Abstract
Sickle cell disease (SCD) is associated with high mortality for children under 5 years of age in sub-Saharan Africa. Newborn sickle screening program and enhanced capacity for SCD treatment are under development to reduce disease burden in Uganda and elsewhere in the region. Based on an international stakeholder meeting and a family-directed conference on SCD in Kampala in 2015, and interviews with parents, multinational experts, and other key informants, we describe health care, community, and family perspectives in support of these initiatives. Key stakeholder meetings, discussions, and interviews were held to understand perspectives of public health and multinational leadership, patients and families, as well as national progress, resource needs, medical and social barriers to program success, and resources leveraged from HIV/AIDS. Partnering with program leadership, professionals, patients and families, multinational stakeholders, and leveraging resources from existing programs are needed for building successful programs in Uganda and elsewhere in sub-Saharan Africa.
Keywords: sickle cell disease, newborn, screening, Uganda, family, community
Introduction
Sickle cell disease (SCD) is a major health burden in sub-Saharan Africa (SSA), affecting 250 000 annual births in more than 40 countries.1 Under 5 (U5) mortality from SCD is estimated at 75% in SSA, accounting for up to 1 in 6 U5 deaths. Many children affected by SCD die prior to diagnosis.1-4 Reducing the toll of SCD is a World Health Organization priority5,6 and is estimated to enable saving of up to 9 million lives globally, 85% in SSA.4
Justification for Screening
Dramatic reductions in U5 mortality from SCD from newborn screening were seen in the United States,5 Jamaica,7 and Brazil, and in regional programs in Ghana and Angola.2,3,8-10 Successful intervention requires a system linking screening to continuous standardized preventive and therapeutic care for affected infants and children before 3 to 4 months of age.2,3,8 Care includes enhanced vaccination, microbial prophylaxis, prevention for endemic malaria, and parental education for seeking care urgent linked to available health facilities4,8 A successful newborn SCD screening program in parts of Ghana has included successful but resource-intensive parent outreach via community-based health workers. These experiences highlight the effectiveness of intensive patient outreach and community-based initiatives in public health approaches, as well as the challenges for sustainability on a national scale.11,12
Why Uganda?
Improved overall U5 mortality rates in Uganda and most countries in SSA still exceed the UN Millennium Development Goal IV (www.ug.undp.org). Uganda has the highest reported birth prevalence of SCD.13 An estimated 20 000 children with SCD are born there each year, constituting 1% to 2% of the 1.5 million annual births and approximately 10% to 15% of all U5 deaths.14,15 To target high-risk children, the Ministry of Health (MoH) has identified improved pediatric outcomes of SCD as a key health priority, focused on newborn screening and follow-up care for affected children.16 Public health leadership in child health has established international collaborations with academic and public health partners to facilitate collective analyses and pilot interventions to launch a sickle screening program.17
Methods
Two Kampala-Based Meetings
To identify current perspectives and challenges for a nascent SCD screening program in Uganda, a stakeholder meeting was help in Kampala in January 2015. Leadership from the Departments of Paediatrics at Makerere University College of Health Sciences and Columbia University organized the meeting in January 2015 held at Makerere University. Discussion focused on SCD screening, notification, and care and treatment for affected children, and the feasibility of designing research to assess ways to effectively notify parents about test results and to enroll affected newborn into special medical care. Participants included officials from the MoH, senior Ugandan clinicians, SCD advocates, and a Tanzanian SCD expert (Table 1).
Table 1.
