Abstract
Our objective was to evaluate the efficacy of daily enemas for the treatment of overactive bladder (OAB) in children. This study was a prospective, controlled trial of 60 children with nonneurogenic OAB. The control patients (40) were treated with standard therapies, including timed voiding, constipation treatment with osmotic laxatives, anticholinergics, and biofeedback physical therapy, whereas the treatment patients (20) received only daily enemas and osmotic laxatives. On assessment of improvement of OAB symptoms, only 30% of the traditionally treated patients’ parents reported resolution of symptoms at 3 months, whereas 85% of enema patients did. At the onset of the study, the average pediatric voiding dysfunction score of all patients was 14, whereas on follow-up, the average scores for traditionally treated patients and enema-treated patients were 12 and 4, respectively. This study demonstrated that daily enema therapy is superior to traditional methods for the treatment of OAB.
Keywords: voiding dysfunction, dysfunctional elimination, constipation, enema, incontinence
Introduction
Overactive bladder (OAB) is a common and vexing problem in children, and despite great advancement in therapies, a certain percentage of patients remains resistant to treatment. We believe that children whose symptoms do not resolve with timed voiding, laxatives, anticholinergic medications, and biofeedback physical therapy do so because of an undiagnosed and inadequately treated megarectum and that therapy directed specifically at the dilated rectum will resolve OAB symptoms most efficaciously. In this study, we evaluated the efficacy of daily enemas for the treatment of OAB in children.
Material and Methods
The study was approved by the institutional review board. This was a prospective, controlled trial of 60 children with nonneurogenic OAB. The inclusion criterion was a diagnosis of pediatric nonneurogenic OAB. Exclusion criteria included neurogenic cause of bladder dysfunction, urinary tract infection, prior lower-urinary-tract surgery, and any diagnosed anatomical abnormalities of the urinary tract that could influence voiding function, such as posterior urethral valves. OAB was defined as uncontrolled daytime urge incontinence, and bladder function was measured using the pediatric voiding dysfunction symptom score (DVSS).
The 40 control patients were treated with traditional therapies, including timed voiding, osmotic laxative PEG3350 (regardless of bowel history to maintain daily, soft bowel movements), and in select cases, anticholinergic medications and/or biofeedback therapy. The 20 remaining patients were prescribed only a daily enema (liquid glycerin suppository for ages 2 to 5, pediatric fleet enema for ages 6 to 11) and enough osmotic laxative to maintain soft spontaneous bowel movements, with no other therapy or voiding schedule. If the voiding symptoms resolved while on the daily enemas, patients were instructed to taper off the daily enemas over a 2-month time period (an enema every other day for a month, and then an enema twice weekly for a month). All patients were evaluated on each visit with complete history and physical, urinalysis, Bristol Stool Scale (BSS), Rome III criteria, KUB X-ray, and Pediatric Voiding Dysfunction Questionnaire. All children were followed up at 3 months.
Data analysis was performed using SPSS Statistics Version 23 (IBM Corp, Armonk, NY). For the nonparametric variables—DVSSs, BSS scores, and Rome III scores—comparisons were made using Mann-Whitney U tests. For the continuous variable—maximum rectal diameter on KUB—a student t test was used. Comparisons were made both within groups for the pretreatment and posttreatment phases as well as between groups.
Results
A total of 60 children (20 experimental and 40 controls) were included in this study. Table 1 demonstrates the mean DVSS, BSS, Rome III, and rectal diameters prior to treatment. There was no significant difference on any of these measurements between the control and treatment groups. Table 2 demonstrates the mean posttreatment measures and the mean change in each metric after the treatment period. Patients who underwent enema had significantly more improvement in DVSSs and significantly greater change in maximum rectal diameters than control patients (Figures 1 and 2). There was no difference in the amount of change between groups. Of note, both control and treatment groups demonstrated significant improvement in all measured variables after the treatment period.
Table 1.
