Abstract
Objective. To determine male vaccination rates with quadrivalent human papillomavirus vaccine (HPV4) before and after the October 2011 national recommendation to routinely immunize adolescent males. Methods. We reviewed HPV4 dose 1 (HPV4-1) uptake in 292 adolescent males in our urban clinic prior to national recommendations and followed-up for HPV4 series completion rates. After national recommendation, 248 urban clinic and 247 suburban clinic males were reviewed for HPV4-1 uptake. Factors associated with HPV4-1 refusal were determined with multiple logistic regression. Results. Of the initial 292 males, 78% received HPV4-1 and 38% received the 3-dose series. After recommendation, HPV4-1 uptake was 59% and 7% in urban and suburban clinics, respectively. Variables associated with HPV4-1 uptake/refusal included time period, race, type of insurance, and receipt of concurrent vaccines. Conclusions. HPV4-1 vaccination rates in our urban clinic were high before and after routine HPV vaccine recommendations for adolescent males. Our vaccination rates were much higher than in a suburban practice.
Keywords: HPV, human papillomavirus, human papillomavirus vaccine, immunizations, male, adolescent
Introduction
In June 2006, the US Food and Drug Administration licensed the use of quadrivalent Human Papillomavirus Vaccine types 6, 11, 16, and 18 (HPV4) in females aged 9 to 26 years for the prevention of cervical, vaginal, vulvar, and perianal cancers; precancerous lesions; and anogenital warts caused by the human papillomavirus (HPV).1 In October 2009, HPV4 approval was extended to males in the same age range under permissive recommendation by the Advisory Committee on Immunization Practices (ACIP) and became a routine recommendation in October 2011. HPV infections in boys and men may result in anogenital condylomata, recurrent respiratory papillomatosis, cancers and precancers of the penis and anus, and various head and neck cancers. Additionally, male vaccination might decrease transmission of the virus to females, thereby reducing disease incidence beyond the effect of female-only vaccination and resulting in a gender-neutral vaccination strategy.2 Studies of HPV4 utilization in males demonstrate a nearly 90% efficacy in preventing male genital warts and >98% success in preventing high-grade neoplastic lesions.3-5 HPV4 vaccine is both highly immunogenic and safe.6-8 The cost for the vaccine is covered by most health insurance plans, and a federal Vaccines for Children program is available.9-10
Despite HPV4’s incredible potential to decrease the cancer burden and to improve reproductive and overall health, national data reveal continued low coverage in adolescent girls receiving one or more doses of the vaccine and even lower rates in males. In its first year of permissive recommendation, the Centers for Disease Control and Prevention (CDC) National Immunization Survey–Teen (NIS-teen) noted a coverage rate of 1.4% among adolescent males.11 This only increased to a rate of 8.3% in 2011 after its new universal recommendation.12 We investigated male HPV4 vaccination uptake in our community after both permissive and universal recommendation, and we compared the rates of HPV4 vaccination in 2 different patient care settings.
Methods
The study was divided into 2 parts. In Part 1, we assessed all 11- to 18-year-old males (N = 292) seen for annual physical examinations in an urban child health clinic serving low-income families in Dayton, Ohio, over a 13-month period ending in April 2011. Participants were offered the first dose (HPV4-1) of the newly approved HPV4 vaccine. The participants and their parents or legal guardians were given comprehensive information on HPV4 by a physician, with a strong recommendation to receive the vaccine. The number of participants consenting to and refusing the vaccine at this initial offering was documented along with any reasons for refusal. Data collected for each participant included the month of visit, participant age, race (black vs white/other), insurance type (public vs private/self-pay), number of siblings, number and type of comorbidities, accompanying parent/guardian, parent/guardian education level, home residence type based on zip code (urban, suburban, rural), care provider (pediatrician or pediatric resident physician), and whether the participant received any other vaccines at the visit. Other vaccines included tetanus toxoid-reduced diphtheria toxoid-acellular pertussis vaccine (TdaP), meningococcal-conjugate vaccine (MCV), varicella zoster vaccine (VZV), and/or hepatitis A doses 1 and 2 (HepA1/A2). TdaP and MCV vaccination rates (received at any time) were also documented. Age was right-skewed and was summarized with median (interquartile range [IQR]) and also categorized as 11.0 to 12.9 versus 13.0 to 17.9 years. Median age and number of comorbidities were compared between the participants who consented to the vaccine and those who refused with Mann-Whitney U tests. Categorical variables were compared with χ2 tests, or Fisher’s exact tests if one or more expected cell frequencies were less than 5. Variables with P values ≤.20 were then entered into a logistic regression analysis and adjusted odds ratios (AORs) with 95% confidence intervals (CIs) were determined for initial HPV4 vaccine refusal. Prior to the logistic regression, independent variables were assessed for multicollinearity. Variance inflation factors greater than 3.0 were considered indicative of multicollinearity.