Attendees at the Stakeholder Planning Meeting of Newborn Sickle Screening in Kampala, Uganda, January 8, 2015.
| Name | Organization |
|---|---|
| Dr Sarah Kiguli | Head, Paediatrics Department, Makerere University School of Medicine |
| Dr Ezekiel Mupere | Senior Lecturer, Paediatrics Department, Makerere University School of Medicine |
| Dr Robert Opoka | Senior Lecturer, Paediatrics Department, Makerere University School of Medicine |
| Dr Phillip Kasirye | Paediatrics Department, Makerere University School of Medicine |
| Dr Phillip LaRussa | Pediatrics Department, Columbia University |
| Dr Nancy Green | Pediatrics Department, Columbia University |
| Dr Sanyukta Mathur | Mailman School of Public Health, Columbia University |
| Dr Magdalena Lyimo | Department of Haematology, Muhimbili University of Health and Allied Sciences, Tanzania |
| Dr Dan Kaye | Department of Obstetrics and Gynecology, Makerere University School of Medicine |
| Dr Jesca Nsungwa | Assistant Commissioner, Child Health, Ministry of Health, Uganda |
| Mr Charles Kiyaga | National Coordinator, Central Public Health Laboratories, Ministry of Health, Uganda |
| Dr Henry Ddungu | Senior Consultant, Haematologist, Uganda Cancer Institute |
| Dr Christopher Ndugwa | Leading Medical Expert, Uganda-American Sickle Rescue Fund (UASCRF) |
| Tusuubira Sharif Kiragga | Executive Secretary, UASCRF |
| Sister Florence Namusisi | Deputy In-charge, Sickle Cell Clinic, Mulago Hospital |
| Sister Twinomuhangi Sulphine | In-charge, Labour Ward, Mulago Hospital |
| Dr Nabirye Loy | Senior House Officer, Year II, Paediatrics Department, Makerere University School of Medicine |
| Dr Katumba Peter | Senior House Officer, Year III, Paediatrics Department, Makerere University School of Medicine |
| Dr Katasi Mwebe Victoria | Senior House Officer, Year I, Paediatrics Department, Makerere University School of Medicine |
| Mr Nsubuga Erostus | Parent and Board Member, UASCRF |
| Ms Victoria Tendo | Trained Sickle Cell Counsellor, UASCRF |
| Mr Sam Sendiwala | UASCRF |
| Annet Dolorence Namirembe | UASCRF office (Nsambya office) |
| Benard Sembyta | UASCRF office |
| Ms Agnes K. Lukoosi | Parent and health worker |
| Namuwaya Stella | Uganda Paediatric Association |
| Evelyn Bakengesa | Makerere University School of Medicine |
A separate meeting also informed this work. It was held in Kampala in June 2015 and was attended by several thousand people. Hosted annually since 2010 by the Uganda-American Sickle Cell Rescue Fund (UASCRF; www.uganda-americansickle.org/), the family-focused conference aimed to increase local awareness of SCD and enhance public health support. The meeting facilitated sharing of perspectives and discussion with patients and families and with local, regional, and international experts.
These meetings and discussions with global program experts in SCD and HIV/AIDS informed the interrelated perspectives to support the implementation of public health programs linking children with SCD to early diagnosis and care for SCD in Uganda and other high SCD burden areas in SSA. Additional perspectives on SCD, screening, and communication of results were obtained from interviews of 60 parents of young children in Kampala and in Rakai, a rural area in Uganda.
Results
Newborn Screening for SCD in Uganda
Leveraging existing capacity is a necessity in a resource-limited setting, in SSA often adapted from anti-HIV/AIDS public health efforts. Fundamental aspects of a new SCD screening program include resourcing the MoH Central Public Health Laboratory in Kampala for adequate capacity for high-volume screening from dried bloodspot samples.18 Logistically existing infrastructure for hub-based sample collection and delivery to a testing laboratory was adapted from the National Sample Referral Transport Network program from early infant HIV detection (EID)19 to ferry samples. The MoH identified the prevalence of sickle trait among young children,17 documenting previously estimated high risk of SCD.13 SCD screening began in regions with higher trait prevalence, indicating a higher population disease risk. Laboratory results are returned to the hubs through transmission of printed reports, and subsequently to local health facilities. The time from sample collection to local delivery of results takes approximately 28 days. However, the system does not include return of results directly to families. To date, mechanisms for informing families are locally determined and may not be systematic.
