Abstract
Necroptosis is a form of regulated necrotic cell death that is mediated by receptor-interacting protein 1 (RIP1) and RIP3 kinases. Diverse receptors, including death receptors, Toll-like receptors, interferon receptors, and DAI DNA receptors are able to trigger necroptosis. The newly identified MLKL protein functions downstream of RIP1/RIP3 and is essential for the execution of necroptosis. Studies also indicate involvement of reactive oxygen species and calcium and sodium ions. Identification of the key mediators of necroptosis is critical for understanding the molecular mechanisms of the necroptotic process.
Keywords: calcium, MLKL, necroptosis, RIP3, ROS, sodium
Abbreviations
- AIF
Apoptosis inducing factor
- BHA
Butylated hydroxnisole
- cIAP1/cIAP2
Cellular inhibitor of apoptosis
- CYLD
Cylindromatosis
- CypD
Cyclophilin D
- DAI
DNA-dependent activator of interferon regulatory factor
- MLKL
Mixed lineage kinase domain-like protein
- mPT
Mitochondrial membrane permeability transition
- NAC
N-acetylcysteine
- NOXs
NADPH oxidase complexes
- NOX1
NADPH oxidase I
- RHIMs
RIP homotypic interaction motifs
- RIP1
Receptor-interacting protein 1
- RIP3
Receptor-interacting protein 3
- ROS
Reactive oxygen species
- TNF
Tumor necrosis factor
- TNF-R1
TNF receptor 1
- TRADD
TNF-R1-associated death domain protein
- TRAF2
TNFR-associated factor 2
- TRP
Transient receptor potential cation channel
- TRPM7
Transient receptor potential cation channel, subfamily M, member 7
Necrosis is a type of cell death that is morphologically characterized by a gain in cell volume, swelling of organelles, plasma membrane rupture, and subsequent loss of intracellular contents.1-3 For a long time necrosis was considered to be accidental cell death caused by extremely harsh physical or chemical insults.4 However, it is now known that necrosis can occur in a controlled and regulated manner. The discovery that a serine/threonine kinase, receptor-interacting protein 1 (RIP1), is required for tumor necrosis factor (TNF)-induced necrotic cell death initiated elucidation of the molecular mechanism underlying programmed necrosis.5,6 When the term necroptosis was first introduced it originally referred to RIP1-mediated programmed necrosis, which is inhibited by necrostatin-1, a chemical inhibitor of RIP1.7 However, after RIP3 was identified as an essential mediator of necroptosis downstream of RIP1,8-10 other types of RIP1-independent but RIP3-dependent programmed necrosis were also reported.11,12 Currently, both RIP1-dependent and RIP1-independent RIP3-mediated programmed necrosis are referred to as necroptosis. In addition, although necroptosis is the most studied form of programmed necrosis, other types of regulated necrosis including pyroptosis, pyronecrosis, parthanatos, oxytosis, ferroptosis, and NETosis have also been described.13 It has been suggested that necroptosis and other forms of programmed necrosis are involved in a variety of diseases including inflammatory bowel diseases, pancreatitis, virus infections, salmonellosis, ischemia-reperfusion injury, and myocardial infarction.14
The initiation of necroptosis can be triggered by the engagement of death receptors, such as TNF receptor 1 (TNFR1), CD95 (FAS), Toll-like receptors, including TLR3 and TLR4, or interferon receptors.11,15-18 The necroptotic signals induced by these receptors lead to formation of the necrosome.8-10 The serine/threonine kinases RIP1 and RIP3 and the recently identified mixed lineage kinase domain-like protein (MLKL) are key components of the necrosome and all of these proteins are essential for TNF-induced necroptosis.19,20 In this review, we will summarize and discuss current knowledge of the regulation of necroptosis and RIP3-mediated execution of the process.
