Abstract
Hypoxia is known to be a major factor in the induction of angiogenesis during tumor development but its role in lymphangiogenesis remains unclear. Blood and lymphatic vasculatures are stimulated by the vascular endothelial family of growth factors – the VEGFs. In this review, we investigate the role of hypoxia in the molecular regulation of synthesis of the lymphangiogenic growth factors VEGF-A, VEGF-C, and VEGF-D. Gene expression can be regulated by hypoxia at either transcriptional or translational levels. In contrast to strong induction of DNA transcription by hypoxia-inducible factors (HIFs), the majority of cellular stresses such as hypoxia lead to inhibition of cap-dependent translation of mRNA and downregulation of protein synthesis. Here, we describe how initiation of translation of VEGF mRNA is induced by hypoxia through an internal ribosome entry site (IRES)-dependent mechanism. Considering the implications of the lymphatic vasculature for metastatic dissemination, it is crucial to understand the molecular regulation of lymphangiogenic growth factors by hypoxia to obtain new insights into cancer therapy.
Keywords: lymphangiogenesis, VEGF, hypoxia, transcription, translation
The Lymphatic Network
The lymphatic vasculature consists of a network of lymph vessels whose main function is to return protein-rich interstitial fluid to the circulating blood. Fluid, macromolecules, and cells, such as leukocytes and activated antigen-presenting cells, enter the lymphatic system through the blind-ended lymphatic capillaries. From here, lymph is transported toward collecting lymphatic vessels and is returned to the blood circulation in the jugular area through the lymphaticovenous junctions.1 On its way, lymph is filtered through the lymph nodes, where foreign particles taken up by antigen-presenting cells initiate specific immune responses.2 In the small intestine, lacteal lymphatic vessels inside the intestinal villi absorb the dietary fat released by enterocytes in the form of lipid particles called chylomicron. In addition to these physiologic functions, the lymphatic system contributes to pathologic conditions such as lymphedema, inflammatory diseases, and tumor metastasis. Many studies have demonstrated the existence of proliferative peri- and intratumoral lymphatic vessels.3 Additionally, tumoral lymphangiogenesis correlates with an increase in metastases,4,5 and detection of lymphangiogenic growth factors is associated with poor prognosis in many human tumors.6-8
Similar to blood capillaries, lymphatic capillaries are thin-walled, relatively large vessels composed of a single layer of endothelial cells, but they are not covered by pericytes or smooth muscle cells and have an absent or poorly developed basement membrane.9 In addition, they lack tight junctions and adherens junctions, which allows easy access for fluid, macromolecules, and cells to enter the vessel lumen.10 Endothelial cells of lymphatic capillaries are oak leaf-shaped and are characterized by discontinuous vascular endothelial (VE)-cadherin–positive button-like junctions. Collecting lymphatic vessels downstream have continuous zipper-like junctions previously described in blood vessels.9 Initial lymphatics combine to form larger vessels called precollectors and collectors, which in turn feed into four major groups of lymph nodes in the axillary and inguinal regions. Collecting lymphatic vessels have a smooth muscle cell layer, basement membrane, and valves.
Lymphatic Markers
Lymphatic vessels were first described in the beginning of the 17th century; however, the first growth factors and molecular markers specific for these vessels were discovered only 10 to 15 y ago. These growth factors include Prox1, the main transcription factor implicated in lymphatic vasculature development;11 lymphatic vascular endothelial-cell hyaluronan receptor-1 (LYVE-1),12 a new homolog of CD44 glycoprotein that is a lymph-specific receptor for hyaluronan;13 and podoplanin, a transmembrane glycoprotein molecule.14 Although the blood and lymphatic vascular systems are structurally related and function in concert, these lymphatic-specific markers have allowed investigation of the specific features of lymph vessels. Vascular endothelial growth factor receptor 3 (VEGFR-3, also known as FLT-4) has primarily been described as an major marker of lymphatics15,16 because its expression in adults becomes restricted to the lymphatic endothelium.17-19 However, recent studies have shown that VEGFR-3 is also upregulated on vascular endothelial cells in angiogenic sprouts and is present on vessels in tumors and wounds.20,21
Lymphangiogenesis in Pathology
In adult organisms, lymphangiogenesis takes place only in certain pathologic conditions. Abnormal function of the lymphatics is implicated in certain disease states, such as lymphedema, inflammation, immune diseases, and tumor metastasis.
Lymphedema is a disorder of the lymphatic vascular system characterized by impaired lymphatic return and swelling of the extremities. When the lymphatic system has been damaged during surgery or radiation treatment, its capacity to absorb excess water and cells from the interstitial space is reduced. If the transport capacity of the lymphatic system is reduced such so that it cannot handle this increase in lymphatic load, an insufficiency of the lymphatic system may occur. Lymphedema can be an unfortunate side effect of cancer treatment and is a chronic condition that, if ignored, can lead to disfigurement, immobilization, and severe infections. Without treatment, the swelling may continue to increase.
Inflammation is thought to contribute to the development and progression of various cancers, including lung,22 breast,23 gastrointestinal,24-26 ovarian,27 prostate,28 skin,29 and liver cancers.30 Inflammatory breast cancer exhibits increased angiogenesis and lymphangiogenesis and has a higher metastatic potential than noninflammatory breast cancer.31 Blocking lymphangiogenesis in chronic inflammatory diseases may be an important means of ameliorating the severity of some of these pathologies.
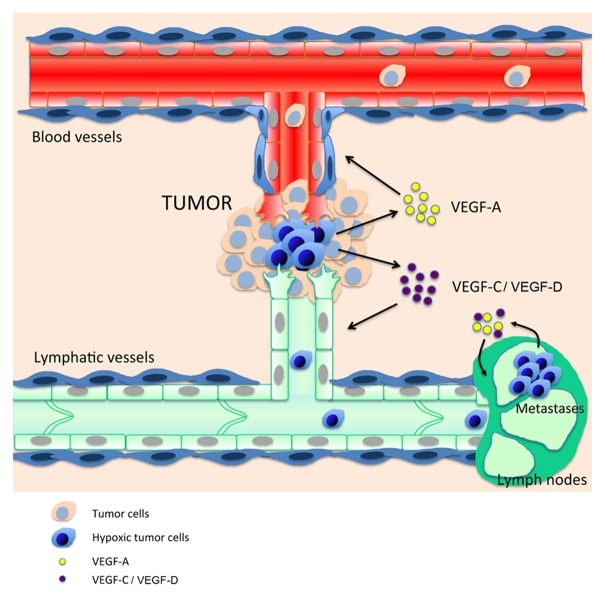
The extent of lymph node metastasis is a major determinant of staging and prognosis of most human malignancies. Although the clinical significance of lymph node involvement is well documented, the molecular mechanisms that promote tumor spread into the lymphatic or blood vascular systems and widespread dissemination are not well understood. Recent studies have provided a large body of evidence indicating that newly visualized lymphatics facilitate the formation of metastases. High tumor interstitial fluid pressure is thought to promote tumor cell entry into lymphatic vessels that have lower fluid pressure.32,33 Intratumoral lymphatic vessel growth often correlates with metastasis of human melanoma, breast, or head and neck cancers34-36 where tumor cells can be observed within lymphatic vessels, demonstrating that lymphatic vessel growth is important for tumor spread (Fig. 1).