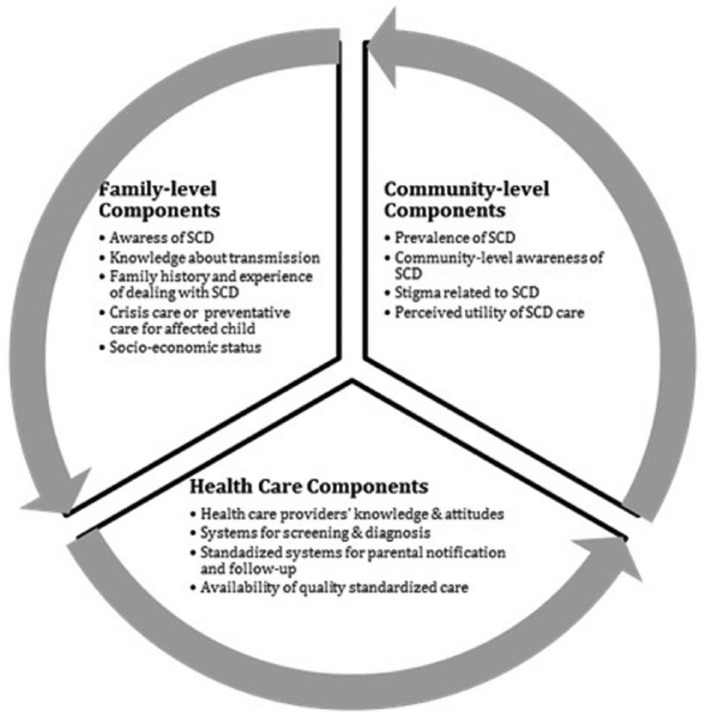
Postscreening, the health care system faces interrelated challenges of providing care by trained health providers, parental notification, linking affected children to timely medical follow-up, social and economic barriers to parental acceptance, education and counseling, and community-level recognition of benefits of screening and care (Figure 1). Understanding these aspects is critical for linking affected children and their families to effective care.
Figure 1.
Key components for multilevel public health response to SCD in Uganda.
Health System Capacity and Health Care Provider Perspectives of SCD Clinical Care
The first and largest specialized clinic for SCD treatment in Uganda was established in Kampala in 1968 at the Makerere University–affiliated Mulago Hospital. The SCD clinic offers care for children and adults and is open daily for routine preventive care or urgent day-long care services. The clinic is staffed by pediatricians, nurses, a medical record officer, a counselor, and volunteers, visiting professors, and rotating medical students. Appointments can be made for regular visits (eg, every 1-3 months), albeit with major challenges for transportation and other costs to families. Most patients receiving care at Mulago SCD clinic live in Kampala and nearby areas. An on-site laboratory performs some basic testing, while its pharmacy distributes some medications for routine preventive care. Families pay for extra laboratory testing, such as SCD diagnostic testing. Opportunities for parental counseling are limited. A comprehensive clinical database was established in 2010 for the 11 000 patients registered. By staff perspective, limitations exist in facilities, diagnostic analyses, staffing, medications, and counseling.
Health care providers acknowledged the need for expansion of SCD care and a system of regional hubs of SCD clinics and smaller local health facilities by general pediatric staff. For example, a smaller hospital in Kampala, Nsambya Hospital, maintains a weekly SCD clinic, currently providing care to approximately 100 patients through a stable cadre of pediatric doctors and nurses. Laboratory capacity includes excellent diagnostic capabilities.
Communication of Screening Results to Families
Stakeholders recognize timely parental notification of SCD aimed at early entry into special and continued care as a critical weak link for program success in SSA, resulting in delayed care and avoidable mortality.20-23 Effective telephone contact is required with parents to relate screening results. Preliminary counseling may be hampered by poor parental understanding, uneven phone contact, and other impediments. In several pilot SCD programs conducted in SSA, only 50% to 60% of parents with affected newborns could be reached by telephone with results, impeding enrollment into early care and diminishing the impact of screening.20,22,24-26 These challenges limit program impact.