RIPK3 is the Essential Player in Necroptosis
The molecular mechanism of TNF signaling has been extensively studied (Fig. 1). It is known that binding of the TNF homotrimer to TNF-receptor 1 (TNF-R1) initiates the formation of a TNF-R1 signaling complex by recruiting several adaptor/effector proteins. TNF-R1–associated death domain protein (TRADD) is the first protein to interact with the receptor through its death domain and subsequently recruits other effector proteins, including RIP1, TNFR-associated factor 2 (TRAF2), and cellular inhibitor of apoptosis (cIAP1/cIAP 2), to form the TNF-R1 surface signaling complex, also named complex I, which leads to the activation of pro-survival pathways involving NF-κB and MAP kinases.21,22 Polyubiquitination of RIP1 by cIAP1/ cIAP 2 and LUBAC is essential for activation of the IKK/NF-κB pathway.23 Under apoptotic conditions, the TNF-R1 complex I is released into the cytosol and recruits other proteins to form complex II and mediate apoptosis.24 Recruitment of Fas-associated protein with death domain (FADD) is essential for the initiation of apoptosis because FADD recruits and induces the dimerization and activation of caspases, which execute the process of apoptosis.24
Figure 1.
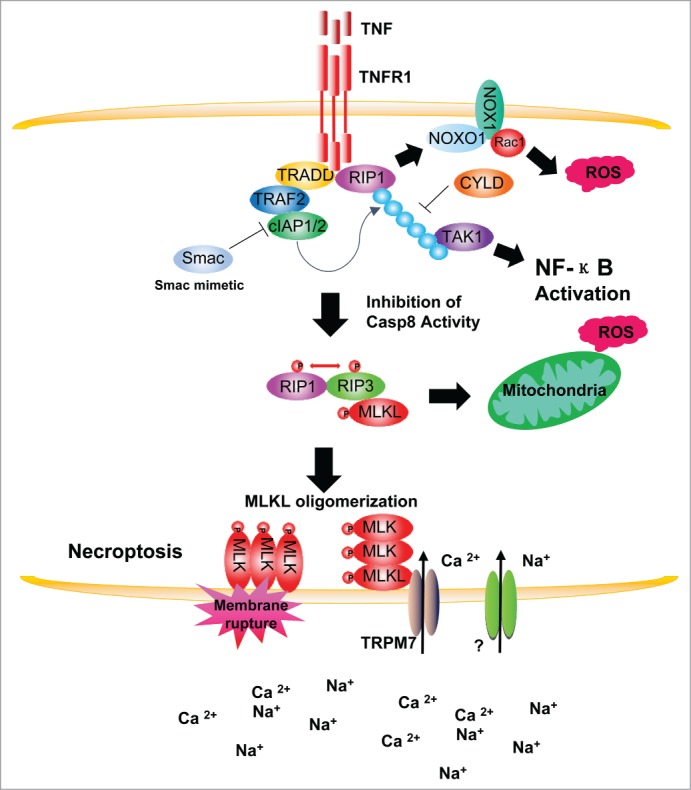
Overview of the execution of RIP3-dependent necrosis. Binding of tumor necrosis factor (TNF) homotrimer to TNF-receptor 1 (TNF-R1) initiates the formation of a TNF-R1 signaling complex through the recruitment of several adaptor/effector proteins including RIP1, TRADD, and TRAF2. Inactivation of cIAP1/2 by Smac or deubiquitination of RIP1 by CYLD induces the formation of complex II and converts signaling from cell survival to cell death pathways. Complex II mediates apoptosis by recruiting FADD and activating caspase-8. When caspase-8 activity is inhibited the amyloid-like RIP1/RIP3 necrosome is formed and recruits the RIP3 kinase downstream substrate MLKL. In certain conditions, such as in L929 cells, the TNF receptor signaling components RIP1 and TRADD form a complex with NADPH oxidases in a TNF-dependent manner to induce the generation of ROS, which are involved in the execution of necroptosis. Phosphorylation of MLKL by RIP3 results in homo-oligomerization of the MLKL protein and its relocation to the plasma membrane. This plasma membrane translocation of MLKL is essential for increasing plasma membrane permeability, either by itself or by association with ion channels, to increase Ca2+ and Na+ ion influx, which may in turn increase osmotic pressure and ultimately lead to cellular membrane rupture.
