Abstract
Although Epstein-Barr virus-associated nasopharyngeal carcinoma (NPC) is a highly radiosensitive cancer, approximately 20% of patients with NPC develop local recurrence after radiation therapy. Multiple proinflammatory cytokines are thought to protect NPC tumor cells from immune surveillance and therapeutic interventions. The cytokine leukemia inhibitory factor (LIF) is a critical component of the NPC microenvironment. LIF influences tumor growth and survival, and is therefore considered a potential therapeutic target and/or prognostic predictor for NPC. High LIF levels have been detected in the circulating blood of patients with recurrent NPC and NPC tumor cells. This review discusses the molecular mechanisms that link LIF to NPC tumor progression and radioresistance.
Keywords: leukemia inhibitory factor, p70S6K, mTOR, nasopharyngeal carcinoma, radioresistance, Epstein-Barr virus
Introduction
In nasopharyngeal carcinoma (NPC), genetic alterations and microenvironmental factors such as Epstein-Barr virus (EBV) and infiltrated leukocytes are considered to be major contributors to tumor growth, immune escape, and survival.1,2 The progression of EBV-associated NPC is profoundly affected by various proinflammatory cytokines that are secreted by tumor cells and surrounding stromal cells, which include lymphocytes, macrophages, T cells, and fibroblasts.3-6 In chronic inflammatory cancers, proinflammatory cytokines (e.g., interleukins IL-6 and IL-8) play critical roles in tumor progression and resistance to treatment, including radioresistance and chemoresistance.7-9 NPC is generally sensitive to radiation therapy, and most NPC patients can be cured if the disease is diagnosed and treated at an early stage. However, some NPC patients develop local recurrence after radiotherapy10 even though they have the same histologic diagnosis as other patients who are cured by the treatment. Although clinical parameters (e.g., a larger tumor volume or advanced tumor stage) may explain some of these treatment failures, many are unexplained. Radioresistance has been recognized as a major cause of treatment failure. Given our current understanding of the determinants of tumor response to radiotherapy, it seems worthwhile to investigate the roles of specific cytokines in tumor radioresistance.
Leukemia inhibitory factor (LIF) is a key factor in the growth of mouse embryonic stem cells and a critical regulator of embryonic development in humans.11 LIF is a member of the IL-6–type cytokine family, which includes IL-6, IL-11, oncostatin M, ciliary neurotrophic factor, cardiotrophin-1, and cardiotrophin-like cytokine.12 The receptor for LIF is a heterodimer of the specific gpl90 subunit (LIFR) and the gpl30 subunit. LIF regulates the proliferation, survival, and differentiation of cells by activating critical signaling pathways, including the Janus tyrosine kinase/signal transducer and activator of transcription 3 (JAK/STAT3), p44/42 mitogen activated protein kinase (ERK1/2), and phosphoinositide 3-kinase (PI3K) signaling pathways.13-15 Although dysregulation of LIF and/or LIFR has been reported in several human malignancies, both prosurvival16-19 and tumor suppressor20,21 properties of LIF have been described. Thus, the cancer-related mechanisms of LIF are likely to be complex.
In this review, we address several issues related to the roles of LIF in NPC progression22 and how LIF is regulated in the NPC tumor microenvironment. We also discuss the potential of LIF as a prognostic biomarker for predicting the radiosensitivity of NPC tumors and as a therapeutic target for this cancer, with the aim of stimulating further translational research on this multifunctional cytokine.
LIF Levels in NPC
Numerous studies have detected human LIF in the circulatory system or body fluids.23-29 Elevated LIF levels are associated with inflammation, autoimmune diseases, blastocyst implantation, and cell proliferation.30-34 LIF can be produced by immune cells and other stromal cells and is induced by various inflammatory factors, including NFκB, TNFα, and IL-1.35 In patients with type III NPC (also known as lymphoepithelioma), the levels of LIF in the circulating blood and the tumor microenvironment are thought to be associated with tumorigenesis.22
LIF in serum samples from NPC patients
The first clue to the clinical involvement of LIF in NPC came from the observation that LIF levels are elevated in serum samples from patients diagnosed with recurrent NPC. In contrast, serum LIF is not detected in approximately 65% of normal individuals (Fig. 1). In NPC patients, a increased serum LIF level (LIF > 4.96 pg/mL) is an independent prognostic factor for recurrence-free survival,22 suggesting that the serum LIF level may serve as a predictor for a patient’s response to radiation therapy. However, LIF levels do not differ significantly between patients with metastasis and those with tumor remission (Fig. 1), suggesting that the paracrine or juxtacrine regulation of LIF in the tumor microenvironment might be more important than its systemic influence. Furthermore, serum LIF levels in pre-treatment NPC patients are not significantly associated with gender, age, or pathologic classification.
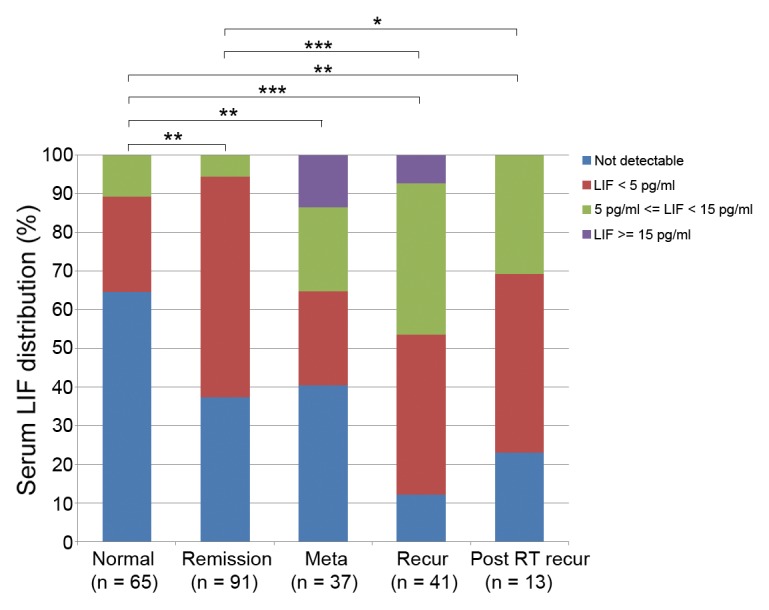
Figure 1. Serum leukemia inhibitory factor (LIF) levels in patients with nasopharyngeal carcinoma (NPC) and normal donors (*P < 0.05, **P < 0.01, ***P < 0.0001; Mann–Whitney test). Meta, metastatic; recur, recurrence; post RT recur, post radiotherapy recurrence.