Figure 1. Crosstalk between tumor hypoxia and the lymphatic and blood vasculatures. Hypoxic tumor cells (blue) near pre-existing blood and lymphatic vessels secrete angiogenic and lymphangiogenic growth factors such as VEGF-A, -C, and –D. Blood vessels bring oxygen and nutriments to tumor cells, whereas lymphatics drain debris and provide new routes for tumor metastasis. Lymphatic metastatic tumor cells maintain lymphangiogenic growth factors synthesis in this poorly oxygenated environment to promote lymph node lymphangiogenesis and establish the “metastatic niche.”
Tumor Growth, Hypoxia, and Lymphangiogenesis
As solid tumors grow, the cells within the expanding mass frequently become hypoxic because of the increasing distance from the nearest blood vessels. Without an adequate vascular supply, solid tumors can grow only to a critical size of 1–2 mm (approximately 106 cells), primarily due to a lack of oxygen and nutrients.37 A number of studies have been performed to characterize and ultimately inhibit tumor angiogenesis. However, since hypoxia also regulates the expression of lymphangiogenic factors, it is crucial to consider tumor hypoxia and tumor lymphangiogenesis as two tightly interlocked phenomena. In contrast to blood vessels, the lymphatic vasculature does not promote tumor growth by providing key elements for cell survival (i.e., oxygen and nutrients), but allows metastatic dissemination of solid tumors through lymph nodes and finally to distant organs.38,39 The lymphatic network is not merely an alternative vehicle to blood vessels for dissemination, but actually constitutes the main vascular system implicated in dissemination because lymphatic vessels have an optimal structure for tumor cell invasion. Indeed, the main difference between blood and lymphatic networks is the structure and permeability of their capillaries: whereas lymphatic capillaries are thin-walled, relatively large vessels, composed of a single layer of endothelial cells, lymphatic capillaries are not ensheathed by pericytes or smooth muscle cells and have little or no basement membrane.3 As a result of this high permeabilty, tumor cells can spread more easily in lymphatics than in blood vessels. Moreover, this invasion is also not just a passive process as tumors induce growth of new lymphatic vessels in draining lymph nodes and enlargment of lymphatic endothelium before metastasis. This remodelling of lymph nodes potentially contributes to the migration, implantation, or survival of metastatic tumor cells by inducing a specific tumor microenvironment. Thus, a hypoxic tumor will not only ensure its survival through activation of angiogenesis but will also become more aggressive. This dual regulation of blood and lymphatic vasculature by hypoxia during tumor growth impairs therapeutic efficacy. First, there is real crosstalk between the tumor and blood and lymphatic endothelial cells. Blood vessel endothelial cells produce lymphangiogenic factors such as vascular endothelial growth factor C (VEGFC), fibroblast growth factor 2 (FGF2), and platelet-derived growth factors (PDGFs) to facilitate tumor-induced lymphangiogenesis.40 Both endothelial cell types produce matrix metalloproteinases that promote tumor spreading. Lymphatic endothelial cells (LECs) also express the CCL21 chemokine that is physiologically implicated in dendritic cell mobilization41 and interacts with the CCR7 receptor expressed by many tumors to stimulate lymphatic dissemination.42 Several treatments have been developed specifically to inhibit tumor angiogenesis (e.g., Avastin) and therefore suppress tumor oxygenation and destroy tumor cells. However, these drugs also target lymphangiogenesis and would generate severe tumor hypoxia, which induces overexpression of lymphangiogenic factors and increased tumor dissemination. This cross talk between hypoxia and the two vascular systems and the resultant spread of the tumor can in part explain the failure of antiangiogenic drugs in cancer treatment (Fig. 1).
A key feature of the lymphatic system is its hypoxic environment as lymphatic vessels do not transport red blood cells. Lymph vessels are often located in remote areas away from oxygen-carrying blood vessels and are therefore exposed to a milieu with very low oxygen levels. Tumor cells have to adapt to this hostile hypoxic environment in order to spread to the lymph nodes.43-45
Normoxia is defined as a milieu where the O2 concentration is sufficient to ensure aerobic metabolism of cells, the basis of eukaryotic physiology.46 In contrast, hypoxia is an environment where the aerobic metabolism of cells is inhibited due to a lack of oxygen. The major cellular response to hypoxia is stabilization of hypoxia-inducible factor 1(HIF1). HIF1 is a transcription factor that controls the expression of a battery of more than 40 target genes.47-49 It is composed of an α subunit that is constitutively expressed and a β subunit that is subject to rapid ubiquitination and proteasomal degradation under normoxic conditions.50 The molecular basis for this regulation is the O2-dependent hydroxylation of proline residues 402 and 564 in HIF-1α by any one of three enzymes in mammals that have been designated prolyl hydroxylase-domain proteins or HIF-1α prolyl hydroxylases.51,52 Prolyl hydroxylation of HIF-1α is required for binding of the von Hippel–Lindau tumor suppressor protein (VHL), which is the recognition component of an E3 ubiquitin-protein ligase that targets HIF-1α for proteasomal degradation.53,54 Hypoxia has been shown to regulate not only angiogenesis, but also lymphangiogenesis by promoting overexpression of specific lymphangiogenic factors (e.g., VEGF-C) and growth factors that are shared by the vascular and the lymphatic vasculature (e.g., VEGF-A, FGF2). In this review we provide an overview of the link between lymphangiogenic factors and hypoxia and the consequences of this relationship in a well-known hypoxic pathology: the development and dissemination of solid tumors.
Hypoxia-Induced Molecular Regulation of VEGFs
The VEGF family is composed of growth factors involved in vascular development. This family includes VEGF-A, -B, -C, -D, and –E, and placental growth factor.55 All members of this family stimulate proliferation and migration of endothelial cells in vitro. These proteins bind and activate specific receptors on the endothelial cell surface: VEGF recognizes VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1); placental growth factor and VEGF-B recognize VEGFR-1; and VEGF-C and VEGF-D recognize VEGFR-2 and VEGFR-3 (Flt-4). Lymphangiogenesis is induced by VEGFs that promote angiogenesis (VEGF-A) or angiogenesis and lymphangiogenesis (VEGF-C and VEGF-D).