Under necroptotic conditions, deubiquitination of RIP1 by cylindromatosis (CYLD) and inhibition of caspase activity allow recruitment of RIP3 to these secondary complexes to form the necrosome.25,26 Inhibition of caspase-8 activity is critical for the formation of the necrosome because otherwise RIP1, RIP3, and CYLD would be cleaved by the caspase.27 In the necrosome, RIP1 and RIP3 interact through their RIP homotypic interaction motifs (RHIMs) and further form large amyloid-like complexes.8,9,28 The recruitment of RIP3 into the secondary complexes switches the death signal from apoptotic to necrotic. Studies with RIP3 knockout mice have demonstrated the essential role of RIP3 in necroptosis in vivo.8–10 Another key component of the necrosome is the MLKL protein, which is recruited to the complex by RIP319,20 and is the downstream substrate of RIP3 kinase.19,20 Phosphorylation of MLKL by RIP3 in the necrosome results in oligomerization and plasma membrane localization of the MLKL protein.29,30 This plasma membrane translocation of MLKL is essential for increasing plasma membrane permeability and ion influx, which in turn increases the cellular osmotic pressure and ultimately results in cell plasma membrane rupture and necroptosis.29-32 Importantly, there is also crosstalk between RIP1-mediated apoptosis and necroptosis pathways in vivo, as demonstrated in mouse models.33-37
In addition to the activation of death receptors, other stimuli can also induce RIP3-dependent necroptosis including activation of TLR 3 and 4, DNA-dependent activator of interferon regulatory factors (DAI/ZBP1/DLM-1), and interferons. TLR-induced necroptosis requires the RHIM-containing TLR adaptor TRIF, which directly interacts with and activates RIP3.11,16 In a similar fashion, the DNA-binding RHIM-containing protein DAI interacts with RIP3 to mediate virus-induced necroptosis.12 Interferons activate the transcription of RNA-responsive protein kinase PKR, which then interacts with RIP1 to trigger necroptosis.18 As RIP1 is only involved in necroptotic death induced by certain stimuli, it is believed that RIP3 is the essential and indispensable component of necroptosis.
Important Mediators Involved in the Execution of RIP3-dependent Necroptosis
MLKL
MLKL is the immediate downstream mediator of RIP3 in necroptosis. MLKL was first identified as a pseudo kinase and was found to be recruited to the necrosome by RIP3.19 MLKL contains N-terminal coiled-coil domains and a C-terminal kinase-like domain. The crystal structure of monomeric MLKL reveals that the N-terminal coiled-coil domains form a 4-helical bundle and tethers to the C-terminal kinase-like domain through a 2-helix linker.38 After phosphorylation by RIP3, MLKL oligomerizes through the N-terminal coiled-coil domains and translocates to the plasma membrane.30 The dimerized N-terminal region of MLKL is sufficient to kill cells when overexpressed29,32 whereas the C-terminal kinase-like domain appears to be important for modulating the mono-oligomeric status of MLKL.29-31,38 Studying the oligomeric structure of MLKL will help us understand the molecular mechanisms of MLKL-mediated necroptosis.
It has been suggested that MLKL mediates disruption of plasma membrane permeability by activating ion channels or forming pore structures directly in the plasma membrane.29-32 In the first scenario, Ca2+ and Na+ are critical for rupture of the plasma membrane.30,32 Since cations such as Ca2+ promote formation of amyloid protein structures that disrupt the plasma membrane,39 MLKL-mediated cation influx may facilitate formation of necrosomes, which have amyloid-like structures, and rupture of the plasma membrane in vivo. Regarding the second scenario, MLKL was found to bind to membrane lipids and disrupt liposome integrity in vitro.29,31 However, as the levels of endogenous MLKL protein are relatively low in necroptotic-sensitive cells, the direct pore-forming activity of MLKL in the plasma membrane needs to be confirmed in vivo.
