severe acute airway obstruction include tumors, foreign bodies, blood clots, and extrinsic compression. Symptoms range between respiratory distress and stridor to full cardiac arrest. Death can occur if airway patency is not maintained by airway management techniques such as flexible or rigid bronchoscopy; novel rescue treatments are required to prevent mortality following airway obstruction.1)
Extracorporeal membrane oxygenation (ECMO) is widely used to treat respiratory distress patients and to prevent mortality during cardiac or respiratory arrest.2) Furthermore, ECMO use is being extended to a wide variety of clinical fields. ECMO represents a novel, effective method of treating airway blockages that cannot be resolved using conventional airway management techniques. In this study, we assessed the utility of ECMO in the treatment of airway obstruction, and developed guidelines for ECMO use in patients with airway obstruction.
Patients and Methods
Between January 2011 and January 2014, 15 patients underwent ECMO for airway obstruction due to various causes. We retrospectively analyzed the clinical data and evaluated the feasibility of ECMO for the treatment of airway blockade. We used ECMO to prevent breath-holding during induction anesthesia or surgery, death during induction therapy and to alleviate airway blockade following massive hemoptysis.
ECMO mode and management
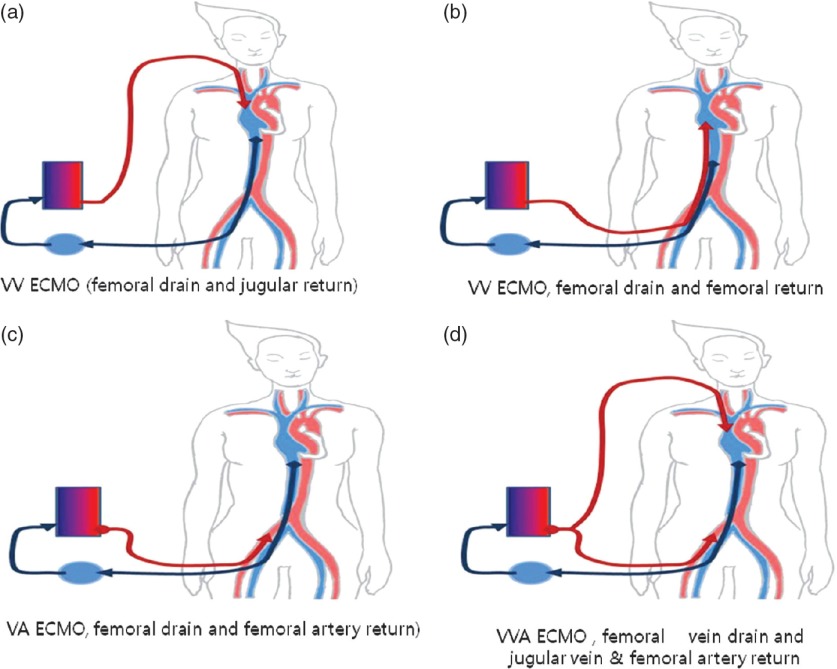
We used veno-venal ECMO (VV ECMO) for elective respiratory support. In the majority of cases involving VV ECMO, the right femoral vein is used for venous drainage and the internal jugular vein is used for venous return (Fig. 1a). If patients were unable to lie down for intubation due to dyspnea or a high risk of respiratory arrest during induction, femoral vein drainage and femoral return to the right atrium was performed in the sitting position (Fig. 1b). If additional heart support was necessary during VV ECMO, we added a return cannula to the femoral artery and employed veno-arterial ECMO (VA ECMO; Fig. 1c). If cardiac arrest occurred due to breath-holding, we initially used VA ECMO (Fig. 1d). A single dose of sodium heparin (5000 IU/ml) was administered intravenously immediately before cannulation. Activated clotting time was maintained for approximately 160–190 s. However, we did not use heparin in cases of ongoing bleeding until bleeding had ceased. The non-heparin-coated cannula was inserted using the Seldinger technique under ultrasound guidance with either a FEM-FLEX2 femoral cannula (Edwards Lifesciences, Irvine, CA, USA) or a Medtronic DLP femoral cannula (Medtronic, Minneapolis, MN, USA). Cannulas were firmly fixed to the patient’s body to prevent decannulation. After cannulation, the heparin-coated ECMO circuit was connected and initiated using a Terumo EBS® (Terumo, Tokyo, Japan) or MAQUET PLS® (MAQUET, NJ, USA) machine.
Fig. 1.