Hypoxia-induced gene expression was first described as a transcriptional mechanism mediated by hypoxia-responsive elements (HREs) present in the promoter region of different genes that are targets of the hypoxia-induced transcription factors (HIFs). In particular, a functional HRE has been identified within the 5′ flanking region element of the human VEGFA gene56,57 that is the target of both HIF1 and HIF2.58 This HRE allows transcriptional induction of VEGFA by hypoxia in several physiologic (i.e., wound healing, inflammation) and pathologic (i.e., ischemia, tumor development) states.
In addition to its transcriptional effects, hypoxia also regulates gene expression post-transcriptionally at the levels of mRNA stability and translation. An important class of mRNAs is stabilized by hypoxia: the so-called AU-rich mRNAs that bear AU-rich elements (AREs) in their 3′ untranslated regions (3′UTR).59,60 AREs are found in mRNAs of most genes coding for cytokines, growth factors, and proto-oncogenes (7–8% of the transcribed genome), indicating that the stabilization of such mRNAs in hypoxic conditions has important consequences regarding cell pathophysiology. In particular, angiogenic cytokines including VEGF-A are regulated by this mechanism.61 mRNA stabilization is controlled by the binding of HuR protein to the ARE in cooperation with polyA-binding protein interacting protein 2 (PAIP2).62 One proposed mechanism is that HuR acts in competition with destabilizing proteins such as AUF1 or tristetraprolin (TTP) to bind to the ARE.61 Another emerging concept is that HuR counteracts the binding of microRNAs to mRNA 3′UTRs.63 In the case of Vegfa mRNA, the HuR binding site overlaps with the binding site of miR-200b, thus HuR antagonizes the suppressive effect of this microRNA.63
Hypoxia also strongly regulates gene expression at the translational level. First, it silences global cell translation by inhibiting mRNA cap-dependent translation through inactivation of mTOR kinase. This results in hypophosphorylation of the 4E-BP protein, which then sequesters the cap-binding factor eIF4E.64,65 In addition, hypoxia induces phosphorylation of the initiation factor eIF2a by activation of PERK kinase, which also generates translational blockade.66 Two major alternative mechanisms are able to overcome this global inhibition of translation that is induced by hypoxia: upstream open reading frames (uORFs) and internal ribosome entry sites (IRESs). uORFs are a key element of translational control in response to stress. These elements precede the initiation codon of the mRNA main coding regions and are present in approximately 40–50% of mRNAs. They are primarily translational inhibitors when eIF2a is dephosphorylated and the complex of initiator tRNA with eIF2 and GTP is available for translation initiation. In contrast, they allow the ribosome to scan and reach the initiation codon of the main coding sequence in conditions of stress when eIF2a is phosphorylated.67
IRESs are RNA structural elements present in the 5′ non-translated regions of a small number of mRNAs that allow recruitment of the ribosome to a site that is a considerable distance from the cap structure, most frequently in the presence of trans-acting factors.64,68 The majority of identified IRESs are found in mRNAs of proteins associated with the control of cell growth and death, including growth factors, proto-oncogenes, and proteins required for apoptosis.65,69 IRES-dependent translation is cap-independent and, in the case of cellular mRNAs, independent of eIF2a phosphorylation; this allows translation to occur in stress conditions.64,70 Notably, HIF1α mRNA possesses an IRES, suggesting that these structure are crucial for translational regulation occurring under hypoxia. IRESs have also been identified in the mRNAs of three major lymphangiogenic growth factors, FGF2, VEGF-A, and VEGF-C.71,72 Interestingly, these IRESs are activated in hypoxic conditions, resulting in translational induction of these factors.45,73 The regulation of VEGF-A and -C expression and their relationship with hypoxia is discussed below.
VEGF-A
VEGF-A, also called vascular permeability factor, is a homodimeric glycoprotein with a molecular weight of approximately 45 kDa. At least 9 VEGF isoforms exist as a result of alternative patterns of splicing.74 Three of these, containing 121, 165, and 189 amino acids respectively, are preferentially expressed by VEGF-A producing cells75-77 Each of these isoforms contributes to formation of a VEGF-A gradient essential for the proper migration of ECs/LECs during angiogenesis or lymphangiogenesis. The larger species, VEGF-165, VEGF-189, and VEGF-206, are basic and bind to isolated heparin and heparin proteoglycans distributed on cellular surfaces and extracellular matrices whereas the smaller species, VEGF-121, is acidic and is freely diffusible.78 Although VEGF-A is mainly known as a growth factor that plays an essential role in physiologic and pathologic angiogenesis during both development and adulthood,79 it also has prolymphangiogenic properties.80,81 The proangiogenic activity of VEGF-A is mediated by interaction with a high-affinity VEGFR2 receptor, whereas the prolymphangiogenic activity is promoted by binding to the VEGFR2/R3 heterodimeric receptor.
In addition to its transcriptional upregulation during hypoxia, is probably the most highly post-transcriptionally regulated factor.74 The gene structure of VEGF-A has been predicted in silico (Fig. 2A). VEGFA mRNA contains two IRESs72 located upstream of the alternative initiation codons CUG and AUG that are responsible for synthesis of alternative isoforms of VEGF-A.74 Both IREs are activated by hypoxia.73 Vegfa IRESs are differentially regulated by an upstream ORF and by binding of Mir16 to the 3′UTR.82,83 A study of VEGF-A IRES trans-acting factors revealed tight regulation by both positive regulators activated by hypoxia (e.g., MAPK3 kinase) and negative regulators that are inhibited during this stress (e.g., DEAD-box RNA helicase 6).84 Another mechanism implicated in translation regulation of VEGF-A is riboswitch. Riboswitch refers to the ability of mRNAs to alter their folding structure and hence rate of translation in response to an environmental modification. During hypoxia, intracellular accumulation of heterogeneous nuclear ribonucleoprotein L (hnRNP L) promotes an active conformation and increases the rate of translation of VEGF-A mRNA.85 VEGF-A expression is strongly regulated at the level of mRNA stability, a process primarily mediated by the AREs present in the Vegfa mRNA. Indeed, the Vegfa mRNA is destabilized by several proteins including AU-rich element RNA-binding protein 1 (AUF1, also known as hnRNPD) and tristetraprolin (TTP), which target the AREs.61 Destabilization of Vegfa mRNA by TTP is responsible for its antiangiogenic activity.86 In contrast, Vegfa mRNA is stabilized by hypoxia.60 This process is mediated by binding of the RNA stabilizing protein HuR and its partner PAIP2 to the AREs, which prevents binding of the destabilizing proteins.60,61 Interestingly, the MDM2 protein, which is translocated from the nucleus to the cytoplasm under hypoxic conditions, increases Vegfa mRNA stabilization.87 Vegfa mRNA stability is thus controlled by interplay between stabilizing and destabilizing proteins that compete for binding to the AREs. Moreover, it has been proposed that export of VEGF-A mRNA from the nucleus and its loading onto ribosomes can be increased during hypoxia by extranuclear shuttling of mRNA-binding proteins such as hnRNP L and A1, which also regulate VEGF-A mRNA stability.88 These mechanisms, combined with transcriptional regulation induced by HIFs, allow fast and massive overexpression of VEGF-A in response to hypoxia.