In November to December 2012, we reviewed the same charts of the 292 participants to determine completion rates for the 3-dose HPV4 series for participants who initially consented to the vaccine. We also determined subsequent acceptance of the HPV4-1 vaccine as well as completion rates for the participants who initially refused the vaccine.
In Part 2 of the study, we assessed HPV4-1 uptake in 2 additional groups of participants following the October 2011 ACIP national recommendation to vaccinate all adolescent males: 248 consecutive adolescent males seen from October 2011 through October 2012 in the same urban clinic as Part 1 participants, and 247 seen during the same time period in a suburban private practice. Data collected for these 2 groups included age, race, type of insurance, residence type based on zip code, whether other vaccines were given at the visit, and vaccination rates for TdaP and MCV. HPV4-1 uptake was then compared among Part 1 and Part 2 participants with a χ2 test followed by 2-group comparisons with Bonferroni corrections applied. All participants were then combined, and comparisons between participants who refused versus consented were made for time period, clinic type, and the variables listed above with χ2 tests. After assessment of multicollinearity, variables with P values ≤.20 were entered into a multiple logistic regression analysis and AORs with 95% CIs were determined for initial HPV4 vaccine refusal. The study was approved by the institutional review board of Dayton Children’s Hospital, Dayton, Ohio.
Results
Part 1: HPV4 Uptake and Completion Rates in Urban Clinic Adolescent Males Prior to the 2011 ACIP Recommendation
Characteristics of Participants and Comparisons Between Those Who Refused Versus Consented
A total of 292 adolescent males were seen during the study period, with 227 (78%) consenting to the HPV4 vaccine at the initial offering. The greatest number of visits occurred during the summer months (41%), and the fewest occurred during the winter months (10%). There was no difference in refusal rates by month of visit. The median (IQR) age of the participants was 13.9 (3.5) years, with no difference between participants who consented versus refused (P = .511). Median (IQR) number of comorbidities was 2.0 (2.0), range 0 to 6, with no differences between groups (P = .424). Table 1 shows other characteristics of the groups and the comparisons between participants who consented versus refused. Seventy-five percent of the participants were of black race, 95% were accompanied by their mothers, and 59% were seen by an attending pediatrician, with no differences for these variables. There were also no differences for the number of siblings, accompanying parent/guardian education level, or area of residence. Private/self-pay insurance type and not receiving other vaccines at the visit were significantly associated with refusal. The private/self-pay group included 4 self-pay participants—2 consented and 2 refused. Although the median number of comorbidities did not differ between groups, a higher proportion of participants who refused had at least one comorbidity compared to participants who consented. The most prevalent comorbidities were respiratory allergies (30%), overweight/obesity (23%), asthma (21%), attention deficit disorder/attention deficit hyperactivity disorder (19%), dermatologic conditions (17%), behavioral (9%), and orthopedic (9%). Of the comorbidities, only asthma was significantly associated with refusal in univariate analyses (18% of those consenting vs 29% of those refusing, P = .049). The majority of participants in both groups (≥94%) had completed both TdaP and MCV vaccinations, suggesting that refusal of the HPV-4 vaccine was not due to refusal of vaccines in general in these urban clinic participants. Table 2 shows AORs (95% CIs) for factors associated with vaccine refusal in Part 1 participants. The first model includes the variables insurance type, other vaccines at visit, comorbidities, asthma, physician type, and parent education level. Because 29% of the participants were missing parent education level, the multiple logistic regression was repeated without parent education level, in order to include all of the participants. In both models, factors that remained significantly associated with vaccine refusal were private/self-pay insurance, not receiving other vaccines at the visit, and the participant having at least one comorbidity. For 47/65 (72%) participants who refused the HPV4-1 vaccine, the accompanying parent/guardian gave the reason for refusal. The reasons are shown in Table 3. The most common reason was that the parent/guardian wanted to review materials first (51%), followed by wanting to think about it first (15%) and wanting to wait until a later date (11%).
Table 1.
Characteristics of Part 1 Participants Consenting to or Refusing the HPV4-1 Vaccine.