Immunohistochemical analysis of LIF expression
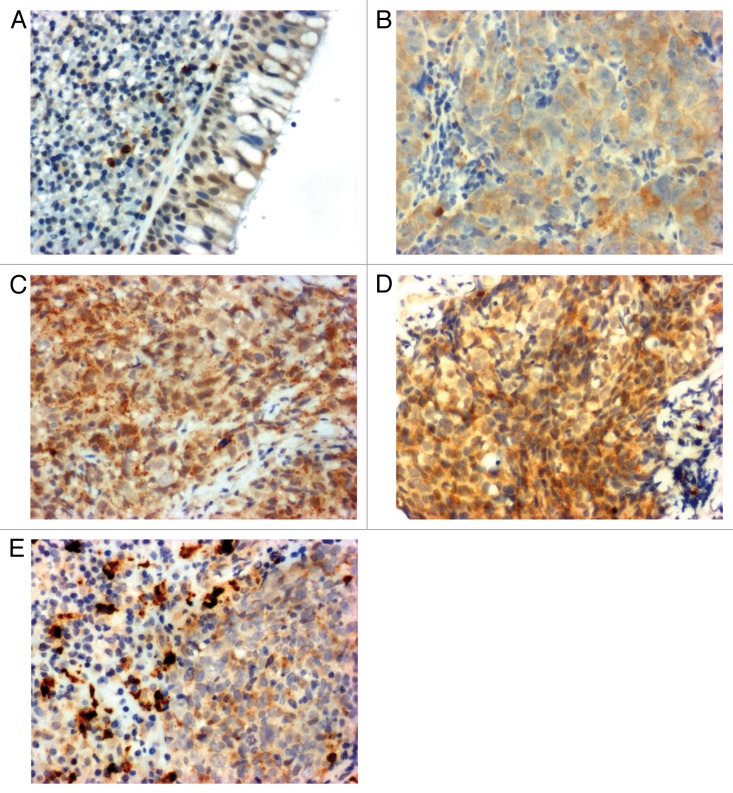
Human LIF exists in at least three isoforms, LIF-D, LIF-M, and LIF-T,36-38 which differ in their first exons: the LIF-D transcript encodes a soluble LIF that is secreted and transduces signaling via LIFR, LIF-T is a truncated isoform found in the nucleus, and the LIF-M transcript is translated into both secreted and intracellular proteins. Secreted LIF-M can also be found as a variant that binds to the extracellular matrix.39 Our immunohistochemical data show that NPC cells express markedly higher levels of LIF compared with adjacent normal epithelial cells. In general, little to no LIF immunoreactivity is found in the nuclei of normal nasopharyngeal basal epithelium cells (Fig. 2A), whereas moderate to strong LIF expression is observed in the cytoplasm of nasopharyngeal tumor cells (Fig. 2B–D). Strong LIF expression is also correlated with poorer prognosis in our samples. However, further studies are needed to examine whether and how the different LIF isoforms contribute to NPC tumorigenesis. In patients with NPC, macrophages infiltrate into the tumor mass. Our laboratory found that tumor-resident macrophages express very high levels of LIF (Fig. 2E), suggesting that macrophages might critically contribute to the high levels of LIF present in the NPC tumor microenvironment.
Figure 2. Leukemia inhibitory factor (LIF) expression in nasopharyngeal tissues. (A) Normal basal epithelium. (B) Nasopharyngeal carcinoma (NPC) tumor diagnosed as complete tumor remission after therapy. (C) NPC tumor diagnosed with relapse after therapy. (D) NPC tumor diagnosed with distant metastasis after therapy. (E) Macrophages residing within NPC tumor areas.
LIF in the microenvironment of NPC
LIF is secreted by many cell types including macrophages, fibroblasts, mesenchymal stem cells, and cancer cells, and exerts its biologic effects via paracrine and autocrine mechanisms.40 Type III NPC is characterized by EBV infection and high lymphocyte infiltration,2 generating a proinflammatory microenvironment that directly influences the fate of tumor cells. LIF levels are markedly higher in NPC biopsies (median value, 135 pg/mg) than in counterpart normal tissue (median value, 41 pg/mg).22 Moreover, the concentrations of LIF detected in NPC biopsy samples are 10-fold higher than those in serum samples. Thus, LIF produced in the tumor microenvironment likely contributes to the elevated serum levels of LIF detected in NPC patients. Cultured NPC cells secrete LIF in a concentration range of approximately 20 pg/mL to 80 pg/mL.22 These observations indicate that tumor cells, macrophages, and possibly other stromal cells collectively contribute to the enhanced production of LIF in the NPC tumor microenvironment.
Functional Roles of LIF in NPC
LIF enhances NPC tumor growth
LIF regulates cell proliferation in various types of human cancer.19,22,41-43 In NPC, LIF increases DNA synthesis and enhances tumor growth. Treatment with soluble LIFR (sLIFR) can counteract the enhancing effect of LIF on cell growth, and treatment with LIF (10 ng/mL) decreases the doubling time of NPC cells by approximately 20%. In a mouse xenograft tumor model, LIF accelerates tumor growth, whereas sLIFR causes growth arrest.22 Future work is needed to define how LIF alters the matrix structure. However, the LIF-induced enhancement of NPC cell growth might be explained by activation of pro-survival signaling-mediated translational control (see “LIF-mediated signaling in NPC” below).