(a) VV ECMO (femoral drain and jugular return). (b) VV ECMO (femoral drain and femoral return). (c) VA ECMO (femoral drain and femoral artery return). (d) VV ECMO (femoral drain and jugular vein and femoral artery return). VV ECMO: veno-venal extracorporeal membrane oxygenation; VA ECMO: veno-arterial extracorporeal membrane oxygenation
Results
ECMO during airway surgery
Elective use for induction anesthesia or surgery
ECMO was used in seven patients to support respiration, and to increase perioperative stability and safety, during trachea surgery. During the same period, we performed trachea surgery without ECMO in 16 cases. ECMO was indicated for four cases of severe tracheal stenosis, which were less than 5 mm, and a single case each of tracheobronchial stenosis, long segmental trachea injury and a large tracheal tumor. Tracheal resection and end-to-end anastomosis was performed in four patients, with carina resection and reconstruction, trachea tumor excision and pericardial patch repair, and direct tracheal repair each performed in a single case. VV ECMO was used in all seven patients; pre-intubation ECMO was additionally performed in three patients. Femoral drain and return ECMO was performed in three non-intubated patients who were not able to lie down for intubation due to dyspnea. In six cases ECMO was ceased immediately following the operation, with a single case of delayed ECMO weaning due to post-operative acute respiratory distress syndrome (ARDS). There was no mortality or morbidity related to ECMO during surgery (Table 1).
Table 1.
Elective ECMO use for induction anesthesia or surgery
| Sex (M/F)/Age (y) | Indication for ECMO | ECMO mode | Comments | Final result |
|---|---|---|---|---|
| M/9 | Posterior membranous portion injury | VA | Primary repair of trachea posterior membranous portion | Discharge |
| F/39 | Large trachea tumor | VV | Pericardial patch repair of posterior membranous portion | Discharge |
| M/15 | Carina stenosis | VV | Carina resection and reconstruction | ECMO weaning, death due to other causes (sepsis) on day 58 |
| F/47 | Trachea stenosis | VV | Trachea resection and end-to-end anastomosis | Discharge |
| M/23 | Trachea stenosis | VV | Trachea resection and end-to-end anastomosis | Discharge |
| F/28 | Trachea stenosis | VV | Trachea resection and end-to-end anastomosis | Discharge |
| M/50 | Trachea stenosis | VV | Trachea resection and end-to-end anastomosis | Discharge |
ECMO: extracorporeal membrane oxygenation; VV: veno-venous; VA: veno-arterial
Emergency use for induction anesthesia or surgery
In three cases of respiratory arrest ECMO was emergently inserted; two other patients developed respiratory failure during induction anesthesia, following total airway collapse due variously to a huge anterior mediastinal mass and tracheal tearing and intubation tube malpositioning. In a single case breath-holding occurred during emergency tracheostomy due to severe tracheal stenosis following thyroid cancer. In all three cases VA ECMO was performed; ECMO weaning failed in two of these patients, who died due to cardiac failure and hypoxic brain damage following delayed ECMO insertion and inappropriate resuscitation; in the remaining patient ECMO weaning was successful (Table 2).
Table 2.
Emergency ECMO use for respiratory arrest during anesthesia induction or surgery
| Sex (M/F)/Age (y) | Indication for ECMO | ECMO mode | Comments | Final result |
|---|---|---|---|---|
| M/74 | Respiratory arrest during induction anesthesia | VA | Trachea laceration from intubation tube during intubation. Delayed ECMO insertion and brain death | Death (day 3 post-ECMO) |
| M/24 | Respiratory arrest during induction anesthesia | VA | Extrinsic tracheal airway compression caused by a huge mediastinal mass during anesthesia | Discharge |
| F/78 | Respiratory arrest during tracheostomy | VV | Delayed ECMO insertion and brain death | Death (day 2 post-ECMO) |
ECMO: extracorporeal membrane oxygenation; VV: veno-venous; VA: veno-arterial
ECMO application during airway obstruction due to life-threatening massive hemoptysis
In five cases ECMO was emergently inserted to prevent death from airway blockade caused by massive hemoptysis due to Rasmussen’s aneurysm rupture, primary pulmonary hypertension, major lung laceration due to blunt trauma, aspergillosis, and lung cancer bleeding. We decided to insert ECMO in patients for whom maintenance of mechanical ventilation was not possible; recurrent hematoma evacuation was also performed to maintain the airway.
VV ECMO was performed initially in four cases, in one of which primary pulmonary hypertension-related bleeding developed during right heart failure; in this case we additionally inserted an arterial return cannula to decrease right ventricle pressure and support heart function. In a single case VA ECMO was performed for respiratory and cardiac support. In all cases ECMO was used to prevent death. Following recovery of respiration, endobronchial tamponade or SURGICEL packing was used to control airway bleeding, following which patients were stable without additional transfusion. In three cases, hemoptysis treatment was administered in the form of lobectomy, wedge resection and primary repair, or bronchial artery embolization. In two further cases no additional hemostasis, such as embolization, was required, and bleeding ceased naturally following SURGICEL packing and/or blood-clotting. In all five cases, absolute respiratory support was provided by ECMO during respiratory failure until hemostasis was successful. ECMO support was required for all patients due to severe ARDS caused by blood aspiration and massive transfusion. All patients were receptive to ECMO and a ventilator, but one patient, who was not discharged from the intensive care unit, died post-ECMO weaning. Another patient was transferred to a general ward but died from other causes post-ECMO weaning (Table 3).