| Variable | Consenting to HPV4-1 (n = 227), n (%) | Refusing HPV4-1 (n = 65), n (%) | P Value |
|---|---|---|---|
| Age (years) | |||
| 11.0-12.9 | 73 (32) | 22 (34) | .798 |
| 13.0-17.9 | 154 (68) | 43 (66) | |
| Race | |||
| Black | 167 (76) | 45 (74) | .738 |
| White/other | 53 (24) | 16 (26) | |
| (n = 220) | (n = 61) | ||
| Insurance type | |||
| Private/self-pay | 31 (14) | 19 (29) | .003 |
| Public | 196 (86) | 46 (71) | |
| Comorbidities | |||
| No | 54 (24) | 8 (12) | .046 |
| Yes | 173 (76) | 57 (88) | |
| Received other vaccines at visit | |||
| No | 79 (35) | 37 (57) | .001 |
| Yes | 148 (65) | 28 (43) | |
| Received TdaP at any time | |||
| No | 4 (2) | 2 (3) | .618 |
| Yes | 223 (98) | 63 (97) | |
| Received MCV at any time | |||
| No | 6 (3) | 4 (6) | .237 |
| Yes | 221 (97) | 61 (94) | |
| Parent/guardian education level | |||
| <High school | 43 (26) | 8 (18) | .064 |
| High school graduate | 82 (51) | 19 (42) | |
| >High school | 37 (23) | 18 (40) | |
| (n = 162) | (n = 45) | ||
| Number of siblings | |||
| None | 59 (26) | 21 (32) | .515 |
| One | 60 (27) | 18 (28) | |
| Two or more | 107 (47) | 26 (40) | |
| (n = 226) | |||
| Residence zip code type | |||
| Urban | 150 (67) | 42 (65) | .299 |
| Suburban | 70 (31) | 19 (29) | |
| Rural | 5 (2) | 4 (6) | |
| (n = 225) | |||
| Physician type | |||
| Attending pediatrician | 128 (56) | 44 (69) | .076 |
| Resident pediatrician | 99 (44) | 20 (31) | |
| (n = 64) | |||
Abbreviations: HPV4-1, quadrivalent human papillomavirus vaccine, dose 1; TdaP, tetanus toxoid-reduced diphtheria toxoid-acellular pertussis vaccine; MCV, meningococcal-conjugate vaccine.
Note. Statistically significant P values are bolded.
Table 2.
Adjusted Odds Ratios and 95% Confidence Intervals for Factors Associated With Refusal of the HPV4-1 Vaccine in Part 1 Participants.
| Models and Variables | AOR (95% CI) |
|---|---|
| Model with parent education level included (n = 207) | |
| Private insurance/self-pay (ref. = public insurance) | 2.76 (1.15-6.61) |
| No other vaccines at visit (ref. = other vaccines given) | 2.47 (1.20-5.08) |
| Comorbidities = yes (ref. = no) | 2.76 (1.02-7.47) |
| Asthma = yes (ref. = no) | 1.67 (0.70-3.95) |
| Physician type = resident (ref. = attending physician) | 0.63 (0.30-1.34) |
| Parent education level (ref. = >high school) | |
| <High school | 0.41 (0.15-1.12) |
| High school graduate | 0.52 (0.23-1.17) |
| Model with parent education level excluded (n = 291) | |
| Private insurance/self-pay (ref. = public insurance) | 3.17 (1.56-6.45) |
| No other vaccines at visit (ref. = other vaccines given) | 2.84 (1.56-5.18) |
| Comorbidities = yes (ref. = no) | 2.57 (1.07-6.18) |
| Asthma = yes (ref. = no) | 1.79 (0.90-3.56) |
| Physician type = resident (ref. = attending physician) | 0.61 (0.32-1.13) |
Abbreviations: HPV4-1, quadrivalent human papillomavirus vaccine, dose 1; AOR, adjusted odds ratio; CI, confidence interval; ref., reference group.
Table 3.
Reasons for HPV4-1 Refusal Cited by Part 1 Participants’ Accompanying Parents/Caregivers.
| Reason for Refusal | n | Percentage of All Refusals (n = 65) | Percentage of Refusals With Cited Reasons (n = 47) |
|---|---|---|---|
| Wants to review materials first | 24 | 37 | 51 |
| Wants to think about it first | 7 | 11 | 15 |
| Wants to wait until next visit or later date | 5 | 8 | 11 |
| Wants to check with insurance first | 3 | 5 | 6 |
| Mother says son is too young | 2 | 3 | 4 |
| Mother says son is not sexually active | 2 | 3 | 4 |
| Mother wants to discuss with father first | 2 | 3 | 4 |
| Because it is a new vaccine | 1 | 1 | 2 |
| Mother not convinced son needs it | 1 | 1 | 2 |
| No reason cited | 18 | 28 | — |
Abbreviation: HPV4-1, quadrivalent human papillomavirus vaccine, dose 1.
HPV4 Completion Rates for Part 1 Participants
Of the 292 eligible participants, 227 (78%) consented when first offered HPV4-1, 17 (6%) initially refused, but later consented, and 48 (16%) refused. One-hundred eighty (74%) of the total 244 consenting participants returned for HPV4-2 and 110/180 (61%) for HPV4-3. Overall, 38% received the complete 3-dose HPV4 series, 24% received 2 doses, and 22% received 1 dose (Figure 1). None of the variables compared between consenting and refusing participants were associated with participants receiving HPV4-1 only, or returning for HPV4-2 alone or both HPV4-2 and -3 (all P values except for insurance type [P = .082] were greater than .250).