LIF induces dedifferentiation of NPC cells
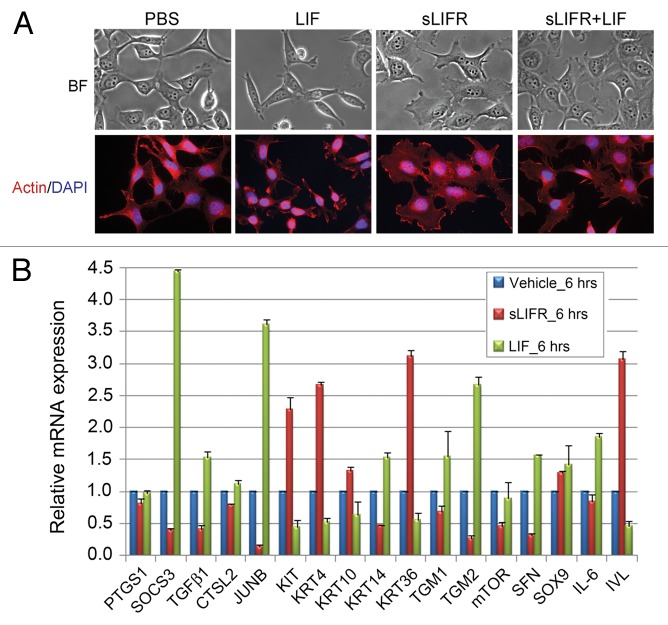
The role of LIF in differentiation has been well documented in numerous cell types,41,44-48 but few studies have focused on its involvement in cancer cell differentiation. Recently, we found that LIF can modulate the expression of genes involved in epithelial differentiation. Supplementation of culture medium with LIF alters the morphology of NPC cells to a more undifferentiated phenotype, whereas the addition of sLIFR leads to terminal differentiation (Fig. 3A). This may be partly explained by increased expression levels of dedifferentiation markers (KRT14, TGM1, TGM2, SFN) and decreased expression levels of differentiation markers (KRT4, KRT 10, KRT36, IVL) (Fig. 3B). Together, available data suggest that the presence of LIF in the tumor microenvironment sustains the characteristics of stem cell-like cancer cells.
Figure 3. Leukemia inhibitory factor (LIF) treatment induces de-differentiation phenotype of nasopharyngeal carcinoma (NPC) cells. (A) Treatment with LIF or soluble LIFR (sLIFR) induces morphologic changes in NPC-TW06 cells. Upper panels, bright field; lower panels, fluorescent staining of actin (red) and DAPI (blue). (B) mRNA expression of genes associated with epithelial differentiation by QRT-PCR. Values are presented as means ± SD of duplicate samples.
LIF-Mediated Signaling in NPC
The mTORC1/p70S6K1 pathway is critically induced by LIF in NPC
LIF mediates critical signaling pathways (e.g., the JAK/STAT3, PI3K, and ERK1/2 signaling pathways) to confer cell type- or developmental stage-specific regulation of multiple biologic processes, including cell proliferation, survival, and differentiation.15,40,49-51 In NPC cells, LIF activates its known targets (e.g., STAT3, MEK1, and p38 MAPK), while additionally activating mTORC1/p70S6K1 signaling. In this pathway, mTORC1 phosphorylates T389 in the linker region of p70S6K1;52,53 activated p70S6K1 in turn phosphorylates mTOR at Ser 2448 via a feedback loop.54 Suppression of mTORC1/p70S6K1 with rapalogs or siRNA-mediated silencing of mTOR attenuates LIF-mediated activation of p70S6K1, mTOR, ERK1/2, and STAT3, and decreases LIF-induced NPC tumor growth.22 In NPC, therefore, mTORC1/p70S6K1 appears to constitute a critical signaling hub that is triggered by LIF. The existence of a LIF-mTORC1-p70S6K1 axis is further supported by close correlations among the levels of LIF, p-p70S6K1 (T389), and mTOR (S2448) found in immunohistochemical analyses of human NPC biopsies and in LIF-treated NPC tumor xenografts.
LIFR contributes to LIF-mediated signaling
Signaling downstream of LIF is mediated by interactions with its two receptor proteins, LIFR and gp130. LIFR belongs to the type I cytokine receptor family. Binding of LIF to LIFR leads to the activation of cytoplasmic tyrosine kinases and triggers intracellular signaling cascades.50 Receptor activation is followed by desensitization and receptor turnover is under tight control.55,56 LIFR expression has been reported in a number of human malignancies, including thyroid cancer,43 rhabdomyosarcoma,42 pancreatic carcinoma,19,57 ovarian cancer,58 breast cancer,21,59 and hepatocellular carcinoma.20 In NPC, LIFR expression is significantly higher in the tumor mass compared with the adjacent normal basal epithelium. Ectopic expression of LIFR in cultured NPC cells enhances the activation of downstream signaling molecules in the absence of ligand stimulation, suggesting that overexpression of LIFR is sufficient to trigger signal transduction, possibly mimicking the ligand-LIFR interaction. Consistent with this observation, siRNA-mediated depletion of LIFR suppresses the LIF-mediated activation of downstream signaling (unpublished observations). NPC tumor cells also express high levels of gp130, but these levels are not significantly different from those observed in normal basal cells.22
LIF Enhances the Resistance of NPC to Ionizing Radiation
Role of LIF in NPC recurrence
Approximately 20% of NPC patients develop local recurrence after radiotherapy, and the relapsed NPC is usually more advanced than the original tumor.10 Various proinflammatory cytokines produced by macrophages, epithelial cells, fibroblasts, and cancer cells in the tumor microenvironment (e.g., transforming growth factor β-1 [TGF-β1], tumor necrosis factor α [TNF-α], interleukin 1 [IL-1], IL-6, COX-2, IL-8 and IL-10) critically modulate the efficacy of radiotherapy.60,61 These proinflammatory cytokines contribute to radioresistance by activating various survival pathways, including the EGFR, STAT3, and PI3K/AKT pathways. LIF levels are elevated in both pretreatment serum samples from NPC patients that developed local recurrence after treatment and in serum samples from post-treatment NPC patients diagnosed with recurrence.22 Consistent with this finding, administration of LIF enhances the radioresistance of NPC xenografts in immunodeficient mice. The LIF-induced activation of prosurvival signaling (e.g., mTORC1/p70S6K1and STAT3 signaling) in NPC cells may explain its role in radioresistance. The high level of LIF may serve a radioprotective function against genotoxic insults.