Figure 2. Schematic representation of VEGF-A, -C, and -D mRNAs. (A) VEGF-A mRNA is characterized by a long 5′UTR (1038 nt) containing two internal ribosome entry sites (IRESs) (A and B). The VEGF-A gene encodes multiple isoforms generated by mRNA splicing of four constitutive and four alternative exons. (B) VEGF-C mRNA possesses a GC-rich 5′UTR containing one IRES. The secondary structure of VEGF-C IRES has been determined by shape analysis and contains 2 motifs (indicated by boxes) showing a similar reactivity pattern between human and mouse mRNA. (C) Similar to VEGF-C, the VEGF-D mRNA is generated from 7 exons.
VEGF-C
The VEGF-C/VEGFR3 signaling pathway is the major pathway implicated in lymphangiogenesis. First identified in 1996,89 VEGF-C is produced as a precursor protein that is activated by intracellular proprotein convertases.89,90 The secreted disulphide-linked VEGF-C subunits only bind VEGFR-3, but the factor is further proteolyzed in the extracellular environment by plasmin and other proteases to generate non–disulfide-linked homodimeric proteins with high affinity for both VEGFR-2 and VEGFR-3.3,90 VEGF-C is crucial for the induction of proliferation, migration, and survival of endothelial cells.91 VEGF-C is also an essential chemotactic and survival factor during embryonic lymphangiogenesis; homozygous deletion of VEGF-C leads to complete absence of lymphatic vasculature in mouse embryos whereas VEGF-C+/− mice display severe lymphatic hypoplasia. In VEGF-C null mice, lymphatic endothelial cells initially differentiate in the cardinal veins but fail to migrate and form primary lymph sacs.92 Although several studies have shown positive correlations between HIF-1α and VEGF-C in various cancers,93-95 for a long time the molecular mechanisms of hypoxia-induced regulation of VEGF-C remained poorly understood. The likelihood of direct transcriptional regulation of VEGF-C by HIF1α is low because the VEGF-C promoter does not contain a HRE sequence.96 Our recent work demonstrated the existence of a single IRES in the 5′UTR of both murine and human VEGF-C mRNA (Fig. 2B). We have demonstrated that VEGF-C IRES activity is upregulated in vivo during tumor growth in three murine models of carcinoma, similar to the IREs of FGF2 and VEGF-A.45 Strikingly, we also observed that VEGF-C IRES activity increases under hypoxia in vitro, but the presence of HIF-1α is not required in cultured cells.
VEGF-D
Binding of VEGF-D, also called c-fos induced growth factor, to its receptor VEGFR-3 promotes lymphangiogenesis. The VEGF-D gene encodes 7 exons (Fig. 2C). Maturation of VEGF-D is similar to that of VEGF-C and occurs by protein cleavage in N and C-terminal regions. VEGF-D has been poorly studied because of the lack of a phenotype resulting from its depletion in mice. Recent reports have shown that overexpression of VEGF-D induces tumor lymphangiogenesis and promotes lymphatic metastasis in mouse tumor models.97 However, few clinical studies have investigated the association between the expression of VEGF-D and lymphatic metastasis. VEGF-D overexpression correlates with an increase in lymphatic vessel growth and lymphatic metastasis.39 Recent studies suggest that VEGF-D is necessary for for the entry of tumor cells into the lymphatic system that results in metastasis.98 VEGF-D promotes structural changes in tumor-draining lymphatic vessels and induces vasodilatation. VEGF-D also increases the endothelial response to prostaglandin E2 (PGE2) by inhibiting the prostaglandin dehydrogenases (PGDH).99,100
The role of hypoxia in the promotion of VEGF-D expression has not been clearly established. Recent studies have demonstrated correlations between VEGF-D and HIF-1α expression in invasive breast ductal carcinoma101 and in resected esophageal squamous cell carcinoma.102
These findings revealed that expression of lymphangiogenic factors is tightly linked to hypoxia, which activates their expression at both transcriptional and translational levels. It is now well known that, at least in solid tumors, hypoxia is a major component of the tumor microenvironment and induces critical changes in tumor cell metabolism, angiogenesis, and lymphangiogenesis.
Concluding Remarks and Perspectives
The lymphatic vasculature has long been considered the poor relation of the blood vasculature. Compared with the vascular network, which provides both oxygen and nutrients and is therefore obviously necessary for life, the lymphatic system appeared to be a less important vascular network. In addition, until recently it was challenging to differentiate lymph from blood vessels due to lack of a specific marker. Recently, however, the lymphatic system has emerged as a crucial player during development and in adulthood. Although it is implicated specifically in chronic inflammatory and vascular pathologies (such as psoriasis and lymphedema), it is also able to interact with blood vessels in cancer. Indeed, recent studies have highlighted hypoxia-induced regulation of lymphangiogenic factors in the tumor microenvironent. Understanding the molecular regulation of lymphangiogenesis in a wide range of organs and pathologies might lead to new therapeutic solutions for diseases such as cancer.