Table 3.
ECMO use during airway obstruction due to life-threatening massive hemoptysis
| Sex (M/F)/Age (y) | Indication for ECMO | EMCO mode | Comments | Final result |
|---|---|---|---|---|
| M/76 | Airway obstruction due to hemoptysis (Rasmussen’s aneurysm) | VA | Bleeding controlled by bronchial artery embolization; post-bleeding ARDS developed. Successful bleeding control but failure of ECMO weaning. | Death due to ARDS (day 29 post-ECMO) |
| M/12 | Airway obstruction due to hemoptysis (pulmonary hypertension) | VV | Bleeding controlled by endobronchial tamponade with blood clot. Post- bleeding ARDS & right heart failure developed. ECMO Mode changed from VV to VA for right heart support & pulmonary artery pressure decompression | Discharge |
| F/38 | Airway obstruction due to hemoptysis (blunt lung trauma) | VV | Bleeding controlled by endobronchial tamponade with Surgicel (conservative treatment). VV ECMO support for 6 days due to post-operative ARDS. | Discharge |
| F/65 | Airway obstruction due to hemoptysis (lung cancer bleeding) | VV | Bleeding controlled by endobronchial tamponade with blood clot. VV ECMO support for t days due to post-operative ARDS. | Discharge |
| F/68 | Airway obstruction due to hemoptysis (Aspergillosis) | VV | Bleeding controlled by endobronchial tamponade with blood clot. Trachea posterior membranous injury developed due to intubation change. Awake VV ECMO support (conservative treatment of trachea injury). Successful ECMO weaning & ICU discharge | Death due to other causes (day 101- post-ECMO) |
ECMO: extracorporeal membrane oxygenation; VV: veno-venous; VA: veno-arterial; ARDS: acute respiratory distress syndrome; ICU: intensive care unit
Discussion
During induction anesthesia for severe airway obstruction, respiratory muscle tone compromises functional residual capacity such that the pressure in the pleural cavity increases causing airway obstruction. When airway obstruction occurs, mechanical ventilation is impossible and rapid maintenance of the airway is necessary. However, in certain cases intubation is problematic despite the application of interventional intubation techniques such as rigid- or fibro-bronchoscopy. In such cases, ECMO should be considered as a secondary option for respiration maintenance. Onozawa et al. first reported the successful use of ECMO in the treatment of adult airway obstruction due to thyroid cancer surgery.3) ECMO confers sufficient gas exchange and hemodynamic support to prevent death during airway surgery, and is now widely used to prevent various airway obstructions during airway surgery involving stenting, tracheostomy, and intubation.4–6)
ECMO use is increasing in cases requiring complicated trachea surgery, such as severe tracheal stenosis (due to intrinsic or extrinsic problems), carinal resection and reconstruction, congenital trachea surgery and long-segment trachea tumor.7–9) ECMO confers superior visualization at the surgical site compared with mechanical ventilation and does not require endotracheal tubes or aggressive ventilation techniques. Several studies have reported that ECMO reduces the risks of surgery in pediatric patients, allowing for more precise airway reconstruction. Although clinical data pertaining to ECMO use in adult patients is scarce, reports of its application during complex airway surgery are beginning to appear. Recently, Lang et al. retrospectively analyzed the data of 10 patients with thoracic malignancies who underwent complex tracheobronchial reconstruction under VA ECMO; complete resection was achieved in eight patients (80%) and there was no perioperative mortality or complications related to ECMO use.10) In addition to preventing death during induction for airway surgery, perioperative ECMO also increases intraoperative stability and safety.
When ECMO support is indicated for airway obstruction surgery, we recommend elective insertion in cases where the observed trachea stenosis was less than 5 mm using a bronchoscope or chest CT. Typically, the results of emergency ECMO for rescue of sudden respiratory arrest are poor, because rapid recovery of oxygen supply cannot be achieved. In a meta-analysis of studies of adult extracorporeal cardiopulmonary resuscitation, the average duration of cardiopulmonary resuscitation before extracorporeal life support was approximately 40 min, indicating that performing ECMO sufficiently rapidly to prevent hypoxic brain damage during sudden respiratory arrest is highly problematic.11) In our study, there was no mortality associated with elective use of ECMO during severe airway disease surgery but two deaths occurred due to hypoxic brain damage following delayed ECMO insertion. It is not necessary to use ECMO for all airway operations but elective ECMO is recommended when sudden respiratory arrest may occur during the perioperative period.