LIF modulates the DNA damage response and radiosensitivity
Cellular radiosensitivity is influenced by both intrinsic factors (e.g., cell cycle distribution, the activation of apoptotic programs, the DNA damage response [DDR], and accumulation of genetic mutations) and extrinsic factors (e.g., oxygen, nutrients, and the elimination of metabolic waste). DNA double-strand breaks (DSBs) are the most critical event in ionizing radiation (IR)-induced cell death. In response to IR, ataxia telangiectasia mutated (ATM) protein functions as a central transducer, triggering a DDR cascade to stimulate apoptosis or DNA repair.62 Activated ATM phosphorylates various checkpoint proteins (e.g., p53, CDC25C, CHK1, CHK2, and NBS1) during all phases of the cell cycle63 and also phosphorylates histone H2AX at Ser139 (γH2AX).64 ATM dysfunction results in abnormal checkpoint responses, genomic instability, cancer predisposition, and high sensitivity to IR, as exhibited in AT cells.65 The presence of LIF in IR-treated NPC cells decreases activation of DDR signaling molecules, including ATM, NBS1, γH2AX, CDC25C, and p53, leading to increased cell survival.22 Furthermore, LIF inhibits IR-induced apoptosis, as evidenced by reduced levels of active caspase-3 and caspase-7 in LIF-treated cells.22 These findings suggest that LIF-mediated radioresistance may result from inhibition of DDR signaling and suppression of apoptosis.
LIF protects cardiac myocytes against oxidative stress under the acute stress condition of ischemia-reperfusion.50 IR stress is known to generate a high level of intracellular reactive oxygen species (ROS). Notably, we found that the presence of LIF in γ-irradiated NPC cells increases the expression of various antioxidant enzymes, including SOD1, SOD2, CAT, PRDX2, and GPX3, and enhances the nuclear localization of nuclear factor erythroid 2-related factor 2 (NRF2), a key transcription factor involved in the antioxidant response (unpublished observations). This antioxidation effect further elucidates the role of LIF in radioresistance of NPC cells.
Blockade of LIF signaling sensitizes NPC cells to ionizing radiation
Based on the signaling pathways activated by LIF, we hypothesized that blockade of LIF-mediated signaling would sensitize tumor cells to radiotherapy. Our laboratory therefore explored whether administration of sLIFR (which prevents cells from responding to LIF) or rapamycin would increase the therapeutic efficacy of IR Notably, sLIFR appears to enhance IR-mediated cell killing both in vitro and in vivo.22 sLIFR-mediated enhancement of radiosensitivity is also observed in NPC xenografts stably expressing sLIFR. Furthermore, sLIFR treatment enhances IR-induced DRR and apoptosis in NPC cells, whereas suppression of mTORC1/p70S6K1 signaling by rapamycin or everolimus markedly blocks the effects of LIF on cell growth and radioresistance.22
The tolerance of normal tissues is the limiting factor for both radiotherapy and chemotherapy. Radiosensitizers such as gemcitabine and nitroimidazole compounds are highly toxic to normal cells. In contrast, sLIFR is a naturally existing protein. As the toxicities of protein drugs are generally much lower than those of chemical compounds, sLIFR could prove useful for the treatment of cancer patients with high serum LIF levels and/or high LIF or LIFR expression levels in their tumor tissues. This could selectively block the effects of LIF in tumor cells with minimal toxicity to normal cells. Additional studies are needed to establish the physiologic effects of sLIFR in humans, but we believe that it will be worthwhile to test the efficacy of inhibiting LIF-LIFR interactions in a Phase I trial in patients with radiation-treated NPC.
In summary, we have described a number of LIF-induced alterations in NPC. Table 1 summarizes the molecular events associated with aberrant activation of LIF-mediated signaling in NPC.
Table 1. Molecular events associated with aberrant activation of leukemia inhibitory factor (LIF) signaling in nasopharyngeal carcinoma (NPC).
| Ontologic function | Component | Observed alterations |
|---|---|---|
| Prosurvival pathway | p70S6K1 | Increased phosphorylation (T389, T421, S424) |
| mTOR | Increased phosphorylation (S2448) | |
| STAT3 | Increased phosphorylation (Y705) | |
| ERK1/2 | Increased phosphorylation (T202/Y204) | |
| GSK-3α/β | Increased phosphorylation (S21/9) | |
| DNA damage responses | ATM | Reduced phosphorylation (S1981) |
| H2AX | Reduced phosphorylation (S139) | |
| NBS1 | Reduced phosphorylation (S343) | |
| CDC25C | Reduced phosphorylation (S216) | |
| Apoptosis | p53 | Reduced phosphorylation (S392) |
| Caspase 3 | Reduced expression | |
| Caspase 7 | Reduced expression | |
| Dedifferentiation | SOCS3 | Increased expression |
| TGFB1 | Increased expression | |
| JUNB | Increased expression | |
| KRT14 | Increased expression | |
| TGM1 | Increased expression | |
| TGM2 | Increased expression | |
| SFN | Increased expression | |
| IL-6 | Increased expression | |
| KRT10 | Reduced expression | |
| KIT | Reduced expression | |
| KRT4 | Reduced expression | |
| KRT36 | Reduced expression | |
| IVL | Reduced expression |
Role of EBV in Regulating LIF Production
In EBV-associated NPC, EBV establishes the latency II program, which is characterized by limited expression of viral proteins and RNA.66 Latent membrane protein 1 (LMP1), the most important EBV-encoded oncoprotein in NPC, functions as a constitutively active tumor necrosis factor receptor (TNFR) by mimicking CD40 signaling.5 The transmembrane regions and the cytoplasmic C-terminal activation regions (CTARs; CTAR1, CTAR2, and CTAR3) of LMP1 can trigger cell type- and latency stage-specific signaling. The LMP1-mediated signaling pathways are described in detail in several excellent reviews.67-69 In NPC, the activation of NF-κB is the most critical event in LMP1-mediated signaling. CTAR1 activates NK-κB through TRAF1, TRAF2, and TRAF3, whereas CTAR2 activates NF-κB through TRADD and TRAF2.70,71 In addition to functioning as a growth promoter for NPC cells, LMP1 also induces multiple immunomodulatory effects that allow tumor cells to circumvent immunosurveillance, at least in part by modulating cytokine expression in the tumor microenvironment.4,69 Promoter analysis suggests that there are three NF-κB/c-Rel binding sites in the promoter region of LIF, and we found that the CTAR1 and CTAR2 domains of LMP1 both contribute to the production of LIF via activation of NF-κB, whereas C-terminally deleted LMP1 does not confer this effect.22 These observations suggest that EBV is a key factor in directing the cytokine profile of the NPC tumor microenvironment.
Concluding Remarks
Although overexpression of LIF has been reported in a number of human malignancies, the functions of LIF in human cancers and the underlying mechanisms remain largely unexplored. As we gain a greater understanding of the roles of LIF in NPC we should be able to further elucidate the multifaceted regulation of LIF in cancer. The next step will be to investigate the potential benefits of sequestering LIF in the tumor microenvironment of NPC patients with high LIF levels in peripheral blood or biopsy samples, for example using a LIF antagonist such as sLIFR or a specific monoclonal antibody.