Glossary
Abbreviations:
- ARE
AU-rich element
- FGF2
fibroblast growth factor 2
- HIF
hypoxia-inducible factor 1
- HRE
hypoxia-responsive element
- IRES
internal ribosome entry site
- LEC
lymphatic endothelial cell
- PDGF
platelet-derived growth factor
- uORF
upstream open reading frame
- UTR
untranslated region
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jeltsch M, Tammela T, Alitalo K, Wilting J. . Genesis and pathogenesis of lymphatic vessels. Cell Tissue Res 2003; 314:69 - 84; http://dx.doi.org/ 10.1007/s00441-003-0777-2; PMID: 12942362 [DOI] [PubMed] [Google Scholar]
- 2.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, et al. . Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest 2005; 115:247 - 57; http://dx.doi.org/ 10.1172/JCI200522037; PMID: 15668734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alitalo K, Tammela T, Petrova TV. . Lymphangiogenesis in development and human disease. Nature 2005; 438:946 - 53; http://dx.doi.org/ 10.1038/nature04480; PMID: 16355212 [DOI] [PubMed] [Google Scholar]
- 4.Williams CS, Leek RD, Robson AM, Banerji S, Prevo R, Harris AL, Jackson DG. . Absence of lymphangiogenesis and intratumoural lymph vessels in human metastatic breast cancer. J Pathol 2003; 200:195 - 206; http://dx.doi.org/ 10.1002/path.1343; PMID: 12754740 [DOI] [PubMed] [Google Scholar]
- 5.Mumprecht V, Detmar M. . Lymphangiogenesis and cancer metastasis. J Cell Mol Med 2009; 13:8A 1405 - 16; http://dx.doi.org/ 10.1111/j.1582-4934.2009.00834.x; PMID: 19583813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renyi-Vamos F, Tovari J, Fillinger J, Timar J, Paku S, Kenessey I, Ostoros G, Agocs L, Soltesz I, Dome B. . Lymphangiogenesis correlates with lymph node metastasis, prognosis, and angiogenic phenotype in human non-small cell lung cancer. Clin Cancer Res 2005; 11:7344 - 53; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-1077; PMID: 16243806 [DOI] [PubMed] [Google Scholar]
- 7.Zeng Y, Opeskin K, Horvath LG, Sutherland RL, Williams ED. . Lymphatic vessel density and lymph node metastasis in prostate cancer. Prostate 2005; 65:222 - 30; http://dx.doi.org/ 10.1002/pros.20288; PMID: 15948136 [DOI] [PubMed] [Google Scholar]
- 8.Stacker SA, Williams RA, Achen MG. . Lymphangiogenic growth factors as markers of tumor metastasis. APMIS 2004; 112:539 - 49; http://dx.doi.org/ 10.1111/j.1600-0463.2004.apm11207-0812.x; PMID: 15563315 [DOI] [PubMed] [Google Scholar]
- 9.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, et al. . Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 2007; 204:2349 - 62; http://dx.doi.org/ 10.1084/jem.20062596; PMID: 17846148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leak LV. . The structure of lymphatic capillaries in lymph formation. Fed Proc 1976; 35:1863 - 71; PMID: 1269772 [PubMed] [Google Scholar]
- 11.Wigle JT, Oliver G. . Prox1 function is required for the development of the murine lymphatic system. Cell 1999; 98:769 - 78; http://dx.doi.org/ 10.1016/S0092-8674(00)81511-1; PMID: 10499794 [DOI] [PubMed] [Google Scholar]
- 12.Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG. . Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem 2001; 276:19420 - 30; http://dx.doi.org/ 10.1074/jbc.M011004200; PMID: 11278811 [DOI] [PubMed] [Google Scholar]
- 13.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. . LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 1999; 144:789 - 801; http://dx.doi.org/ 10.1083/jcb.144.4.789; PMID: 10037799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breiteneder-Geleff S, Soleiman A, Horvat R, Amann G, Kowalski H, Kerjaschki D. . [Podoplanin--a specific marker for lymphatic endothelium expressed in angiosarcoma]. Verh Dtsch Ges Pathol 1999; 83:270 - 5; PMID: 10714221 [PubMed] [Google Scholar]
- 15.Karkkainen MJ, Ferrell RE, Lawrence EC, Kimak MA, Levinson KL, McTigue MA, Alitalo K, Finegold DN. . Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat Genet 2000; 25:153 - 9; http://dx.doi.org/ 10.1038/75997; PMID: 10835628 [DOI] [PubMed] [Google Scholar]
- 16.Kilic N, Oliveira-Ferrer L, Neshat-Vahid S, Irmak S, Obst-Pernberg K, Wurmbach JH, Loges S, Kilic E, Weil J, Lauke H, et al. . Lymphatic reprogramming of microvascular endothelial cells by CEA-related cell adhesion molecule-1 via interaction with VEGFR-3 and Prox1. Blood 2007; 110:4223 - 33; http://dx.doi.org/ 10.1182/blood-2007-06-097592; PMID: 17761831 [DOI] [PubMed] [Google Scholar]
- 17.Breslin JW, Gaudreault N, Watson KD, Reynoso R, Yuan SY, Wu MH. . Vascular endothelial growth factor-C stimulates the lymphatic pump by a VEGF receptor-3-dependent mechanism. Am J Physiol Heart Circ Physiol 2007; 293:H709 - 18; http://dx.doi.org/ 10.1152/ajpheart.00102.2007; PMID: 17400713 [DOI] [PubMed] [Google Scholar]
- 18.Bridenbaugh E. . Literature watch. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. Lymphat Res Biol 2005; 3:87 - 8; http://dx.doi.org/ 10.1089/lrb.2005.3.87; PMID: 16000057 [DOI] [PubMed] [Google Scholar]
- 19.Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, et al. . Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J 2001; 20:4762 - 73; http://dx.doi.org/ 10.1093/emboj/20.17.4762; PMID: 11532940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tammela T, Zarkada G, Wallgard E, Murtomäki A, Suchting S, Wirzenius M, Waltari M, Hellström M, Schomber T, Peltonen R, et al. . Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 2008; 454:656 - 60; http://dx.doi.org/ 10.1038/nature07083; PMID: 18594512 [DOI] [PubMed] [Google Scholar]
- 21.Petrova TV, Bono P, Holnthoner W, Chesnes J, Pytowski B, Sihto H, Laakkonen P, Heikkilä P, Joensuu H, Alitalo K. . VEGFR-3 expression is restricted to blood and lymphatic vessels in solid tumors. Cancer Cell 2008; 13:554 - 6; http://dx.doi.org/ 10.1016/j.ccr.2008.04.022; PMID: 18538738 [DOI] [PubMed] [Google Scholar]
- 22.Ardies CM. . Inflammation as cause for scar cancers of the lung. Integr Cancer Ther 2003; 2:238 - 46; http://dx.doi.org/ 10.1177/1534735403256332; PMID: 15035887 [DOI] [PubMed] [Google Scholar]
- 23.Van der Auwera I, Van Laere SJ, Van den Eynden GG, Benoy I, van Dam P, Colpaert CG, Fox SB, Turley H, Harris AL, Van Marck EA, et al. . Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin Cancer Res 2004; 10:7965 - 71; http://dx.doi.org/ 10.1158/1078-0432.CCR-04-0063; PMID: 15585631 [DOI] [PubMed] [Google Scholar]
- 24.Jaiswal M, LaRusso NF, Gores GJ. . Nitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesis. Am J Physiol Gastrointest Liver Physiol 2001; 281:G626 - 34; PMID: 11518674 [DOI] [PubMed] [Google Scholar]