Figure 1.
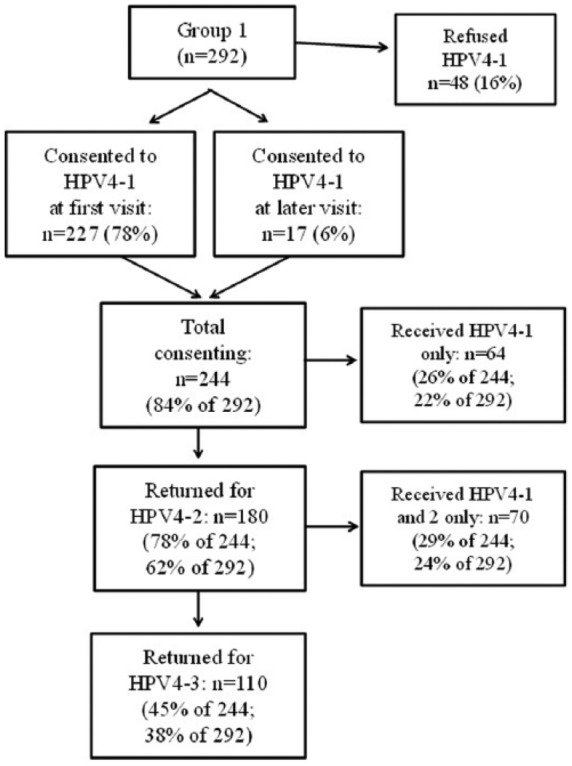
Flow chart of rates of HPV4 vaccine completion for Part 1 participants.
Part 2: HPV4 Uptake in Urban and Suburban Male Adolescents After the October 2011 ACIP Recommendation, and Refusal Rates for All Part 1 and Part 2 Participants Combined
The HPV4-1 uptake rates were higher in the urban clinic than the suburban private practice for participants in the post–October 2011 time period. For the urban clinic participants, 145/248 (59%) consented, while only 18/247 (7%) of suburban clinic participants consented (P < .01). Both were significantly lower than the 78% of urban clinic participants consenting prior to the October 2011 recommendation (P < .01). For postrecommendation participants, the median (IQR) age was 13.2 (3.3) for both clinics, but all other demographics were significantly different (compared with χ2 tests). The differences in proportions (urban vs suburban) were black race (74% vs 1%, P < .001), public insurance (85% vs 17%, P < .001), receipt of other vaccines at the visit (69% vs 21%, P < .001), TdaP and/or MCV at any time (98% vs 74%, P < .001), and residence type (64% urban, 31% suburban, 5% rural vs 6% urban, 90% suburban, 4% rural, P < .001).
Table 4 shows the comparisons between participants who consented to versus refused the HPV-4 vaccine. Overall, 390/787 (49%) consented. Compared to participants who consented, a higher proportion of participants who refused were white, had private/self-pay insurance type, lived in a suburban or rural zip code, did not receive other vaccines at the visit, never received TdaP or MCV vaccines, had visits in the postrecommendation time period, and were from the suburban private practice. The variable “clinic type” exhibited collinearity with residence type and race, and was excluded from a multiple logistic regression model incorporating all other significant variables (n = 774). The AORs (95% CIs) are shown in Table 5. All variables remained statistically significant except for living in a suburban zip code. The participants living in rural zip codes had a higher adjusted odds of refusal, but the sample size was small (n = 31).
Table 4.
Comparisons Between Participants Consenting to or Refusing the HPV4-1 Vaccine for All Part 1 and Part 2 Participants Combined.
| Variable | Consenting to HPV4-1 (n = 390), n (%) | Refusing HPV4-1 (n = 397), n (%) | P Value |
|---|---|---|---|
| Time period | |||
| Pre–October 2011 | 227 (58) | 65 (16) | <.001 |
| Post–October 2011 | 163 (42) | 332 (84) | |
| Clinic type | |||
| Urban | 372 (95) | 168 (42) | <.001 |
| Suburban | 18 (5) | 229 (58) | |
| Age (years) | |||
| 11.0-12.9 | 155 (40) | 171 (43) | .343 |
| 13.0-17.9 | 235 (60) | 226 (57) | |
| Race | |||
| Black | 273 (71) | 124 (32) | <.001 |
| White/other | 110 (29) | 269 (68) | |
| (n = 383) | (n = 393) | ||
| Insurance type | |||
| Private/self-pay | 70 (18) | 222 (56) | <.001 |
| Public | 320 (82) | 175 (44) | |
| Received other vaccines at visit | |||
| No | 120 (31) | 269 (68) | <.001 |
| Yes | 270 (69) | 128 (32) | |
| Received TdaP at any time | |||
| No | 5 (1) | 61 (15) | <.001 |
| Yes | 385 (99) | 336 (85) | |
| Received MCV at any time | |||
| No | 8 (2) | 69 (17) | <.001 |
| Yes | 382 (98) | 328 (83) | |
| Residence zip code type | |||
| Urban | 249 (64) | 117 (29) | <.001 |
| Suburban | 130 (34) | 258 (65) | |
| Rural | 9 (2) | 22 (6) | |
| (n = 388) | |||
Abbreviations: HPV4-1, quadrivalent human papillomavirus vaccine, dose 1; TdaP, tetanus toxoid-reduced diphtheria toxoid-acellular pertussis vaccine; MCV, meningococcal-conjugate vaccine.