Glossary
Abbreviations:
- ATM
ataxia telangiectasia mutated
- CDC25C
cell division cycle 25C
- CTAR
cytoplasmic C-terminal activation regions of LMP1
- DDR
DNA damage response
- DSB
DNA double-strand break
- EBV
Epstein-Barr virus
- ERK1/2
p44/42 mitogen activated protein kinase
- γH2AX
phosphorylated (Ser139) histone H2AX
- Gp130
glycoprotein 130
- IL-1
interleukin 1
- IL-6
interleukin 6
- IL-8
interleukin 8
- IL-10
interleukin 10
- JAK
Janus tyrosine kinase
- LIF
leukemia inhibitory factor
- LIFR
leukemia inhibitory factor receptor
- LMP1
latent membrane protein 1 of EBV
- mTOR
mammalian target of rapamycin
- mTORC1
mammalian target of rapamycin complex 1
- NBS1
nibrin
- NPC
nasopharyngeal carcinoma
- NRF2
nuclear factor erythroid 2-related factor 2
- p70S6K1
70 kDa ribosomal protein S6 kinase 1
- PI3K
phosphoinositide 3-kinase
- ROS
reactive oxygen species
- sLIFR
soluble leukemia inhibitory factor receptor
- STAT3
signal transducer and activator of transcription 3
- TGFβ1
transforming growth factor beta-1
- TNFα
tumor necrosis factor alpha
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was funded by the Ministry of Science and Technology, Taiwan (NSC101–2320-B-182–010-MY3) and a grant from the Ministry of Education, Taiwan to Chang Gung University (EMRPD1C0031).
References
- 1.Lo KW, To KF, Huang DP. . Focus on nasopharyngeal carcinoma. Cancer Cell 2004; 5:423 - 8; http://dx.doi.org/ 10.1016/S1535-6108(04)00119-9; PMID: 15144950 [DOI] [PubMed] [Google Scholar]
- 2.Wei WI, Sham JS. . Nasopharyngeal carcinoma. Lancet 2005; 365:2041 - 54; http://dx.doi.org/ 10.1016/S0140-6736(05)66698-6; PMID: 15950718 [DOI] [PubMed] [Google Scholar]
- 3.Mosialos G. . Cytokine signaling and Epstein-Barr virus-mediated cell transformation. Cytokine Growth Factor Rev 2001; 12:259 - 70; http://dx.doi.org/ 10.1016/S1359-6101(00)00035-6; PMID: 11325606 [DOI] [PubMed] [Google Scholar]
- 4.Pai S, Khanna R. . Role of LMP1 in immune control of EBV infection. Semin Cancer Biol 2001; 11:455 - 60; http://dx.doi.org/ 10.1006/scbi.2001.0412; PMID: 11669607 [DOI] [PubMed] [Google Scholar]
- 5.Morris MA, Dawson CW, Young LS. . Role of the Epstein-Barr virus-encoded latent membrane protein-1, LMP1, in the pathogenesis of nasopharyngeal carcinoma. Future Oncol 2009; 5:811 - 25; http://dx.doi.org/ 10.2217/fon.09.53; PMID: 19663731 [DOI] [PubMed] [Google Scholar]
- 6.Tang KF, Tan SY, Chan SH, Chong SM, Loh KS, Tan LK, Hu H. . A distinct expression of CC chemokines by macrophages in nasopharyngeal carcinoma: implication for the intense tumor infiltration by T lymphocytes and macrophages. Hum Pathol 2001; 32:42 - 9; http://dx.doi.org/ 10.1053/hupa.2001.20886; PMID: 11172294 [DOI] [PubMed] [Google Scholar]
- 7.Lin WW, Karin M. . A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest 2007; 117:1175 - 83; http://dx.doi.org/ 10.1172/JCI31537; PMID: 17476347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balkwill FR, Mantovani A. . Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol 2012; 22:33 - 40; http://dx.doi.org/ 10.1016/j.semcancer.2011.12.005; PMID: 22210179 [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Zhou BP. . Inflammation: a driving force speeds cancer metastasis. Cell Cycle 2009; 8:3267 - 73; http://dx.doi.org/ 10.4161/cc.8.20.9699; PMID: 19770594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suárez C, Rodrigo JP, Rinaldo A, Langendijk JA, Shaha AR, Ferlito A. . Current treatment options for recurrent nasopharyngeal cancer. Eur Arch Otorhinolaryngol 2010; 267:1811 - 24; http://dx.doi.org/ 10.1007/s00405-010-1385-x; PMID: 20865269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimitriadis E, Menkhorst E, Salamonsen LA, Paiva P. . Review: LIF and IL11 in trophoblast-endometrial interactions during the establishment of pregnancy. Placenta 2010; 31:Suppl S99 - 104; http://dx.doi.org/ 10.1016/j.placenta.2009.12.027; PMID: 20129664 [DOI] [PubMed] [Google Scholar]
- 12.Metcalf D. . The unsolved enigmas of leukemia inhibitory factor. Stem Cells 2003; 21:5 - 14; http://dx.doi.org/ 10.1634/stemcells.21-1-5; PMID: 12529546 [DOI] [PubMed] [Google Scholar]
- 13.Dahéron L, Opitz SL, Zaehres H, Lensch MW, Andrews PW, Itskovitz-Eldor J, Daley GQ. . LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells 2004; 22:770 - 8; http://dx.doi.org/ 10.1634/stemcells.22-5-770; PMID: 15342941 [DOI] [PubMed] [Google Scholar]
- 14.Niwa H, Ogawa K, Shimosato D, Adachi K. . A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature 2009; 460:118 - 22; http://dx.doi.org/ 10.1038/nature08113; PMID: 19571885 [DOI] [PubMed] [Google Scholar]
- 15.Pera MF, Tam PP. . Extrinsic regulation of pluripotent stem cells. Nature 2010; 465:713 - 20; http://dx.doi.org/ 10.1038/nature09228; PMID: 20535200 [DOI] [PubMed] [Google Scholar]
- 16.Kellokumpu-Lehtinen P, Talpaz M, Harris D, Van Q, Kurzrock R, Estrov Z. . Leukemia-inhibitory factor stimulates breast, kidney and prostate cancer cell proliferation by paracrine and autocrine pathways. Int J Cancer 1996; 66:515 - 9; http://dx.doi.org/; PMID: 8635867 [DOI] [PubMed] [Google Scholar]