- 25.Brower V. . Researchers attempting to define role of cytokines in cancer risk. J Natl Cancer Inst 2005; 97:1175 - 7; http://dx.doi.org/ 10.1093/jnci/dji269; PMID: 16106019 [DOI] [PubMed] [Google Scholar]
- 26.Biarc J, Nguyen IS, Pini A, Gossé F, Richert S, Thiersé D, Van Dorsselaer A, Leize-Wagner E, Raul F, Klein JP, et al. . Carcinogenic properties of proteins with pro-inflammatory activity from Streptococcus infantarius (formerly S.bovis). Carcinogenesis 2004; 25:1477 - 84; http://dx.doi.org/ 10.1093/carcin/bgh091; PMID: 14742316 [DOI] [PubMed] [Google Scholar]
- 27.Altinoz MA, Korkmaz R. . NF-kappaB, macrophage migration inhibitory factor and cyclooxygenase-inhibitions as likely mechanisms behind the acetaminophen- and NSAID-prevention of the ovarian cancer. Neoplasma 2004; 51:239 - 47; PMID: 15254653 [PubMed] [Google Scholar]
- 28.Wang W, Bergh A, Damber JE. . Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate 2004; 61:60 - 72; http://dx.doi.org/ 10.1002/pros.20061; PMID: 15287094 [DOI] [PubMed] [Google Scholar]
- 29.Hussein MR, Ahmed RA. . Analysis of the mononuclear inflammatory cell infiltrate in the non-tumorigenic, pre-tumorigenic and tumorigenic keratinocytic hyperproliferative lesions of the skin. Cancer Biol Ther 2005; 4:819 - 21; http://dx.doi.org/ 10.4161/cbt.4.8.1864; PMID: 16210913 [DOI] [PubMed] [Google Scholar]
- 30.Bartsch H, Nair J. . Oxidative stress and lipid peroxidation-derived DNA-lesions in inflammation driven carcinogenesis. Cancer Detect Prev 2004; 28:385 - 91; http://dx.doi.org/ 10.1016/j.cdp.2004.07.004; PMID: 15582261 [DOI] [PubMed] [Google Scholar]
- 31.Angelo LS, Kurzrock R. . Vascular endothelial growth factor and its relationship to inflammatory mediators. Clin Cancer Res 2007; 13:2825 - 30; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-2416; PMID: 17504979 [DOI] [PubMed] [Google Scholar]
- 32.Jain RK. . Barriers to drug delivery in solid tumors. Sci Am 1994; 271:58 - 65; http://dx.doi.org/ 10.1038/scientificamerican0794-58; PMID: 8066425 [DOI] [PubMed] [Google Scholar]
- 33.Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, et al. . Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 2002; 296:1883 - 6; http://dx.doi.org/ 10.1126/science.1071420; PMID: 11976409 [DOI] [PubMed] [Google Scholar]
- 34.Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa S, Mihm MC, et al. . Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol 2005; 18:1232 - 42; http://dx.doi.org/ 10.1038/modpathol.3800410; PMID: 15803182 [DOI] [PubMed] [Google Scholar]
- 35.Maula SM, Luukkaa M, Grénman R, Jackson D, Jalkanen S, Ristamäki R. . Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res 2003; 63:1920 - 6; PMID: 12702584 [PubMed] [Google Scholar]
- 36.Choi WW, Lewis MM, Lawson D, Yin-Goen Q, Birdsong GG, Cotsonis GA, Cohen C, Young AN. . Angiogenic and lymphangiogenic microvessel density in breast carcinoma: correlation with clinicopathologic parameters and VEGF-family gene expression. Mod Pathol 2005; 18:143 - 52; http://dx.doi.org/ 10.1038/modpathol.3800253; PMID: 15297858 [DOI] [PubMed] [Google Scholar]
- 37.Bergers G, Benjamin LE. . Tumorigenesis and the angiogenic switch. Nat Rev Cancer 2003; 3:401 - 10; http://dx.doi.org/ 10.1038/nrc1093; PMID: 12778130 [DOI] [PubMed] [Google Scholar]
- 38.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. . Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 2001; 7:192 - 8; http://dx.doi.org/ 10.1038/84643; PMID: 11175850 [DOI] [PubMed] [Google Scholar]
- 39.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. . VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 2001; 7:186 - 91; http://dx.doi.org/ 10.1038/84635; PMID: 11175849 [DOI] [PubMed] [Google Scholar]
- 40.Petrova TV, Mäkinen T, Mäkelä TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Ylä-Herttuala S, Alitalo K. . Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J 2002; 21:4593 - 9; http://dx.doi.org/ 10.1093/emboj/cdf470; PMID: 12198161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tal O, Lim HY, Gurevich I, Milo I, Shipony Z, Ng LG, Angeli V, Shakhar G. . DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med 2011; 208:2141 - 53; http://dx.doi.org/ 10.1084/jem.20102392; PMID: 21930767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Issa A, Le TX, Shoushtari AN, Shields JD, Swartz MA. . Vascular endothelial growth factor-C and C-C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res 2009; 69:349 - 57; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-1875; PMID: 19118020 [DOI] [PubMed] [Google Scholar]
- 43.Ivanovic Z. . Hypoxia or in situ normoxia: The stem cell paradigm. J Cell Physiol 2009; 219:271 - 5; http://dx.doi.org/ 10.1002/jcp.21690; PMID: 19160417 [DOI] [PubMed] [Google Scholar]
- 44.Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT. . Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol Cell Biol 2008; 28:718 - 31; http://dx.doi.org/ 10.1128/MCB.01338-07; PMID: 17967865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morfoisse F, Kuchnio A, Frainay C, Gomez-Brouchet A, Delisle MB, Marzi S, Helfer AC, Hantelys F, Pujol F, Guillermet-Guibert J, et al. . Hypoxia induces VEGF-C expression in metastatic tumor cells via a HIF-1α-independent translation-mediated mechanism. Cell Rep 2014; 6:155 - 67; http://dx.doi.org/ 10.1016/j.celrep.2013.12.011; PMID: 24388748 [DOI] [PubMed] [Google Scholar]
- 46.Ivanovic Z. . Physiological, ex vivo cell oxygenation is necessary for a true insight into cytokine biology. Eur Cytokine Netw 2009; 20:7 - 9; PMID: 19318314 [DOI] [PubMed] [Google Scholar]
- 47.Gerber HP, Condorelli F, Park J, Ferrara N. . Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem 1997; 272:23659 - 67; http://dx.doi.org/ 10.1074/jbc.272.38.23659; PMID: 9295307 [DOI] [PubMed] [Google Scholar]
- 48.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, et al. . Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 1998; 12:149 - 62; http://dx.doi.org/ 10.1101/gad.12.2.149; PMID: 9436976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryan HE, Lo J, Johnson RS. . HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J 1998; 17:3005 - 15; http://dx.doi.org/ 10.1093/emboj/17.11.3005; PMID: 9606183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang LE, Gu J, Schau M, Bunn HF. . Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A 1998; 95:7987 - 92; http://dx.doi.org/ 10.1073/pnas.95.14.7987; PMID: 9653127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruick RK, McKnight SL. . A conserved family of prolyl-4-hydroxylases that modify HIF. Science 2001; 294:1337 - 40; http://dx.doi.org/ 10.1126/science.1066373; PMID: 11598268 [DOI] [PubMed] [Google Scholar]