Pretreatment Measurements.a
| Treatment Group | Control Group | Difference (P) | |
|---|---|---|---|
| DVSS | 14 | 14 | 0 (.68) |
| BSS | 3.55 | 3.95 | 0.4 (.12) |
| Rome III | 0.55 | 0.85 | 0.3 (.3) |
| Rectal diameter (cm) | 6.35 | 6.2 | 0.15 (.66) |
Abbreviations: DVSS, pediatric voiding dysfunction symptom score; BSS, Bristol Stool Scale.
There were no variables that were significantly different between the control and treatment groups prior to enema.
Table 2.
Measurements Done After the Treatment Phase.a
| Treatment Group | Control Group | Difference (P) | |
|---|---|---|---|
| DVSS | 4 | 12 | |
| Change from pretreatment | −10 | −2 | 8 (<.01) |
| BSS | 3.85 | 4.05 | |
| Change from pretreatment | 0.3 | 0.1 | 0.2 (.08) |
| Rome III | 0.1 | 0.2 | |
| Change from pretreatment | 0.45 | 0.65 | 0.2 (.45) |
| Rectal diameter (cm) | 2.15 | 5 | |
| Change from pretreatment | 4.2 | 1.2 | 3 (<.01) |
Abbreviations: DVSS, pediatric voiding dysfunction symptom score; BSS, Bristol Stool Scale.
Participants who underwent enemas had a significantly greater improvement in DVSSs and maximum rectal diameters.
Figure 1.
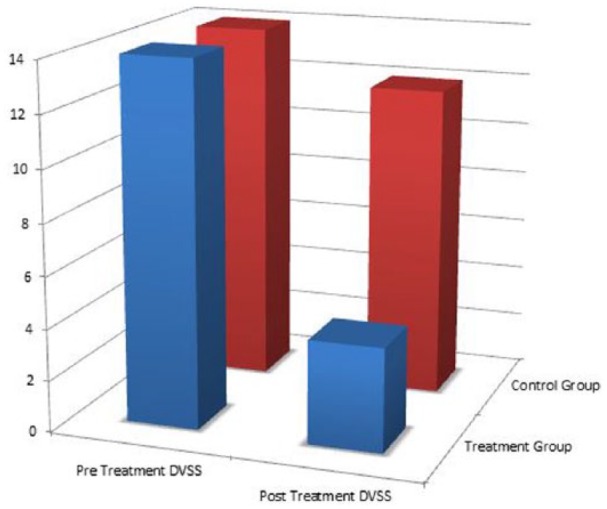
Pretreatment and posttreatment pediatric voiding dysfunction symptom score (DVSSs): the treatment group showed a significantly greater improvement in DVSS when compared with the control group.
Figure 2.
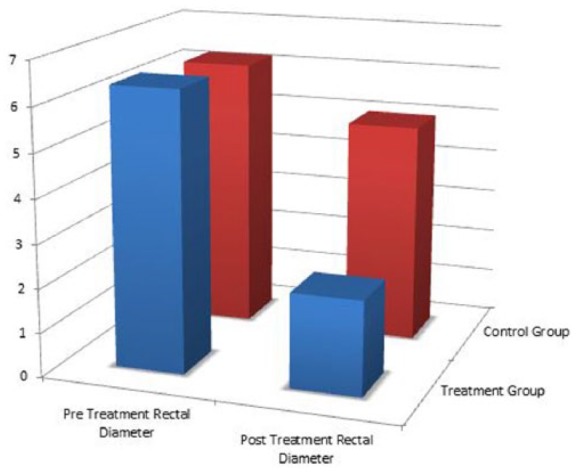
Pretreatment and posttreatment maximum rectal diameters: the treatment group also showed a significantly greater improvement in maximum rectal diameter on KUB when compared with the control group.