Ghana’s regional newborn screening program for SCD has taken a more proactive approach for enrolling newborns into special care. While notification rates were nearly 90%, substantial resources were required to deploy community workers to parents’ homes to inform them about sickle cell results.20 Other potential communication tools include trained village health workers to perform counseling and guidance to parents to encourage visits to health facilities. However, sustaining the village health worker model of outreach might strain existing limited financial and human resources, unless tied to multiple key public health services.
Alternate approaches to parental notification are of interest. Uganda’s infant health care passport could support communication about SCD status between parents and providers. Passports are already provided to new mothers at birthing sites for recording immunizations and other routine care, and now include a section for SCD screening results. Pediatric providers could offer enhanced pediatric immunizations and reinforce to parents the need for ongoing medical attention. Despite the opportunities, experience with HIV EID has demonstrated that a more active communication system is needed to ensure timely notification and enrollment into care.
Opportunities Through Novel Approaches to Communication
Opportunities may exist for testing novel mobile health (mHealth) technologies to help reach parents with screening results. In recent years, use of mHealth has been proposed as an effective and efficient extension of “health information systems along the continuum of care.”27,28 Rapid growth of telecommunication networks provides the ability to reach a widely disperse population for patient and family communication, even in rural settings with low cost and scalability.29 SMS text messaging allows for multiple, flexible, automated, low cost, and scalable communications. Global applications of text messaging have included use for treatment compliance such as for TB or HIV/AIDS. For instance, the “Tanzania Youth Alliance” used text messaging in its male circumcision campaign as part of its HIV prevention efforts (http://www.tayoa.org/tayoa/?p=107).
An efficient, effective, scalable method to link screening, parental notification, and early entry into standardized care is needed to support public health efforts to strengthen programs for SCD screening and care, perhaps even for sickle trait identification, and it would greatly improve program feasibility, impact, and efficiency in Uganda and in SSA. Mobile health communication by cellular telephones and text messaging are in widespread use and are well accepted in Uganda.24,25 Texting has been effective in SSA for communication about HIV health care26-28 but has not been tested to support newborn screening notification.
Family and Community Needs
A study in Uganda found poor parental knowledge of the genetic cause of SCD, awareness of the potential for transmission of SCD to one’s children, and knowledge of one’s own sickle cell status.30 Limited understanding about SCD, confusion with HIV/AIDS, and social stigma around the disease could be highly stigmatizing for the family.31-33 Research in neighboring Kenya shows that, while parents supported treatment for SCD, they had concerns about disclosure of SCD and carrier status.31 Mothers are often blamed when their children are diagnosed with SCD and may lose the support of their husbands and extended family.32,34 Community perceptions of a hereditary and chronic condition greatly affect uptake of service uptake.
Two additional critical aspects must be recognized: (1) Financial burden to families from a serious chronic illness is a notable challenge. Families faced considerable costs for treatment and related travel and loss of parental productivity in caring for chronically ill children. Families may also be burdened with care of multiple family members with SCD. Further problematized could be costs and travel for specialty care at times when the child appears medically well. (2) Availability of carrier screening and counseling as part of prenatal and premarital services are also part of addressing SCD. Additional efforts on these aspects of care are of public health importance and should be built into disease measures, although perhaps triaged to later in the national approach to SCD.
Parent Barriers
Sixty parent interviews were conducted to inquire about barriers to the follow-up of newborn sickle screening for SCD and the use of mHealth for communication to guide follow-up of positive screens. One third of these parents had children with SCD. Parents identified multiple barriers to follow-up, such as travel cost or difficulties with missing work time. Despite proliferation of mobile phones, parents cited irregular telephone access from lost, stolen, or broken phones or challenges with charging phone batteries. Parents were not universally willing to communicate about their newborn’s health via text messaging due to lack of familiarity with personal messaging and the potential for misunderstanding of message content.