Note. Statistically significant P values are bolded.
Table 5.
Adjusted Odds Ratios and 95% Confidence Intervals for Factors Associated With Refusal of the HPV4-1 Vaccine in Part 1 and Part 2 Participants Combined (N = 774).
| Variables | AOR (95% CI) |
|---|---|
| Time period = post–October 2011 (ref = pre–October 2011) | 4.39 (2.98-6.47) |
| White/other race (ref. = black) | 1.67 (1.12-2.49) |
| Private insurance/self-pay (ref. = public insurance) | 2.71 (1.80-4.10) |
| No other vaccines at visit (ref. = other vaccines given) | 3.79 (2.63-5.47) |
| TdaP and MCV never received (ref = 1 or both received at any time) | 3.96 (1.14-13.71) |
| Residence zip code type (ref. = urban) | |
| Suburban | 1.37 (0.92-2.04) |
| Rural | 3.18 (1.16-8.72) |
Abbreviations: HPV4-1, quadrivalent human papillomavirus vaccine, dose 1; AOR, adjusted odds ratio; CI, confidence interval; ref., reference group; TdaP, tetanus toxoid-reduced diphtheria toxoid-acellular pertussis vaccine; MCV, meningococcal-conjugate vaccine.
Discussion
HPV vaccination of adolescent girls and boys has been recommended by the ACIP since 2006 and 2011, respectively.1 Both the available quadrivalent (HPV4) and bivalent (HPV2) vaccines protect against HPV types that cause 70% of cervical cancers. HPV4 vaccine protects against infection with HPV types that can cause 90% of genital warts. Despite these afforded protective effects, there has been slow uptake of the vaccine overall.13 While rates of HPV vaccination are low in both sexes, the rates among boys and young men are much lower than for girls. Our analysis occurred shortly after the ACIP recommendations for vaccination of males. The initial recommendations for male vaccination were considered permissive (category). Such a recommendation required individual clinical decision making by the provider to actively consider the benefits and risks of HPV vaccination for each patient and help the patient/parent decide whether to be vaccinated. A formal recommendation to routinely vaccinate all boys came shortly after that. Our study addressed HPV vaccine acceptance among males in our area during these time periods.
In Part 1 of our study, we analyzed vaccination rates among our low-income, urban clinic. Most of the adolescents were black (75%) and almost all were accompanied by their mothers (95%). Each of our physicians utilized a strong recommendation with their patients and parents for HPV vaccine delivery. We found that subject acceptance of the initial vaccine in the HPV4 vaccine series among males at our low-income urban clinic was high during the first year of permissive recommendation (78%). These rates are much higher than reported national rates among males, with the most recent analysis of adolescent HPV immunization rates noting that only 34.6% of boys received ≥1 HPV vaccine in 2013.13 Vaccine refusal occurred significantly more often among those who had either private insurance or were self-pay. Conversely, individuals were more likely to receive the HPV vaccine if they received additional vaccines during the visit. A recent study demonstrated similar findings in that patients were apt to receive the HPV vaccine when it was discussed in context with other due vaccines and when it was offered in the context of expectant provision.14 Subjects with a history of asthma were also seemingly less likely to accept the HPV4 vaccine. Importantly, among both HPV vaccine acceptors and refusers, the vast majority (94%) had received both TdaP and MCV vaccines. This would suggest that refusal to receive HPV vaccine was not associated with vaccine refusal in general within this population. Approximately 40% of the participants completed the entire 3-dose series. Of reasons cited for vaccine refusal, most wanted to better review supplied informational materials prior to vaccination.