- 17.Estrov Z, Samal B, Lapushin R, Kellokumpu-Lehtinen P, Sahin AA, Kurzrock R, Talpaz M, Aggarwal BB. . Leukemia inhibitory factor binds to human breast cancer cells and stimulates their proliferation. J Interferon Cytokine Res 1995; 15:905 - 13; http://dx.doi.org/ 10.1089/jir.1995.15.905; PMID: 8564713 [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald JS, Tsareva SA, Poehlmann TG, Berod L, Meissner A, Corvinus FM, Wiederanders B, Pfitzner E, Markert UR, Friedrich K. . Leukemia inhibitory factor triggers activation of signal transducer and activator of transcription 3, proliferation, invasiveness, and altered protease expression in choriocarcinoma cells. Int J Biochem Cell Biol 2005; 37:2284 - 96; http://dx.doi.org/ 10.1016/j.biocel.2005.02.025; PMID: 16125646 [DOI] [PubMed] [Google Scholar]
- 19.Kamohara H, Ogawa M, Ishiko T, Sakamoto K, Baba H. . Leukemia inhibitory factor functions as a growth factor in pancreas carcinoma cells: Involvement of regulation of LIF and its receptor expression. Int J Oncol 2007; 30:977 - 83; PMID: 17332938 [PubMed] [Google Scholar]
- 20.Okamura Y, Nomoto S, Kanda M, Li Q, Nishikawa Y, Sugimoto H, Kanazumi N, Takeda S, Nakao A. . Leukemia inhibitory factor receptor (LIFR) is detected as a novel suppressor gene of hepatocellular carcinoma using double-combination array. Cancer Lett 2010; 289:170 - 7; http://dx.doi.org/ 10.1016/j.canlet.2009.08.013; PMID: 19733004 [DOI] [PubMed] [Google Scholar]
- 21.Iorns E, Ward TM, Dean S, Jegg A, Thomas D, Murugaesu N, Sims D, Mitsopoulos C, Fenwick K, Kozarewa I, et al. . Whole genome in vivo RNAi screening identifies the leukemia inhibitory factor receptor as a novel breast tumor suppressor. Breast Cancer Res Treat 2012; 135:79 - 91; http://dx.doi.org/ 10.1007/s10549-012-2068-7; PMID: 22535017 [DOI] [PubMed] [Google Scholar]
- 22.Liu SC, Tsang NM, Chiang WC, Chang KP, Hsueh C, Liang Y, Juang JL, Chow KP, Chang YS. . Leukemia inhibitory factor promotes nasopharyngeal carcinoma progression and radioresistance. J Clin Invest 2013; 123:5269 - 83; http://dx.doi.org/ 10.1172/JCI63428; PMID: 24270418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lédée-Bataille N, Laprée-Delage G, Taupin JL, Dubanchet S, Frydman R, Chaouat G. . Concentration of leukaemia inhibitory factor (LIF) in uterine flushing fluid is highly predictive of embryo implantation. Hum Reprod 2002; 17:213 - 8; http://dx.doi.org/ 10.1093/humrep/17.1.213; PMID: 11756390 [DOI] [PubMed] [Google Scholar]
- 24.Mashayekhi F, Salehi Z. . Expression of leukemia inhibitory factor in the cerebrospinal fluid of patients with multiple sclerosis. J Clin Neurosci 2011; 18:951 - 4; http://dx.doi.org/ 10.1016/j.jocn.2010.12.031; PMID: 21570299 [DOI] [PubMed] [Google Scholar]
- 25.Mikolajczyk M, Wirstlein P, Skrzypczak J. . The impact of leukemia inhibitory factor in uterine flushing on the reproductive potential of infertile women--a prospective study. Am J Reprod Immunol 2007; 58:65 - 74; http://dx.doi.org/ 10.1111/j.1600-0897.2007.00492.x; PMID: 17565549 [DOI] [PubMed] [Google Scholar]
- 26.Thum MY, Abdalla HI, Bhaskaran S, Harden EL, Ford B, Sumar N, Shehata H, Bansal AS. . The effect of serum concentration of leukaemia inhibitory factor on in vitro fertilization treatment outcome. Am J Reprod Immunol 2006; 55:76 - 80; http://dx.doi.org/ 10.1111/j.1600-0897.2005.00328.x; PMID: 16364015 [DOI] [PubMed] [Google Scholar]
- 27.Ren SG, Seliktar J, Li X, Hershman JM, Braunstein GD, Melmed S. . In vivo and in vitro regulation of thyroid leukemia inhibitory factor (LIF): marker of hypothyroidism. J Clin Endocrinol Metab 1999; 84:2883 - 7; PMID: 10443695 [DOI] [PubMed] [Google Scholar]
- 28.Ren SG, Seliktar J, Li X, Braunstein GD, Melmed S. . Measurement of leukemia inhibitory factor in biological fluids by radioimmunoassay. J Clin Endocrinol Metab 1998; 83:1275 - 83; PMID: 9543156 [DOI] [PubMed] [Google Scholar]
- 29.Suman P, Malhotra SS, Gupta SK. . LIF-STAT signaling and trophoblast biology. JAKSTAT 2013; 2:e25155; http://dx.doi.org/ 10.4161/jkst.25155; PMID: 24416645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paiva P, Menkhorst E, Salamonsen L, Dimitriadis E. . Leukemia inhibitory factor and interleukin-11: critical regulators in the establishment of pregnancy. Cytokine Growth Factor Rev 2009; 20:319 - 28; http://dx.doi.org/ 10.1016/j.cytogfr.2009.07.001; PMID: 19647472 [DOI] [PubMed] [Google Scholar]
- 31.Gadient RA, Patterson PH. . Leukemia inhibitory factor, Interleukin 6, and other cytokines using the GP130 transducing receptor: roles in inflammation and injury. Stem Cells 1999; 17:127 - 37; http://dx.doi.org/ 10.1002/stem.170127; PMID: 10342555 [DOI] [PubMed] [Google Scholar]
- 32.Grant SL, Douglas AM, Goss GA, Begley CG. . Oncostatin M and leukemia inhibitory factor regulate the growth of normal human breast epithelial cells. Growth Factors 2001; 19:153 - 62; http://dx.doi.org/ 10.3109/08977190109001083; PMID: 11811789 [DOI] [PubMed] [Google Scholar]