Calcium
Under physiological conditions, the cytoplasmic Ca2+ concentration is approximately 0.1 μM while the extracellular Ca2+ concentration is approximately 1.2 mM. The plasma membrane is impermeable to extracellular Ca2+ and most of the intracellular Ca2+ is stored in the endoplasmic reticulum (ER). Intracellular Ca2+ can act as a second messenger in many signaling transduction pathways including neurotransmitter release, contraction of muscle cells, and fertilization.40–42 Furthermore, many enzymes require intracellular Ca2+ as a cofactor for their activity43 whereas extracellular Ca2+ is important for maintaining the electric potential between the interior and the exterior of a cell. Thus, calcium ions play a pivotal role in maintaining cellular homeostasis.
Under certain pathological conditions, apoptosis or necrosis can be initiated when ER Ca2+ is released into the cytosol or when extracellular Ca2+ crosses the plasma membrane. The increase in cytosolic Ca2+ triggers mitochondrial Ca2+ overload, leading to mitochondrial membrane permeability transition (mPT) and ATP depletion.44,45 Cytosolic Ca2+ also induces the activation of Ca2+-dependent proteases such as calpains and Ca2+/calmodulin-dependent protein kinases such as CaMKII.46,47 These events will ultimately lead to apoptosis or necrosis.48 The cytoplasmic Ca2+ concentration may even determine the nature of cell death; it has been reported that induction of apoptosis is related to a low increase in cytosolic Ca2+ from the ER, whereas induction of necrosis is related to more severe insults from plasma membrane influx.49-51
Several ion channels have been shown to mediate extracellular Ca2+ entry into necrotic cells including NMDA receptors and voltage-sensitive Ca2+ channels,52 acid-sensing ion channels (ASICs),53,54 transient receptor potential (TRP) cation channels,30 or capacitive entry channels that are activated by emptying of the ER Ca2+ storage.55,56 Moreover, Ca2+ influx activates calpain, which proteolytically inactivates the Na+/Ca2+ exchanger responsible for the extrusion of Ca.2+57-59 This process constitutes a positive-feedback loop that ensures an irreversible increase in intracellular Ca2+ in necrotic cells. Several mechanisms for the cell death induced by cytosolic Ca2+ overload have been proposed. For example, it has been suggested that Ca2+ overload can destabilize lysosomal membranes,60 induce cleavage of the plasma membrane Na+/Ca2+ exchanger,61 and promote the release of apoptosis-inducing factor (AIF) from mitochondria, which is required for necrotic cell death.62,63
Ca2+-mediated programmed necrosis has also been reported in Caenorhabditis elegans. Hyperactive mutation of the ion channel MEC-4 in C. elegans induces a toxic Ca2+ influx that leads to necrotic neurodegeneration. For TNF-induced necroptosis in L929 mouse cells, a similar increase in cytosolic Ca2+ has been observed after TNF-α treatment.53,64 Moreover, Ca2+ chelators exhibit a protective effect in L929 cells upon necroptosis induction, suggesting a potential role of Ca2+ in the execution of TNF-induced necroptosis.3 We recently demonstrated that MLKL translocates to the plasma membrane and regulates calcium influx through the TPRM7 ion channel during TNF-induced necroptosis in human HT29 cells.30 Translocation of MLKL to the plasma membrane leads to a robust Ca2+ influx, which is an early event of necroptosis. We demonstrated that TRPM7, a non–voltage-sensitive ion channel that has previously been implicated in necroptosis, is the Ca2+ channel activated by MLKL. Importantly, knockdown of TRPM7 did not interfere with MLKL phosphorylation by RIP3, MLKL trimerization, or MLKL translocation to the plasma membrane, suggesting that TRPM7 functions downstream of MLKL in necroptosis. However, it is worth noting that some cells, such as mouse embryonic fibroblast (MEF) cells, are not protected as well as HT29 cells in Ca2+-free medium.30 Additionally, if the cell culture medium contains HEPES, Ca2+ is less protective against necroptosis (unpublished data). Since TRPM7 and other TRPMs are cation channels, it is possible that other cations also contribute to the execution of necroptosis.65,66
Sodium