ECMO may also be indicated by incidental airway obstruction following endobronchial hematoma due to massive hemoptysis. In such cases, airway patency maintenance and control of bleeding should be performed simultaneously to prevent death, followed by definitive treatment such as bronchial arterial embolization (BAE); surgery should be undertaken for causative treatment. However, during massive hemoptysis hemostasis and airway maintenance are impossible; blood floods the airway such that the majority of patients die from hypovolemia or airway obstruction before BAE or surgery can be performed. Therefore, to increase patient survival during life-threatening hemoptysis, novel methods are required to prevent death until definitive treatment can be provided.
ECMO appears to be a highly effective method of preventing death; although not used widely for massive hemoptysis, in several case reports ECMO has prevented mortality before treatment. Bédard et al.12) performed ECMO before lobectomy in hypoxemia patients with repetitive hemoptysis due to an aortopulmonary collateral artery following a Fontan operation. Similarly, Yuan et al.13) applied ECMO in patients with massive hemoptysis due to blunt trauma. As these reports indicate, during ECMO respiratory maintenance and hemostasis is possible without ventilation. Control of bleeding represents the priority for treatment; after respiratory function has been recovered using ECMO, bleeding can be addressed without concern for airway blockade.
In conclusion, ECMO is useful during anesthesia in patients at high risk of airway blockade due to endobronchial bleeding, and during complex thoracic surgery. ECMO confers a safer environment during airway surgery, and its complication rate is acceptable. We recommend elective perioperative insertion of ECMO during thoracic surgery to reduce the risk of mortality.
Disclosure Statement
The authors report to Annals of Thoracic and Cardiovascular Surgery that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Acknowledgments
This study was supported by the Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital.
References
- 1).Granholm T, Farmer DL. The surgical airway. Respir Care Clin N Am 2001; 7: 13-23. [DOI] [PubMed] [Google Scholar]
- 2).Johnson NJ, Acker M, Hsu CH, et al. Extracorporeal life support as rescue strategy for out-of-hospital and emergency department cardiac arrest. Resuscitation 2014; 85: 1527-32. [DOI] [PubMed] [Google Scholar]
- 3).Onozawa H, Tanaka T, Takinami M, et al. Anesthetic management using extracorporeal circulation of a patient with severe tracheal stenosis by thyroid cancer. Masui 1999; 48: 658-61. [PubMed] [Google Scholar]
- 4).Chen A. ECMO-assisted rigid bronchoscopy for tracheal obstruction. J Bronchology Interv Pulmonol 2009; 16: 296-7. [DOI] [PubMed] [Google Scholar]
- 5).Kaneko T, Itani M, Komasawa N, et al. Anesthesia for tracheal metal stent management utilizing venovenous extracorporeal life support. Masui 2012; 61: 1269-72. [PubMed] [Google Scholar]
- 6).Hackner K, Bein T, Kuehnel T, et al. Elective use of extracorporeal lung assist: prevention of an airway disaster. Anaesthesist 2010; 59: 1008-12. [DOI] [PubMed] [Google Scholar]
- 7).Kunisaki SM, Fauza DO, Craig N, et al. Extracorporeal membrane oxygenation as a bridge to definitive tracheal reconstruction in neonates. J Pediatr Surg 2008; 43: 800-4. [DOI] [PubMed] [Google Scholar]
- 8).Hines MH, Hansell DR. Elective extracorporeal support for complex tracheal reconstruction in neonates. Ann Thorac Surg 2003; 76: 175-8. [DOI] [PubMed] [Google Scholar]
- 9).Keeyapaj W, Alfirevic A. Carinal resection using an airway exchange catheter-assisted venovenous ECMO technique. Can J Anaesth 2012; 59: 1075-6. [DOI] [PubMed] [Google Scholar]
- 10).Lang G, Ghanim B, Hötzenecker K, et al. Extracorporeal membrane oxygenation support for complex tracheo-bronchial procedures. Eur J Cardiothorac Surg 2015; 47: 250-6. [DOI] [PubMed] [Google Scholar]
- 11).Cardarelli MG, Young AJ, Griffith B. Use of extracorporeal membrane oxygenation for adults in cardiac arrest (E-CPR): a meta-analysis of observational studies. ASAIO J 2009; 55: 581-6. [DOI] [PubMed] [Google Scholar]
- 12).Bédard E, Lopez S, Perron J, et al. Life-threatening hemoptysis following the Fontan procedure. Can J Cardiol 2008; 24: 145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Yuan KC, Fang JF, Chen MF. Treatment of endobronchial hemorrhage after blunt chest trauma with extracorporeal membrane oxygenation (ECMO). J Trauma 2008; 65: 1151-4. [DOI] [PubMed] [Google Scholar]