After universal HPV immunization was recommended, both vaccine uptake and setting comparisons were made between adolescent males at our urban clinic and at a suburban private practice. Many of the demographic characteristics of the clinic population studied were statistically different than that seen in the private practice including higher percentage of black race, residence in an urban location, receipt of concurrent adolescent vaccines at the visit, and public insurance status. The number of consenting subjects in the private practice was markedly lower than that of the urban clinic (7% vs 59%, respectively, P < .01), and was similar to the poor HPV4 vaccination numbers among males seen nationally.11 Compared to HPV4, receipt of other adolescent vaccines, TdaP and MCV, was found to be higher in both locations. Again, the suburban practice had statistically lower rates. In Part 2 of the study, after all participants were included in analyses of vaccine uptake following formal ACIP recommendations, nonvaccinators were more likely to be white, have private insurance, not receive concurrent vaccines at the initial visit, had never received prior MCV or TdaP vaccines, and were patients of the suburban practice. Other studies document that vaccines tend to be more often refused by above-poverty whites.15
It is not surprising that a participant would be more likely to receive a vaccine if insurance paid for the vaccine, especially if its cost were high and required multiple doses. Similarly, the expectation of receiving a number of vaccines at a single visit appeared to increase the likelihood that one also receives HPV4 vaccine. This is somewhat contrary to the apprehensions that some parents have when it comes to simultaneously administering multiple vaccines to their child, as some caretakers limit the number of vaccinations given to their children in a single visit. Studies addressing parental requests to “spread out” the vaccination schedule highlight the reality that certain parents are concerned about simultaneous vaccinations.16 While it is unclear as to why increased rates of HPV4 uptake occurred when concurrent immunizations were administered, it may simply reflect subject assent to an additional recommended vaccine in the setting of an expectant provision of other scheduled immunizations.
Discussions with patients and their parents addressing the importance of HPV prevention through immunization is a critical educational piece. Strong practitioner recommendations are important factors in increasing patient vaccine utilization. Prior studies have shown a positive correlation between immunization and physician recommendation of the HPV4 vaccine in females.17,18 A recent survey noted that more parents of male teens undergoing HPV vaccination (71.7%) reported receiving a recommendation compared to parents of unvaccinated teens (25.7%).13 Similarly, another study found that the most common reason cited by parents for nonvaccination of their adolescent sons was “My doctor or health care provider has not recommended it.”19 Such a strong “pro-vaccination culture” of a practice could positively influence vaccine acceptance. Additionally, the high HPV4 vaccine acceptance among our urban clinic subjects could be reflective of the generally high acceptance rate of all recommended vaccines among our overall clinic population: 93% in 2011 for non-HPV4 vaccines among patients 2 years of age or greater.
While important, not all studies, however, correlate a strong practitioner recommendation with acceptance of HPV4 vaccination. In one study, patient-provider communication did not correlate with HPV4 vaccination despite patients’ high satisfaction ratings of their providers,20 suggesting that still other factors contribute to HPV4 vaccine acceptance or refusal. Another study noted that despite physician recommendations, parents increasingly intended not to vaccinate their adolescent daughters with HPV4.21 Reasons for refusal included “not sexually active,” “safety concerns/side effects,” “not recommended,” or “not needed or necessary,” similar to the responses given in our clinic. This suggests that physician recommendation alone may not be enough to increase uptake and only reinforces the multifactorial nature of vaccine reception. Moreover, males of all ages generally have poor knowledge and awareness of HPV infection, morbidity, transmission, and prevention.22 Knowledge gaps and misconceptions about the HPV4 vaccine continue to exist, including the continued misbelief that the vaccine is intended only for those who are sexually active. Both lack of knowledge and the belief that HPV vaccine is not needed are frequently cited by parents as reasons not to vaccinate their adolescent child.13 The role of the vaccine as a preventive measure prior to sexual activity must be stressed in patient education.
In our urban clinic, HPV4 vaccine acceptance was quite high after ACIP recommendations for universal adolescent male vaccination. Yet we experienced a significant decrease in coverage rate when compared to the previous permissive-recommendation period. The reasons for this drop are not apparent. It is possible that the decreased rates of vaccine acceptance is due partly to either continued lack of patient knowledge about the significance of HPV infection and vaccine recommendations and/or lack of that particular topic’s emphasis by the provider. The recommended vaccine changes may not be prioritized in parental decision-making. The drop in the acceptance of HPV4 vaccine could also be secondary to an increasing concern for safety and efficacy: the percentage of parents concerned about vaccine safety increased from 4.5% in 2008 to 17% in 2010.21 Despite a federally authorized recommendation for routine coverage, parents increasingly intend not to vaccinate female teens.21
The concept of male HPV4 vaccination is generally seen as acceptable to parents and adolescents. Consequently, why are HPV vaccine rates still low? Studies have highlighted the mismatch between apparent vaccine approval and the willingness to vaccinate one’s own self or son: one study detected a striking difference between the general acceptability of the HPV4 vaccine in men (prior to recommendation in 2009) and actual uptake rates.21 Another documented a discrepancy between parents’ general support of male HPV4 vaccination and intentions to have their own sons vaccinated.23 Some patients would be willing to encourage vaccination of everyone except themselves. We did not explore our patients’ intentions or thoughts about vaccinating others and may foresee this as future project.