- 33.Kellokumpu-Lehtinen P, Talpaz M, Harris D, Van Q, Kurzrock R, Estrov Z. . Leukemia-inhibitory factor stimulates breast, kidney and prostate cancer cell proliferation by paracrine and autocrine pathways. Int J Cancer 1996; 66:515 - 9; http://dx.doi.org/; PMID: 8635867 [DOI] [PubMed] [Google Scholar]
- 34.Spangenburg EE, Booth FW. . Multiple signaling pathways mediate LIF-induced skeletal muscle satellite cell proliferation. Am J Physiol Cell Physiol 2002; 283:C204 - 11; http://dx.doi.org/ 10.1152/ajpcell.00574.2001; PMID: 12055089 [DOI] [PubMed] [Google Scholar]
- 35.Auernhammer CJ, Melmed S. . Leukemia-inhibitory factor-neuroimmune modulator of endocrine function. Endocr Rev 2000; 21:313 - 45; PMID: 10857556 [DOI] [PubMed] [Google Scholar]
- 36.Haines BP, Voyle RB, Pelton TA, Forrest R, Rathjen PD. . Complex conserved organization of the mammalian leukemia inhibitory factor gene: regulated expression of intracellular and extracellular cytokines. J Immunol 1999; 162:4637 - 46; PMID: 10202003 [PubMed] [Google Scholar]
- 37.Haines BP, Voyle RB, Rathjen PD. . Intracellular and extracellular leukemia inhibitory factor proteins have different cellular activities that are mediated by distinct protein motifs. Mol Biol Cell 2000; 11:1369 - 83; http://dx.doi.org/ 10.1091/mbc.11.4.1369; PMID: 10749936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hisaka T, Desmoulière A, Taupin JL, Daburon S, Neaud V, Senant N, Blanc JF, Moreau JF, Rosenbaum J. . Expression of leukemia inhibitory factor (LIF) and its receptor gp190 in human liver and in cultured human liver myofibroblasts. Cloning of new isoforms of LIF mRNA. Comp Hepatol 2004; 3:10; http://dx.doi.org/ 10.1186/1476-5926-3-10; PMID: 15566573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mereau A, Grey L, Piquet-Pellorce C, Heath JK. . Characterization of a binding protein for leukemia inhibitory factor localized in extracellular matrix. J Cell Biol 1993; 122:713 - 9; http://dx.doi.org/ 10.1083/jcb.122.3.713; PMID: 8335694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trouillas M, Saucourt C, Guillotin B, Gauthereau X, Taupin JL, Moreau JF, Boeuf H. . The LIF cytokine: towards adulthood. Eur Cytokine Netw 2009; 20:51 - 62; PMID: 19541590 [DOI] [PubMed] [Google Scholar]
- 41.Peñuelas S, Anido J, Prieto-Sánchez RM, Folch G, Barba I, Cuartas I, García-Dorado D, Poca MA, Sahuquillo J, Baselga J, et al. . TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell 2009; 15:315 - 27; http://dx.doi.org/ 10.1016/j.ccr.2009.02.011; PMID: 19345330 [DOI] [PubMed] [Google Scholar]
- 42.Wysoczynski M, Miekus K, Jankowski K, Wanzeck J, Bertolone S, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. . Leukemia inhibitory factor: a newly identified metastatic factor in rhabdomyosarcomas. Cancer Res 2007; 67:2131 - 40; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-1021; PMID: 17332343 [DOI] [PubMed] [Google Scholar]
- 43.Lumachi F, Basso SM, Orlando R. . Cytokines, thyroid diseases and thyroid cancer. Cytokine 2010; 50:229 - 33; http://dx.doi.org/ 10.1016/j.cyto.2010.03.005; PMID: 20381375 [DOI] [PubMed] [Google Scholar]
- 44.Liu H, Jiang D, Chi F, Zhao B. . The Hippo pathway regulates stem cell proliferation, self-renewal, and differentiation. Protein Cell 2012; 3:291 - 304; http://dx.doi.org/ 10.1007/s13238-012-2919-3; PMID: 22549587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graf U, Casanova EA, Cinelli P. . The Role of the Leukemia Inhibitory Factor (LIF) - Pathway in Derivation and Maintenance of Murine Pluripotent Stem Cells. Genes (Basel) 2011; 2:280 - 97; http://dx.doi.org/ 10.3390/genes2010280; PMID: 24710148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bozec A, Bakiri L, Hoebertz A, Eferl R, Schilling AF, Komnenovic V, Scheuch H, Priemel M, Stewart CL, Amling M, et al. . Osteoclast size is controlled by Fra-2 through LIF/LIF-receptor signalling and hypoxia. Nature 2008; 454:221 - 5; http://dx.doi.org/ 10.1038/nature07019; PMID: 18548006 [DOI] [PubMed] [Google Scholar]
- 47.Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, Preisser L, Anegon I, Catala L, Ifrah N, et al. . Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood 2007; 110:4319 - 30; http://dx.doi.org/ 10.1182/blood-2007-02-072587; PMID: 17848619 [DOI] [PubMed] [Google Scholar]
- 48.Takahashi Y, Carpino N, Cross JC, Torres M, Parganas E, Ihle JN. . SOCS3: an essential regulator of LIF receptor signaling in trophoblast giant cell differentiation. EMBO J 2003; 22:372 - 84; http://dx.doi.org/ 10.1093/emboj/cdg057; PMID: 12554639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burdon T, Smith A, Savatier P. . Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol 2002; 12:432 - 8; http://dx.doi.org/ 10.1016/S0962-8924(02)02352-8; PMID: 12220864 [DOI] [PubMed] [Google Scholar]