Promoting Public Awareness of SCD
Social barriers exist for improving SCD care and outcomes, including an overall lack of awareness about SCD, understanding of SCD as a medical condition, and its inheritance. As an example, a major local language, Luganda, does not have a term for SCD. Furthermore, the potential for improved outcomes through early and continuous preventative approaches and aggressive medical care for acute complications is generally poorly understood. Fundamental approaches to enhance social support for families include enhanced public awareness through a campaign for communicating about SCD; publication of evidence around care, treatment, and burden of SCD in Uganda; improve messaging about SCD to enhance social partnerships among affected families and communities; and to identify SCD-related services that are available throughout Uganda. Ultimately, sickle cell facilities could be mapped to enhance public access.
Advocacy is needed to garner public and even private resources for medical, counseling, and social needs of families affected by SCD. Stakeholders supported development of innovative community-based programs to provide support to families (www.uganda-americansickle.org). Such programs could be initiated at critical times, such as when newborns are diagnosed with SCD through screening. Participants reiterated that follow-up of screening-positive patients should be based in their homes, either by telephone call or through visits from volunteers or village health workers.
Lessons From Other Screening Efforts: Newborn Screening for SCD in Tanzania
Tanzania is another East African country with a large burden from SCD and child mortality.3 Like Uganda, relevant national health policies support the reduction of mortality from SCD. The Ministry of Health and Social Welfare in Tanzania has included SCD in the strategy for noncommunicable diseases and has developed a strategy for introducing SCD in the public health system. More recently, the “Newborn Screening for Child Survival - Sickle Cell Disease” Program was established, supported by the Ministry, with funding from the Wellcome Trust (www.muhimbili-wellcome.org/research/haematology) and technical support from the American Public Health Laboratories (www.aphl.org/globalhealth/pages/countries.aspx?country=Tanzania). Program aims are to screen newborn children for sickle cell and link them to comprehensive care. This follows a study of newborn screening, which demonstrated an 18.2% incidence of hemoglobinopathies.35 These efforts for widespread newborn screening need to be supported through concurrent development of regional SCD centers.9,22 As has been noted elsewhere in the region, increased public awareness and focus on communication with families about results and instructions for medical follow-up are critical aspects of programmatic success to improve health and decrease disease-associated mortality.10,20
Infant Anti-HIV/AIDS Screening Programs
Over the past decade, many countries in SSA have developed the capacity to diagnose and treat infants with HIV infection in the context of the global scale-up of HIV care and treatment services.36 Untreated, infant mortality is 50%.37 Similar to SCD, diagnosing infants early in life can translate into provision of lifesaving treatments for babies who are otherwise at high risk for death or significant morbidity from infections and other HIV-related conditions. Identifying infected infants from HIV-positive mothers requires EID testing. To support ongoing medical management, many high-HIV-burden countries have acquired sophisticated laboratory capacity for testing, most often at central or regional labs. Infant dried blood spot specimens obtained in routine maternal-child health clinics are transported to a central laboratory hub for testing. Establishing laboratory capacity has been a great accomplishment but only one critical step along the cascade of early infant diagnosis to successful treatment.36
Establishing effective systems for sending specimens and receiving results back in a timely fashion for EID have encountered significant challenges. Innovations that have been implemented include models for specimen transport and technological approaches to returning results.19,36,38 In parallel, engaging families and health care workers about the importance of continued care and early antiretroviral treatment initiation and identifying optimal messaging for communities and for service delivery has been an iterative and laborious process.36,39 Decentralization of care and treatment to the primary care level, coupled with enabling nurses and other nonphysician clinicians to prescribe antiretroviral treatments, are service delivery innovations that have been essential to the success of EID and infant HIV treatment programs.36,39
HIV EID programs in SSA provide crucial lessons for SCD, as well as a feasible infrastructure for early diagnosis.36 However, EID was only one component of a comprehensive scale-up of HIV services supported by unprecedented global investment.40 Funding through major international donors supports public sector costs for the majority of services, including laboratory reagents, health worker salaries, and antiretroviral and prophylactic treatments.39,40 Sufficiency of resources is critical for efforts to establish diagnostic and treatment services for children with SCD. As described for in Ugandan, adaptation of infrastructure originally developed for HIV to newborn screening systems will add to growing public health capacity needed for SCD.