Perhaps the most striking finding in Part 2 of our study was the difference in uptake rates between the urban and suburban settings. Of the site characteristics, race, insurance type, and receipt of concurrent vaccines were statistically significant. The difference in racial makeup was very large, and it is possible that cultural and ethnic contributions factor into the differences in vaccine rates. However, a previous investigation demonstrated little difference among racial groups in receiving the HPV4 vaccine. The study characterized HPV4 vaccination attitudes among a low-income minority patient group very similar to our urban clinic population. However, across all racial groups represented in their study (black, Latino, white), there were no differences in acceptance among parents of the patients.24 In contrast, the CDC has reported preliminary racial uptake pattern differences: in both males and females, HPV4 initiation among whites was lower than for blacks and Hispanics, but receipt of subsequent HPV4 doses among those who received HPV4-1 was higher among Hispanics and whites than blacks.12 This suggests that white patients are less likely to receive the first dose but, once received, were more likely to complete the series. Consistent with this, our data show that the predominantly white suburban clinic had much lower rates of HPV4-1 uptake than the predominantly African American clinic.
An additional factor influencing HPV4 vaccination is that our urban clinic is a teaching clinic with residents and learners. These individuals generally are permitted more time with each patient, which, in turn, might contribute toward acceptance of the vaccine secondary to sustained efforts in recommending HPV vaccine. Further understanding of these factors and their influences are critical to developing more effective vaccination strategies.
Completion rates for male HPV4 immunization in the urban clinic during Part 2 of the study prior to universal recommendation were in line with the national average for females.12 National tracking data for male completion rates have not yet been published. Our full-series completion rate of 38% is not optimal, but the most likely contributing factor to this is a well-known loss to follow-up trend that occurs with adolescents. Low adolescent compliance can account for a majority of visit losses for any clinical reason, not just for administration of HPV4 vaccine. A recent randomized clinical trial has proposed a 2-dose schedule for adolescent girls.25 Should this prove effective, such a recommendation could directly affect the not infrequent occurrence of teenagers inability to return and receive all of their vaccines.
HPV4 vaccination rates continue to lag behind those of other recommended adolescent vaccines (TdaP and MCV).12 Our data are consistent with this, as TdaP and MCV coverage is higher than HPV4-1 in both urban and suburban settings. However, adolescent coverage of TdaP and MCV is also higher in the low-income clinic than the private practice, reinforcing the low vaccination trend in the latter site. Attitudes toward HPV4 differ from TdaP and MCV predominantly because of the relationship of HPV infection to sexual activity, and the stigma associated with sexually transmitted diseases. Studies have not demonstrated an association between HPV vaccination and increased risky sexual behaviors.26 Still there continues to be concern among some in the public about the vaccine promoting risky sexual behavior.
A limitation of the study includes the fact that several participants in the urban clinic were lost to follow-up such that HPV vaccine completion rates may have been effected. Finally, findings from our study sites and participant population should not be directly generalized to other patient groups and clinical settings.
Conclusions
In summary, HPV4 vaccine acceptance among males remained high during both the permissive and universal routine vaccine recommendation periods. We documented a decrease in vaccine acceptance after national recommendations for universal vaccination of male adolescents. HPV4-1 uptake was greater in the low-income clinic, while the rates in the suburban private practice paralleled lower national rates. TdaP and MCV coverage was also greater in the low-income clinic than the private practice. Our male HPV4 completion rates were comparable to the generally higher rates noted among adolescent females. Overall, HPV4-1 uptake rates lag behind those of other recommended adolescent vaccines. We suggest that improved acceptance of HPV4 vaccination among teenage males might occur if parents and patients are provided with education and given strong practitioner recommendations during a clinic visit. Administration of HPV4 vaccine in concert with other recommended adolescent immunizations might also promote increased receipt of the vaccine.
Author Contributions
VCN: Contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
AS: Contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
MTN: Contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
GME: Contributed to conception; contributed to acquisition; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
SJA: Contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Acknowledgments
Special thanks to Sherry Hile, RN, BSN, Jan Gabriel, RN, BSN, Judy Bayes, RN, and the staff of Children’s Health Clinic and Cornerstone Pediatrics.