- 52.Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, et al. . Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 2009; 136:839 - 51; http://dx.doi.org/ 10.1016/j.cell.2009.01.020; PMID: 19217150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al. . Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001; 292:468 - 72; http://dx.doi.org/ 10.1126/science.1059796; PMID: 11292861 [DOI] [PubMed] [Google Scholar]
- 54.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr.. . HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 2001; 292:464 - 8; http://dx.doi.org/ 10.1126/science.1059817; PMID: 11292862 [DOI] [PubMed] [Google Scholar]
- 55.Ferrara N. . Vascular endothelial growth factor as a target for anticancer therapy. Oncologist 2004; 9:Suppl 1 2 - 10; http://dx.doi.org/ 10.1634/theoncologist.9-suppl_1-2; PMID: 15178810 [DOI] [PubMed] [Google Scholar]
- 56.Pagès G, Pouysségur J. . Transcriptional regulation of the Vascular Endothelial Growth Factor gene--a concert of activating factors. Cardiovasc Res 2005; 65:564 - 73; http://dx.doi.org/ 10.1016/j.cardiores.2004.09.032; PMID: 15664382 [DOI] [PubMed] [Google Scholar]
- 57.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. . Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 1996; 16:4604 - 13; PMID: 8756616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blancher C, Moore JW, Talks KL, Houlbrook S, Harris AL. . Relationship of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression to vascular endothelial growth factor induction and hypoxia survival in human breast cancer cell lines. Cancer Res 2000; 60:7106 - 13; PMID: 11156418 [PubMed] [Google Scholar]
- 59.Gruber AR, Fallmann J, Kratochvill F, Kovarik P, Hofacker IL. . AREsite: a database for the comprehensive investigation of AU-rich elements. Nucleic Acids Res 2011; 39:D66 - 9; http://dx.doi.org/ 10.1093/nar/gkq990; PMID: 21071424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levy AP, Levy NS, Goldberg MA. . Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem 1996; 271:2746 - 53; http://dx.doi.org/ 10.1074/jbc.271.5.2746; PMID: 8576250 [DOI] [PubMed] [Google Scholar]
- 61.Griseri P, Pagès G. . Control of pro-angiogenic cytokine mRNA half-life in cancer: the role of AU-rich elements and associated proteins. J Interferon Cytokine Res 2014; 34:242 - 54; http://dx.doi.org/ 10.1089/jir.2013.0140; PMID: 24697202 [DOI] [PubMed] [Google Scholar]
- 62.Onesto C, Berra E, Grépin R, Pagès G. . Poly(A)-binding protein-interacting protein 2, a strong regulator of vascular endothelial growth factor mRNA. J Biol Chem 2004; 279:34217 - 26; http://dx.doi.org/ 10.1074/jbc.M400219200; PMID: 15175342 [DOI] [PubMed] [Google Scholar]
- 63.Chang SH, Hla T. . Post-transcriptional gene regulation by HuR and microRNAs in angiogenesis. Curr Opin Hematol 2014; 21:235 - 40; http://dx.doi.org/ 10.1097/MOH.0000000000000040; PMID: 24714527 [DOI] [PubMed] [Google Scholar]
- 64.Holcik M, Sonenberg N. . Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 2005; 6:318 - 27; http://dx.doi.org/ 10.1038/nrm1618; PMID: 15803138 [DOI] [PubMed] [Google Scholar]
- 65.Baird SD, Turcotte M, Korneluk RG, Holcik M. . Searching for IRES. RNA 2006; 12:1755 - 85; http://dx.doi.org/ 10.1261/rna.157806; PMID: 16957278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silvera D, Schneider RJ. . Inflammatory breast cancer cells are constitutively adapted to hypoxia. Cell Cycle 2009; 8:3091 - 6; http://dx.doi.org/ 10.4161/cc.8.19.9637; PMID: 19755858 [DOI] [PubMed] [Google Scholar]
- 67.Somers J, Pöyry T, Willis AE. . A perspective on mammalian upstream open reading frame function. Int J Biochem Cell Biol 2013; 45:1690 - 700; http://dx.doi.org/ 10.1016/j.biocel.2013.04.020; PMID: 23624144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vagner S, Galy B, Pyronnet S. . Irresistible IRES. Attracting the translation machinery to internal ribosome entry sites. EMBO Rep 2001; 2:893 - 8; http://dx.doi.org/ 10.1093/embo-reports/kve208; PMID: 11600453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bushell M, Stoneley M, Sarnow P, Willis AE. . Translation inhibition during the induction of apoptosis: RNA or protein degradation?. Biochem Soc Trans 2004; 32:606 - 10; http://dx.doi.org/ 10.1042/BST0320606; PMID: 15270687 [DOI] [PubMed] [Google Scholar]
- 70.Thakor N, Holcik M. . IRES-mediated translation of cellular messenger RNA operates in eIF2α- independent manner during stress. Nucleic Acids Res 2012; 40:541 - 52; http://dx.doi.org/ 10.1093/nar/gkr701; PMID: 21917851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vagner S, Gensac MC, Maret A, Bayard F, Amalric F, Prats H, Prats AC. . Alternative translation of human fibroblast growth factor 2 mRNA occurs by internal entry of ribosomes. Mol Cell Biol 1995; 15:35 - 44; PMID: 7799942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huez I, Créancier L, Audigier S, Gensac MC, Prats AC, Prats H. . Two independent internal ribosome entry sites are involved in translation initiation of vascular endothelial growth factor mRNA. Mol Cell Biol 1998; 18:6178 - 90; PMID: 9774635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bornes S, Prado-Lourenco L, Bastide A, Zanibellato C, Iacovoni JS, Lacazette E, Prats AC, Touriol C, Prats H. . Translational induction of VEGF internal ribosome entry site elements during the early response to ischemic stress. Circ Res 2007; 100:305 - 8; http://dx.doi.org/ 10.1161/01.RES.0000258873.08041.c9; PMID: 17255526 [DOI] [PubMed] [Google Scholar]
- 74.Arcondéguy T, Lacazette E, Millevoi S, Prats H, Touriol C. . VEGF-A mRNA processing, stability and translation: a paradigm for intricate regulation of gene expression at the post-transcriptional level. Nucleic Acids Res 2013; 41:7997 - 8010; http://dx.doi.org/ 10.1093/nar/gkt539; PMID: 23851566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, Connolly DT. . Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 1989; 246:1309 - 12; http://dx.doi.org/ 10.1126/science.2479987; PMID: 2479987 [DOI] [PubMed] [Google Scholar]
- 76.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. . Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989; 246:1306 - 9; http://dx.doi.org/ 10.1126/science.2479986; PMID: 2479986 [DOI] [PubMed] [Google Scholar]
- 77.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. . The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem 1991; 266:11947 - 54; PMID: 1711045 [PubMed] [Google Scholar]