Discussion
The Uganda MoH and other major stakeholders are committed to the success of the newborn screening program and medical care for SCD to reduce SCD-associated mortality in children. Key parties include providers, patients, patient advocates and families affected by SCD, multinational leaders, and researchers. Beyond Uganda, several countries in the region are now initiating or expanding their SCD-related efforts (www.muhimbili-wellcome.org/research/haematology), and informing others of program creation, problem solving, and lessons learned will be critical for successful new programs. International collaboration of academic and advocacy efforts will be critical for addressing the disease burden.
Identifying social, logistic, and financial barriers for children to receive timely and continuous care for SCD are critical aspects of research. Research is needed on behavioral attitudes, logistics, and motivators for parental follow-up. No single communication system will solve all of the barriers of notification and linkage to care, including challenges inherent to use of mobile phone technologies. Incentivizing the seeking of regular care will need to include education and counseling. Assessing public awareness and misperceptions of SCD can inform public health leadership and advocacy efforts.
Decreasing the burden of SCD in Uganda and throughout SSA requires multilevel public health commitments addressing familial, health service, and community perspectives and challenges. Innovation, collaboration, and adaptation will be needed to develop, test, and implement successful efforts for diagnosis, notification, and engagement into early and continuous care. To improve child health, newborn screening programs must extend beyond laboratory-focused practices to address impediments to screening effectiveness. Following newborn screening for SCD, effective systems that address health care and family and social components are needed to alleviate the burden of SCD. Expanding health systems to address medical needs related to SCD, enhancing public awareness about SCD and its treatability, and the social context for communication are critical for linking parents to effective care for their children. Ultimately, these efforts need to be formulated and implemented to optimize the impact of screening on U5 morbidity or mortality.
Author Contributions
NSG: Contributed to conception and design; contributed to analysis; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
SM: Contributed to conception and design; contributed to analysis; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
SK: Contributed to conception and design; contributed to analysis; drafted the manuscript; critically revised the manuscript; and gave final approval.
JM: Contributed to conception and design; contributed to analysis; critically revised the manuscript; and gave final approval.
VF: Contributed to analysis and drafted the manuscript.
PL: Contributed to conception and design; contributed to analysis; drafted the manuscript; critically revised the manuscript; and gave final approval.
ML: Contributed to conception and design; contributed to analysis; and critically revised the manuscript; and gave final approval.
EJA: Contributed to analysis and drafted the manuscript.
LM: Contributed to conception and design and contributed to analysis.
EM: Contributed to conception and design; contributed to analysis; critically revised the manuscript; and gave final approval.
Acknowledgments
Participants in the stakeholder meeting included representatives from Makerere University, Mulago Hospital, Uganda-American Sickle Cell Rescue Fund, Columbia University, and the Uganda Ministry of Health. The authors acknowledge a grant from the Columbia Policy Initiative in support of this initiative, and input from Dr Kwaku Ohene-Frempong, Professor of Pediatric Hematology at the University of Pennsylvania and consultant to the sickle cell newborn screening program in Ghana.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received a grant from the Columbia Policy Initiative.
References
- 1. Piel FB, Rees DC, Williams TN. Managing the burden of sickle-cell disease in Africa. Lancet Haematol. 2014;1(1):e11-e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in Africa: a neglected cause of early childhood mortality. Am J Prev Med. 2011;41(6 suppl 4):S398-S405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Makani J, Cox SE, Soka D, et al. Mortality in sickle cell anemia in Africa: a prospective cohort study in Tanzania. PLoS One. 2011;6:e14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010-2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10:e1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO Regional Office for Africa. Sickle-cell Disease: A Strategy for the WHO African Region. Brazzaville, Congo: WHO Regional Office for Africa; 2010. [Google Scholar]
- 6. WHO Regional Office for Africa. Sickle cell disease prevention and control. http://www.afro.who.int/en/clusters-a-programmes/dpc/non-communicable-diseases-managementndm/programme-components/sickle-cell-disease.html. Accessed February 28, 2015.