For cells under physiological conditions, the extracellular concentration of Na+ ions is much higher than the intracellular concentration. This Na+ concentration gradient provides the potential energy to form the membrane potential, which is critical for maintaining cellular osmotic balance.67 Intracellular Na+ increase can be caused by either defective outward pumping of Na+ resulting from metabolic depletion or increased Na+ influx via activated membrane transporters. As one key characteristic of necrosis is an increase in cell volume, the Na+ and water overload in necrotic cells is considered an important step leading to a marked increase in cell volume and subsequent cell lysis.1 It has been reported that oxygen and glucose deprivation cause an increase in intracellular Na+ and induce neuronal necrotic cell death68 and that ionotropic glutamate receptors,69 voltage-dependent Na+ channels,70 and both plasma membrane and mitochondrial Na+/Ca2+exchangers65,71 are required for necrosis.61,67 Furthermore, oxidative stress can also induce Na+ influx through the activation of non-selective cation channels and lead to necrosis.72 In addition, Ca2+ influx can be elicited by Na+ influx through various mechanisms, such as reverse operation of the Na+/Ca2+ exchanger or non-selective Ca2+ entry via voltage-sensitive Na+ channels.73
The involvement of Na+ influx in TNF-induced necroptosis has recently been reported.32 Translocation of MLKL to the plasma membrane of L929 cells leads to a robust influx of Na+ before the plasma membrane becomes permeable. Depletion of Na+ from the culture medium of L929 cells protects against necrotic, but not apoptotic, cell death.32 Therefore, both Ca2+ and Na+ might play a role in mediating necroptosis. This is in fact quite likely because there are multiple TRPM channel proteins that are expressed in different cell types. For example, TRPM4, which is known to mediate Na+ influx, might be involved in necrosis in a similar manner to TRPM7 in HT29 cells.74
Reactive Oxygen Species
Reactive oxygen species (ROS) are normally generated as natural byproducts of cell metabolism and are involved in diverse cellular processes.75,76 ROS are produced from 3 different sources within the cell. First, mitochondria are the major site of ROS generation. It is known that ROS are predominantly generated by complex I and complex III of the mitochondrial respiratory chain.77 Second, the plasma membrane-associated NADPH oxidase complexes (NOXs) use NADPH as a substrate to reduce molecular oxygen and generate superoxide anions, which play an important role in innate immunity against infectious pathogens and function as second messengers mediating signal transduction pathways.76,78,79 Third, although less robustly than NOXs, other enzymes including xanthine oxidase, cyclooxygenases, lipoxygenases, myeloperoxidases, heme oxygenase, monoamine oxidases, and aldehyde oxidase, as well as cytochrome P450-based enzymes, can also generate ROS in a variety of cell types.80
Under physiological conditions cells possess multiple antioxidant systems to counteract excessive production of ROS and maintain the balance between the production and scavenging of ROS, leading to general homeostasis.81,82 However, under pathological conditions excessive uncontrolled ROS production results in deleterious effects and induces oxidative stress, which causes cell damage and may eventually kill the cell.79 ROS may directly oxidize cellular proteins, lipids, or nucleic acids and therefore cause general cellular damage.78 Additionally, ROS can function as second messengers to initiate or mediate multiple signaling pathways involved in cell death. For example, ROS have been shown to induce production of ceramide and activate the p53 pathway during cell death.79,80 The uncontrolled production of ROS induced by TNF was first observed in the 1990s,83 and since then the important role of ROS in the execution of TNF-induced necroptosis has been proposed. It has been reported that inhibition of mitochondrial complexes I and II protects L929 cells against TNF-induced necroptosis.84,85 Moreover, treatment with the ROS scavengers butylated hydroxnisole (BHA) or N-acetylcysteine (NAC) delays TNFα-induced necroptosis in several cell types.85,86 In addition to the production of ROS from mitochondria, another important source of ROS is the plasma membrane-associated NADPH oxidase. We have reported that TNF is a direct activator of NADPH oxidase 1 (NOX1) in the L929 cell line and in p65-/- MEFs subjected to caspase inhibition.86 In TNF-induced necroptosis, the death domain-containing TNF-R1 adaptor proteins RIP1 and TRADD associate with NOX1 and the small GTPase RAC1 to regulate ROS production at the plasma membrane, which is involved in the execution of necroptosis.86