Discussion
The historical teaching regarding OAB of childhood was that a congenital obstruction interrupted urine flow and led to the development of detrusor hypertrophy and hyperactivity, and the accepted therapy was serial and repeated dilation of this obstruction.1 As time progressed, this anatomical obstruction was found to be the result of a willful dyssynergic contraction of the pelvic floor during voiding, and the treatment was changed to biofeedback physical therapy.2 This nonphsyiological contraction of the urethral sphincter was no medical curiosity but a disease process so severe that it could influence the natural history of many childhood disorders, such as vesicoureteral reflux and nocturnal enuresis, and in extreme cases induce renal failure.3,4
There is some debate among scientists as to whether this dyssynergic sphincter contraction is a learned or inborn condition.5 It is the author’s opinion that in children with an intact nervous system, uninhibited voiding dominates the infantile period of voiding prior to toilet training. This is most clearly represented by the progressive bladder growth in pre–toilet-trained children, with increasing compliance.6 This is mirrored in children with cerebral palsy, who demonstrate progressive bladder growth when they maintain an uninhibited, infantile voiding pattern.7
The teaching of bladder overactivity as the natural progression of an obstructed voiding pattern evolved from pathological models of voiding dysfunction such as posterior urethral valves and the neurogenic bladder, which develops in myelodysplastic patients with discoordinated sphincters.8-10 This led to the development of the modern model of dysfunctional elimination, an acquired condition where children paradoxically fail to relax their pelvic floor during elimination, resulting in bowel and bladder pathology.11,12 This study sought to investigate an alternative theory regarding the origins of dysfunctional elimination, with a therapy directed toward that cause to examine its benefits.
We have long known of the association between bowel and bladder dysfunction. This was first noticed in the 1960s, largely because of the presence of urinary symptoms in children with Hirschsprung’s disease. In his seminal work, Shopfner13 noted that the distention of the colon, especially the rectum, could have profound effects on bladder function.
O’Regan furthered this work with several groundbreaking studies linking rectal distention to bladder overactivity, with excellent success in treating nocturnal enuresis, urinary tract infections, and vesicoureteral reflux simply by alleviating this rectal distention. What has unfortunately hampered the development of this work has been the lack of a uniform definition of constipation.14-17
O’Regan defined constipation not as functional constipation, but mainly as the presence of fecal soiling, incomplete rectal emptying, and/or grossly decreased level of perception to balloon insufflation on anorectal manometry. The interesting discovery that O’Regan made was that often children with OAB symptoms presented with no functional signs of constipation, yet had markedly abnormal anorectal manometry studies. In other words, they often volitionally delayed defecation until the rectum distended to fill the anatomical pelvis and then would reach a new, abnormal homeostasis where stools would evacuate at regular intervals and with surprisingly normal appearance; yet the rectal tone would be so diminished as to have abnormal manometry studies. Put another way, these children were changing the rectum from a sensing organ (which in normal circumstances provides cues on the need to defecate), to a storage organ, with decreased sensation, and often resultant fecal soiling, but more often than not—normal stooling patterns.16
O’Regan early on discovered what we have also demonstrated in this study that parental reporting of their children’s bowel habits by BSS or Rome III criteria is often inaccurate, and even when accurate, often not helpful in diagnosing rectal distention in children with OABs.17 Not only that, but in children with completely normal bowel habits, rectal distention can be the main or sole cause of urinary symptoms, leading to compression of the bladder, uninhibited contractions, and often urethral obstruction, all of which has been proved years ago.13
The ability of rectal stool to induce uninhibited bladder contractions is well understood and was described as early as the 1980s; this work has been supported by numerous studies, although the exact mechanisms have yet to be defined. The uncanny ability of children to be cured of nocturnal enuresis by simply restoring normal rectal tone is a great testament to this relationship.16
What O’Regan proposed was that “in chronically constipated children; the rectum is never empty, necessitating the repetition or maintenance of rectal sphincter complex contraction to maintain fecal continence. Consequent concomitant urethral sphincter contraction occurs”16 p. 261. In other words, the pelvic floor contractions during voiding are not a willful process that can be unlearned, but a physiological response to stool withholding. And this could be reversed by directing therapy specifically at rectal dilation, in other words daily enemas, with excellent results.