Footnotes
Authors’ Note: This article was presented in abstract form, in part, at the 2012 PAS Meeting in Boston, MA, and the 2013 PAS Meeting in Washington, D.C.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee for Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56(RR-2):1-24. [PubMed] [Google Scholar]
- 2. Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28:6858-6867. [DOI] [PubMed] [Google Scholar]
- 3. Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Georgousakis M, Jayasinghe S, Brotherton J, Gilroy N, Chiu C, Macartney K. Population-wide vaccination against human papillomavirus in adolescent boys: Australia as a case study. Lancet Infect Dis. 2012;12:627-634. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705-1708. [PubMed] [Google Scholar]
- 6. Pomfret TC, Gagnon JM, Jr, Gilchrist AT. Quadrivalent human papillomavirus (HPV) vaccine: a review of safety, efficacy, and pharmacoeconomics. J Clin Pharm Ther. 2011;36:1-9. [DOI] [PubMed] [Google Scholar]
- 7. Einstein MH, Baron M, Levin MJ, et al. ; HPV-010 Study Group. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: follow-up from months 12-24 in a phase III randomized study of healthy women aged 18-45 years. Hum Vaccin. 2011;7:1343-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302:750-757. [DOI] [PubMed] [Google Scholar]
- 9. Alexander KA. HPV vaccine: evolving indications and recommendations. Infect Dis Child. http://www.healio.com/pediatrics/vaccine-preventable-diseases/news/online/%7B18a3eb02-7655-4492-8552-1e1f794f08f0%7D/hpv-vaccine-evolving-indications-and-recommendations. Published April 1, 2011. Accessed March 22, 2016.
- 10. Centers for Disease Control and Prevention. HPV vaccine information for young women—fact sheet. http://www.cdc.gov/std/hpv/stdfact-hpv-vaccine-young-women.htm. Updated July 18, 2012. Accessed August 18, 2014.
- 11. Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:1117-1123. [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:844. [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescents, 2007-2013, and postlicensure vaccine safety monitoring, 2006-2014—United States. MMWR Morb Mortal Wkly Rep. 2014;63:620-624. [PMC free article] [PubMed] [Google Scholar]
- 14. Darden PM, Pope CA, Davis BH, et al. HPV vaccine and provider recommendation: what does that mean? Paper presented at: PAS Annual Meeting; April 26, 2015; San Diego, CA. [Google Scholar]
- 15. Smith PJ, Chu SY, Barker LE. Children who have received no vaccines: who are they and where do they live? Pediatrics. 2004;114:187-195. [DOI] [PubMed] [Google Scholar]
- 16. Kempe A, Daley MF, McCauley MM, et al. Prevalence of parental concerns about childhood vaccines: the experience of primary care physicians. Am J Prev Med. 2011;40:548-555. [DOI] [PubMed] [Google Scholar]
- 17. Block SL. Practicalities of implementing HPV vaccination in your pediatric practice. Infect Dis Child. http://www.healio.com/pediatrics/vaccine-preventable-diseases/news/online/%7Be1734dcf-7e84-4b4e-b7ae-1f2730d005fa%7D/practicalities-of-implementing-hpv-vaccination-in-your-pediatric-practice. Published April 1, 2011. Accessed March 22, 2016.
- 18. Caskey R., Lindau ST, Alexander GC. Knowledge and early adoption of the HPV vaccine among girls and young women: results of a national survey. J Adolesc Health. 2009;45:453-462. [DOI] [PubMed] [Google Scholar]
- 19. Donahue KL, Stupiansky NW, Alexander AB, Zimet GD. Acceptability of the human papillomavirus vaccine and reasons for non-vaccination among parents of adolescent sons. Vaccine. 2014;32:3883-3885. [DOI] [PubMed] [Google Scholar]
- 20. Rand CM, Schaffer SJ, Humiston SG, et al. Patient-provider communication and human papillomavirus vaccine acceptance. Clin Pediatr (Phila). 2011;50:106-113. [DOI] [PubMed] [Google Scholar]
- 21. Darden PM, Thompson DM, Roberts JR, et al. Reasons for not vaccinating adolescents: national immunization survey of teens, 2008-2010. Pediatrics. 2013;131:645-651. [DOI] [PubMed] [Google Scholar]
- 22. Zimet GD, Rosenthal SL. HPV vaccine and males: issues and challenges. Gynecol Oncol. 2010;117(2 suppl):S26-S31. [DOI] [PubMed] [Google Scholar]
- 23. Hernandez BY, Wilkens LR, Thompson PJ, et al. Acceptability of prophylactic human papillomavirus vaccination among adult men. Hum Vaccin. 2010;6:467-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perkins RB, Tipton H, Shu E, et al. Attitudes toward HPV vaccination among low-income and minority parents of sons: a qualitative analysis. Clin Pediatr (Phila). 2013;52:231-240. [DOI] [PubMed] [Google Scholar]
- 25. Dobson SR, McNeil S, Dionne M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309:1793-1802. [DOI] [PubMed] [Google Scholar]
- 26. Liddon NC, Leichliter JS, Markowitz LE. Human papillomavirus vaccine and sexual behavior among adolescent and young women. Am J Prev Med. 2012;42:44-52. [DOI] [PubMed] [Google Scholar]