- 78.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. . Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem 1992; 267:26031 - 7; PMID: 1464614 [PubMed] [Google Scholar]
- 79.Carmeliet P. . Angiogenesis in life, disease and medicine. Nature 2005; 438:932 - 6; http://dx.doi.org/ 10.1038/nature04478; PMID: 16355210 [DOI] [PubMed] [Google Scholar]
- 80.Dellinger MT, Brekken RA. . Phosphorylation of Akt and ERK1/2 is required for VEGF-A/VEGFR2-induced proliferation and migration of lymphatic endothelium. PLoS One 2011; 6:e28947; http://dx.doi.org/ 10.1371/journal.pone.0028947; PMID: 22174934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wuest TR, Carr DJ. . VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med 2010; 207:101 - 15; http://dx.doi.org/ 10.1084/jem.20091385; PMID: 20026662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bastide A, Karaa Z, Bornes S, Hieblot C, Lacazette E, Prats H, Touriol C. . An upstream open reading frame within an IRES controls expression of a specific VEGF-A isoform. Nucleic Acids Res 2008; 36:2434 - 45; http://dx.doi.org/ 10.1093/nar/gkn093; PMID: 18304943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karaa ZS, Iacovoni JS, Bastide A, Lacazette E, Touriol C, Prats H. . The VEGF IRESes are differentially susceptible to translation inhibition by miR-16. RNA 2009; 15:249 - 54; http://dx.doi.org/ 10.1261/rna.1301109; PMID: 19144909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Casanova CM, Sehr P, Putzker K, Hentze MW, Neumann B, Duncan KE, Thoma C. . Automated high-throughput RNAi screening in human cells combined with reporter mRNA transfection to identify novel regulators of translation. PLoS One 2012; 7:e45943; http://dx.doi.org/ 10.1371/journal.pone.0045943; PMID: 23029333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ray PS, Jia J, Yao P, Majumder M, Hatzoglou M, Fox PL. . A stress-responsive RNA switch regulates VEGFA expression. Nature 2009; 457:915 - 9; http://dx.doi.org/ 10.1038/nature07598; PMID: 19098893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Essafi-Benkhadir K, Onesto C, Stebe E, Moroni C, Pagès G. . Tristetraprolin inhibits Ras-dependent tumor vascularization by inducing vascular endothelial growth factor mRNA degradation. Mol Biol Cell 2007; 18:4648 - 58; http://dx.doi.org/ 10.1091/mbc.E07-06-0570; PMID: 17855506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou S, Gu L, He J, Zhang H, Zhou M. . MDM2 regulates vascular endothelial growth factor mRNA stabilization in hypoxia. Mol Cell Biol 2011; 31:4928 - 37; http://dx.doi.org/ 10.1128/MCB.06085-11; PMID: 21986500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vumbaca F, Phoenix KN, Rodriguez-Pinto D, Han DK, Claffey KP. . Double-stranded RNA-binding protein regulates vascular endothelial growth factor mRNA stability, translation, and breast cancer angiogenesis. Mol Cell Biol 2008; 28:772 - 83; http://dx.doi.org/ 10.1128/MCB.02078-06; PMID: 18039850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. . A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 1996; 15:1751; PMID: 8612600 [PMC free article] [PubMed] [Google Scholar]
- 90.Karpanen T, Alitalo K. . Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol 2008; 3:367 - 97; http://dx.doi.org/ 10.1146/annurev.pathmechdis.3.121806.151515; PMID: 18039141 [DOI] [PubMed] [Google Scholar]
- 91.Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmén C, Oike Y, Pajusola K, Thurston G, Suda T, et al. . Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood 2005; 105:4642 - 8; http://dx.doi.org/ 10.1182/blood-2004-08-3327; PMID: 15746084 [DOI] [PubMed] [Google Scholar]
- 92.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, et al. . Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 2004; 5:74 - 80; http://dx.doi.org/ 10.1038/ni1013; PMID: 14634646 [DOI] [PubMed] [Google Scholar]
- 93.Liang X, Yang D, Hu J, Hao X, Gao J, Mao Z. . Hypoxia inducible factor-alpha expression correlates with vascular endothelial growth factor-C expression and lymphangiogenesis/angiogenesis in oral squamous cell carcinoma. Anticancer Res 2008; 28:3A 1659 - 66; PMID: 18630523 [PubMed] [Google Scholar]
- 94.Schoppmann SF, Fenzl A, Nagy K, Unger S, Bayer G, Geleff S, Gnant M, Horvat R, Jakesz R, Birner P. . VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery 2006; 139:839 - 46; http://dx.doi.org/ 10.1016/j.surg.2005.12.008; PMID: 16782443 [DOI] [PubMed] [Google Scholar]
- 95.Tao J, Li T, Li K, Xiong J, Yang Z, Wu H, Wang C. . Effect of HIF-1alpha on VEGF-C induced lymphangiogenesis and lymph nodes metastases of pancreatic cancer. J Huazhong Univ Sci Technolog Med Sci 2006; 26:562 - 4; http://dx.doi.org/ 10.1007/s11596-006-0520-9; PMID: 17219968 [DOI] [PubMed] [Google Scholar]
- 96.Chilov D, Kukk E, Taira S, Jeltsch M, Kaukonen J, Palotie A, Joukov V, Alitalo K. . Genomic organization of human and mouse genes for vascular endothelial growth factor C. J Biol Chem 1997; 272:25176 - 83; http://dx.doi.org/ 10.1074/jbc.272.40.25176; PMID: 9312130 [DOI] [PubMed] [Google Scholar]
- 97.Stacker SA, Achen MG. . From anti-angiogenesis to anti-lymphangiogenesis: emerging trends in cancer therapy. Lymphat Res Biol 2008; 6:165 - 72; http://dx.doi.org/ 10.1089/lrb.2008.1015; PMID: 19093789 [DOI] [PubMed] [Google Scholar]
- 98.Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, Zhang YF, Williams SP, Farnsworth RH, Chai MG, et al. . VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell 2012; 21:181 - 95; http://dx.doi.org/ 10.1016/j.ccr.2011.12.026; PMID: 22340592 [DOI] [PubMed] [Google Scholar]
- 99.Karnezis T, Shayan R, Fox S, Achen MG, Stacker SA. . The connection between lymphangiogenic signalling and prostaglandin biology: a missing link in the metastatic pathway. Oncotarget 2012; 3:893 - 906; PMID: 23097685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. . Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 2014; 14:159 - 72; http://dx.doi.org/ 10.1038/nrc3677; PMID: 24561443 [DOI] [PubMed] [Google Scholar]
- 101.Okada K, Osaki M, Araki K, Ishiguro K, Ito H, Ohgi S. . Expression of hypoxia-inducible factor (HIF-1alpha), VEGF-C and VEGF-D in non-invasive and invasive breast ductal carcinomas. Anticancer Res 2005; 25:3003 - 9; PMID: 16080559 [PubMed] [Google Scholar]
- 102.Tzao C, Lee SC, Tung HJ, Hsu HS, Hsu WH, Sun GH, Yu CP, Jin JS, Cheng YL. . Expression of hypoxia-inducible factor (HIF)-1alpha and vascular endothelial growth factor (VEGF)-D as outcome predictors in resected esophageal squamous cell carcinoma. Dis Markers 2008; 25:141 - 8; http://dx.doi.org/ 10.1155/2008/468323; PMID: 19096126 [DOI] [PMC free article] [PubMed] [Google Scholar]