- 7. King L, Fraser R, Forbes M, Grindley M, Ali S, Reid M. Newborn sickle cell disease screening: the Jamaican experience (1995-2006). J Med Screen. 2007;14:117-122. [DOI] [PubMed] [Google Scholar]
- 8. Hiraki S, Green NS. Newborn screening for treatable genetic conditions: past, present and future. Obstet Gynecol Clin North Am. 2010;37:11-21. [DOI] [PubMed] [Google Scholar]
- 9. Makani J, Soka D, Rwezaula S, et al. Health policy for sickle cell disease in Africa: experience from Tanzania on interventions to reduce under-five mortality. Trop Med Int Health. 2015;20:184-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohene-Frempong K, Oduro J, Tetteh H, Nkrumah F. Screening newborns for sickle cell disease in Ghana. Pediatrics. 2008;121(suppl 2):S120-S121. [Google Scholar]
- 11. Phillips JF, Jackson EF, Bawah AA, et al. The long-term fertility impact of the Navrongo project in northern Ghana. Stud Fam Plann. 2012;43:175-190. [DOI] [PubMed] [Google Scholar]
- 12. Simmons R, Fajans P, Ghiron L. eds. Scaling Up Health Service Delivery: From Pilot Innovations to Policies and Programmes. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 13. Okwi AL, Byarugaba W, Ndugwa CM, Parkes A, Ocaido M, Tumwine JK. An up-date on the prevalence of sickle cell trait in Eastern and Western Uganda. BMC Blood Disord. 2010;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Serjeant G, Ndugwa C. Sickle cell disease in Uganda: a time for action. East Afr Med J. 2004;80:384-387. [DOI] [PubMed] [Google Scholar]
- 15. UNICEF. Uganda, 26 November 2013: Government of Uganda launches national drive to end preventable child and maternal deaths. http://www.unicef.org/esaro/5440_uganda_preventable-deaths.html. Accessed September 24, 2014.
- 16. Ministry of Health. Monitoring & Evaluation Plan for Health Sector Strategic & Investment Plan 2010/11-2014/15. Entebbe, Uganda: Government of Uganda; 2011. [Google Scholar]
- 17. Ndeezi G, Kiyaga C, Hernandez AG, et al. Burden of sickle cell trait and disease in the Uganda Sickle Surveillance Study (US3): a cross-sectional study. Lancet Glob Health. 2016;4:e195-e200. [DOI] [PubMed] [Google Scholar]
- 18. Kiyaga C, Sendagire H, Joseph E, et al. Consolidating HIV testing in a public health laboratory for efficient and sustainable early infant diagnosis (EID) in Uganda. J Public Health Policy. 2015;36:153-169. [DOI] [PubMed] [Google Scholar]
- 19. Kiyaga C, Sendagire H, Joseph E, et al. Uganda’s new national laboratory sample transport system: a successful model for improving access to diagnostic services for Early Infant HIV Diagnosis and other programs. PLoS One. 2013;8:e78609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McGann PT, Ferris MG, Ramamurthy U, et al. A prospective newborn screening and treatment program for sickle cell anemia in Luanda, Angola. Am J Hematol. 2013;88:984-989. [DOI] [PubMed] [Google Scholar]
- 21. Tshilolo L, Kafando E, Sawadogo M, et al. Neonatal screening and clinical care programmes for sickle cell disorders in sub-Saharan Africa: lessons from pilot studies. Public Health. 2008;122:933-941. [DOI] [PubMed] [Google Scholar]
- 22. Makani J, Ofori-Acquah S, Nnodu O, Wonkam A, Ohene-Frempong K. Sickle cell disease: new opportunities and challenges in Africa. ScientificWorldJournal. 2013;2013:193252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Therrell BL, Padilla CD, Loeber JG, et al. Current status of newborn screening worldwide. Semin Perinatol. 2015;39:171-187. [DOI] [PubMed] [Google Scholar]