- 50.Zouein FA, Kurdi M, Booz GW. . LIF and the heart: just another brick in the wall?. Eur Cytokine Netw 2013; 24:11 - 9; PMID: 23661360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathieu ME, Saucourt C, Mournetas V, Gauthereau X, Thézé N, Praloran V, Thiébaud P, Bœuf H. . LIF-dependent signaling: new pieces in the Lego. Stem Cell Rev 2012; 8:1 - 15; http://dx.doi.org/ 10.1007/s12015-011-9261-7; PMID: 21537995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dennis PB, Pullen N, Kozma SC, Thomas G. . The principal rapamycin-sensitive p70(s6k) phosphorylation sites, T-229 and T-389, are differentially regulated by rapamycin-insensitive kinase kinases. Mol Cell Biol 1996; 16:6242 - 51; PMID: 8887654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pearson RB, Dennis PB, Han JW, Williamson NA, Kozma SC, Wettenhall RE, Thomas G. . The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J 1995; 14:5279 - 87; PMID: 7489717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fenton TR, Gout IT. . Functions and regulation of the 70kDa ribosomal S6 kinases. Int J Biochem Cell Biol 2011; 43:47 - 59; http://dx.doi.org/ 10.1016/j.biocel.2010.09.018; PMID: 20932932 [DOI] [PubMed] [Google Scholar]
- 55.Blanchard F, Duplomb L, Wang Y, Robledo O, Kinzie E, Pitard V, Godard A, Jacques Y, Baumann H. . Stimulation of leukemia inhibitory factor receptor degradation by extracellular signal-regulated kinase. J Biol Chem 2000; 275:28793 - 801; http://dx.doi.org/ 10.1074/jbc.M003986200; PMID: 10858440 [DOI] [PubMed] [Google Scholar]
- 56.Blanchard F, Wang Y, Kinzie E, Duplomb L, Godard A, Baumann H. . Oncostatin M regulates the synthesis and turnover of gp130, leukemia inhibitory factor receptor alpha, and oncostatin M receptor beta by distinct mechanisms. J Biol Chem 2001; 276:47038 - 45; http://dx.doi.org/ 10.1074/jbc.M107971200; PMID: 11602599 [DOI] [PubMed] [Google Scholar]
- 57.Kamohara H, Takahashi M, Ishiko T, Ogawa M, Baba H. . Induction of interleukin-8 (CXCL-8) by tumor necrosis factor-alpha and leukemia inhibitory factor in pancreatic carcinoma cells: Impact of CXCL-8 as an autocrine growth factor. Int J Oncol 2007; 31:627 - 32; PMID: 17671691 [PubMed] [Google Scholar]
- 58.Ye F, Hu Y, Lu W, Zhou C, Xie X. . Expression of leukaemia inhibitory factor in epithelial ovarian carcinoma: correlation with clinical characteristics. Histopathology 2008; 53:224 - 8; http://dx.doi.org/ 10.1111/j.1365-2559.2008.03068.x; PMID: 18540977 [DOI] [PubMed] [Google Scholar]
- 59.Chen D, Sun Y, Wei Y, Zhang P, Rezaeian AH, Teruya-Feldstein J, Gupta S, Liang H, Lin HK, Hung MC, et al. . LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med 2012; 18:1511 - 7; http://dx.doi.org/ 10.1038/nm.2940; PMID: 23001183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schaue D, McBride WH. . Links between innate immunity and normal tissue radiobiology. Radiat Res 2010; 173:406 - 17; http://dx.doi.org/ 10.1667/RR1931.1; PMID: 20334512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deorukhkar A, Krishnan S. . Targeting inflammatory pathways for tumor radiosensitization. Biochem Pharmacol 2010; 80:1904 - 14; http://dx.doi.org/ 10.1016/j.bcp.2010.06.039; PMID: 20599771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lavin MF, Kozlov S. . ATM activation and DNA damage response. Cell Cycle 2007; 6:931 - 42; http://dx.doi.org/ 10.4161/cc.6.8.4180; PMID: 17457059 [DOI] [PubMed] [Google Scholar]
- 63.Khanna KK, Lavin MF, Jackson SP, Mulhern TD. . ATM, a central controller of cellular responses to DNA damage. Cell Death Differ 2001; 8:1052 - 65; http://dx.doi.org/ 10.1038/sj.cdd.4400874; PMID: 11687884 [DOI] [PubMed] [Google Scholar]
- 64.Kastan MB, Lim DS. . The many substrates and functions of ATM. Nat Rev Mol Cell Biol 2000; 1:179 - 86; http://dx.doi.org/ 10.1038/35043058; PMID: 11252893 [DOI] [PubMed] [Google Scholar]
- 65.Lavin MF. . Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol 2008; 9:759 - 69; http://dx.doi.org/ 10.1038/nrm2514; PMID: 18813293 [DOI] [PubMed] [Google Scholar]
- 66.Young LS, Rickinson AB. . Epstein-Barr virus: 40 years on. Nat Rev Cancer 2004; 4:757 - 68; http://dx.doi.org/ 10.1038/nrc1452; PMID: 15510157 [DOI] [PubMed] [Google Scholar]
- 67.Lam N, Sugden B. . CD40 and its viral mimic, LMP1: similar means to different ends. Cell Signal 2003; 15:9 - 16; http://dx.doi.org/ 10.1016/S0898-6568(02)00083-9; PMID: 12401515 [DOI] [PubMed] [Google Scholar]
- 68.Eliopoulos AG, Young LS. . LMP1 structure and signal transduction. Semin Cancer Biol 2001; 11:435 - 44; http://dx.doi.org/ 10.1006/scbi.2001.0410; PMID: 11669605 [DOI] [PubMed] [Google Scholar]
- 69.Middeldorp JM, Pegtel DM. . Multiple roles of LMP1 in Epstein-Barr virus induced immune escape. Semin Cancer Biol 2008; 18:388 - 96; http://dx.doi.org/ 10.1016/j.semcancer.2008.10.004; PMID: 19013244 [DOI] [PubMed] [Google Scholar]
- 70.Chen CC, Chen LC, Liang Y, Tsang NM, Chang YS. . Epstein-Barr virus latent membrane protein 1 induces the chemotherapeutic target, thymidine phosphorylase, via NF-kappaB and p38 MAPK pathways. Cell Signal 2010; 22:1132 - 42; http://dx.doi.org/ 10.1016/j.cellsig.2010.03.008; PMID: 20214978 [DOI] [PubMed] [Google Scholar]
- 71.Zheng H, Li LL, Hu DS, Deng XY, Cao Y. . Role of Epstein-Barr virus encoded latent membrane protein 1 in the carcinogenesis of nasopharyngeal carcinoma. Cell Mol Immunol 2007; 4:185 - 96; PMID: 17601372 [PubMed] [Google Scholar]