So what if the modern theory of dysfunctional elimination and the resultant OAB is wrong? Our research points to a different cause altogether. We have proved that simply emptying the rectum repeatedly with the goal of restoring normal rectal tone resolves OAB in children more efficaciously than the standard of care. In fact, if the current model of voiding dysfunction were accurate, it should be impossible for our treatment to have been beneficial at all because we made no effort to influence pelvic floor function. Some would argue that our therapy would make pelvic floor contractions worse. Yet, in almost all children, the bladder symptoms resolved. And in children whose symptoms did not resolve, the enemas were often not successful in restoring rectal tone.
Conclusion
A daily enema regimen specifically targeted at restoring normal rectal tone is more effective than the standard of care for the treatment of OAB in children.
Author Contributions
SJH contributed to the conception and design; contributed to the acquisition, analysis, and interpretation of data; drafted the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. MC contributed to the analysis and interpretation of data and drafted the manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Milanovic D, Sremcevic D, Perovic S, Scepanovic D, Krstic Z. Stenosis of the external urethral opening as one of the causes of recurrent urinary infections in girls: surgical treatment [in Serbian]. Acta Chir Iugosl. 1989;36:229-237. [PubMed] [Google Scholar]
- 2. Palmer LS. Biofeedback in the management of urinary continence in children. Curr Urol Rep. 2010;11:122-127. [DOI] [PubMed] [Google Scholar]
- 3. Hinman F., Jr. Nonneurogenic neurogenic bladder (the Hinman syndrome): 15 years later. J Urol. 1986;136:769-777. [DOI] [PubMed] [Google Scholar]
- 4. Sillen U. Bladder dysfunction and vesicoureteral reflux. Adv Urol. 2008;(2008):815472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yeung CK. The normal infant bladder. Scand J Urol Nephrol Suppl. 1995;173:19-23. [PubMed] [Google Scholar]
- 6. Zerin JM, Chen E, Ritchey ML, Bloom DA. Bladder capacity as measured at voiding cystourethrography in children: relationship to toilet training and frequency of micturition. Radiology. 1993;187:803-806. [DOI] [PubMed] [Google Scholar]
- 7. Richardson I, Palmer LS. Clinical and urodynamic spectrum of bladder function in cerebral palsy. J Urol. 2009;182:1945-1948. [DOI] [PubMed] [Google Scholar]
- 8. Hodges SJ, Patel B, McLorie G, Atala A. Posterior urethral valves. ScientificWorldJournal. 2009;9:1119-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chung DE, Sandhu JS. Overactive bladder and outlet obstruction in men. Curr Urol Rep. 2011;12:77-85. [DOI] [PubMed] [Google Scholar]
- 10. Madersbacher H. Neurogenic bladder dysfunction in patients with myelomeningocele. Curr Opin Urol. 2002;12:469-472. [DOI] [PubMed] [Google Scholar]
- 11. Franco I. New ideas in the cause of bladder dysfunction in children. Curr Opin Urol. 2011;21:334-338. [DOI] [PubMed] [Google Scholar]
- 12. Koff SA. Relationship between dysfunctional voiding and reflux. J Urol. 1992;148:1703-1705. [DOI] [PubMed] [Google Scholar]
- 13. Shopfner CE. Urinary tract pathology associated with constipation. Radiology. 1968;90:865-877. [DOI] [PubMed] [Google Scholar]
- 14. O’Regan S, Schick E, Hamburger B, Yazbeck S. Constipation associated with vesicoureteral reflux. Urology. 1986;28:394-396. [DOI] [PubMed] [Google Scholar]
- 15. O’Regan S, Yazbeck S. Constipation: a cause of enuresis, urinary tract infection and vesico-ureteral reflux in children. Med Hypotheses. 1985;17:409-413. [DOI] [PubMed] [Google Scholar]
- 16. O’Regan S, Yazbeck S, Hamberger B, Schick E. Constipation a commonly unrecognized cause of enuresis. Am J Dis Child. 1986;140:260-261. [DOI] [PubMed] [Google Scholar]
- 17. O’Regan S, Yazbeck S, Schick E. Constipation, bladder instability, urinary tract infection syndrome. Clin Nephrol. 1985;23:152-154. [PubMed] [Google Scholar]