- 24. Hettiarachchi M, Amarasena S. Indicators of newborn screening for congenital hypothyroidism in Sri Lanka: program challenges and way forward. BMC Health Serv Res. 2014;14:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rahimy M, Gangbo A, Ahouignan G, Alihonou E. Newborn screening for sickle cell disease in the Republic of Benin. J Clin Pathol. 2009;62:46-48. [DOI] [PubMed] [Google Scholar]
- 26. Rahimy MC, Gangbo A, Adjou R, Deguenon C, Goussanou S, Alihonou E. Effect of active prenatal management on pregnancy outcome in sickle cell disease in an African setting. Blood. 2000;96:1685-1689. [PubMed] [Google Scholar]
- 27. Aranda-Jan CB, Mohutsiwa-Dibe N, Loukanova S. Systematic review on what works, what does not work and why of implementation of mobile health (mHealth) projects in Africa. BMC Public Health. 2014;14:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Catalani C, Philbrick W, Fraser H, Mechael P, Israelski DM. mHealth for HIV treatment & prevention: a systematic review of the literature. Open AIDS J. 2013;7:17-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mookherji S, Mehl G, Kaonga N, Mechael P. Unmet need: improving mHealth evaluation rigor to build the evidence base. J Health Commun. 2015;20:1224-1229. [DOI] [PubMed] [Google Scholar]
- 30. Okwi A, Byarugaba W, Ndugwa C, Parkes A, Ocaido M, Tumwine J. Knowledge gaps, attitude and beliefs of the communities about sickle cell disease in Eastern and Western Uganda. East Afr Med J. 2009;86:442-449. [DOI] [PubMed] [Google Scholar]
- 31. Marsh V, Kombe F, Fitzpatrick R, Williams TN, Parker M, Molyneux S. Consulting communities on feedback of genetic findings in international health research: sharing sickle cell disease and carrier information in coastal Kenya. BMC Med Ethics. 2013;14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marsh VM, Kamuya DM, Molyneux SS. “All her children are born that way”: gendered experiences of stigma in families affected by sickle cell disorder in rural Kenya. Ethn Health. 2011;16:343-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nzewi E. Malevolent Ogbanje: recurrent reincarnation or sickle cell disease? Soc Sci Med. 2001;52:1403-1416. [DOI] [PubMed] [Google Scholar]
- 34. Fullwiley D. Biosocial suffering: order and illness in urban West Africa. BioSocieties. 2006;1:421-438. [Google Scholar]
- 35. Rwezaula SS. Tanzania Scholarly Digital Library, Muhumbili University and Allied Health Sciences, Thesis, 2010. [Google Scholar]
- 36. Fayorsey RN, Saito S, Carter RJ, et al. Decentralization of pediatric HIV care and treatment in five sub-Saharan African countries. J Acquir Immune Defic Syndr. 2013;62:e124-e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ciaranello A, Lu Z, Ayaya S, et al. Incidence of World Health Organization stage 3 and 4 events, tuberculosis and mortality in untreated, HIV-infected children enrolling in care before 1 year of age: an IeDEA (International Epidemiologic Databases to Evaluate AIDS) East Africa regional analysis. Pediatr Infect Dis J. 2014;33:623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chib A, Wilkin H, Hoefman B. Vulnerabilities in mHealth implementation: a Ugandan HIV/AIDS SMS campaign. Glob Health Promot. 2013;20(1 suppl):26-32. [DOI] [PubMed] [Google Scholar]
- 39. El-Sadr WM, Holmes CB, Mugyenyi P, et al. Scale-up of HIV treatment through PEPFAR: a historic public health achievement. J Acquir Immune Defic Syndr. 2012;60(suppl 3):S96-S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Howard AA, Gasana M, Getahun H, et al. PEPFAR support for the scaling up of collaborative TB/HIV activities. J Acquir Immune Defic Syndr. 2012;60(suppl 3):S136-S144. [DOI] [PMC free article] [PubMed] [Google Scholar]