The regulation of ROS generation by RIP3 and MLKL is a crucial component of TNF-induced necroptosis. It has been demonstrated that ROS production upon induction of necroptosis is inhibited in RIP3- or MLKL-deficient cells.8,9,20 It is worth noting that the requirement for ROS for the execution of necroptosis seems to be dependent on cell type and that ROS are not required for necroptosis in certain types of cells. For example, it has been demonstrated that ROS scavengers do not inhibit necroptosis in colon carcinoma HT-29 cells and Jurkat T cells.8 In addition, ROS scavengers may exhibit some off-target effects in necroptosis.85 Moreover, a recent study suggested that depletion of mitochondria prevented ROS production but had no effect on necroptotic cell death in NIH3T3 cells, suggesting that players other than mitochondrial ROS are necessary for the execution of necrosis.87 Thus, the function and source of the ROS that are generated in TNF-induced necroptosis are currently a subject of some debate within the field and future studies are necessary to clarify the functions of ROS from different sources in TNF-induced necroptosis.
The Role of Mitochondria in Necroptosis
The role of mitochondria in necroptosis was first implicated when ROS produced from mitochondrial complex I were found to be required for TNF-induced necrosis.83 More recently, the identification of RIP3 as a key regulator of necroptosis and mitochondrial ROS production further indicated the possible role of ROS in necroptosis.8–10 It has been shown that RIP3 physically interacts with and activates several metabolic enzymes including the cytosol localized GLUL and the mitochondrial localized GLUD1.10 Activation of these metabolic enzymes causes ROS generation by the mitochondrial respiratory chain and promotes necroptosis.10 In addition, 2 mitochondrial proteins, PGAM5 and Drp-1, have been suggested as crucial downstream effectors of RIP3 in the execution of necroptosis in HT29 cells88 although recent studies suggest that both PGAM5 and Drp-1 are dispensable for RIP3-dependent necroptosis in other cell types.89,90 Furthermore, a recent study indicated that depletion of mitochondria had little effect on necroptosis, which argues against the role of mitochondria in the execution of necroptosis.87 This conclusion is supported by the observation that MLKL localizes to the plasma membrane and regulates plasma membrane permeability upon necroptosis induction.29-32 Thus, mitochondria may function as facilitators, but not as executors, in the process of RIP3-mediated necroptosis.
As a key modulator of the mitochondrial permeability transition pore, cyclophilin D (CypD) is known to play a role in programmed necrosis.91 Current knowledge of necrosis suggests that both RIP1 and CypD are key players in regulated necrosis and that the RIP1 pathway is responsible for the plasma membrane rupture whereas CypD regulates the mitochondrial permeability transition (mPT).30,74 Work by Linkermann et al.92 elegantly demonstrated that both of these pathways are activated and distinct in regulated necrosis during ischemia/perfusion injury. Importantly, this study raised the possibility that CypD may also function downstream of RIP1-mediated Ca2+ elevation. Therefore, it is important to test whether RIP1-mediated Ca2+ elevation leads to mPT and whether CypD is required for this process. If so, CypD-mediated mPT might be a component of the RIP1-regulated machinery that accelerates the process, although this mitochondrial event may not be essential for execution of RIP3-mediated necroptosis.
Conclusion
In the last few years our understanding of the molecular mechanism of necroptosis has greatly improved. Although RIP1 and RIP3 are crucial for initiating the process, MLKL is an essential downstream executor of necroptosis. Other potential executors of necroptosis include ROS and calcium and sodium ions, which may directly or indirectly cause damage to proteins, lipids, and DNA to facilitate necroptosis. However, many aspects of the execution of necroptosis remain elusive, for example how MLKL is targeted to the plasma membrane and how it regulates the permeability of the cell plasma membrane. Therefore, further investigation is necessary for full understanding of the execution process of RIP3-mediated necroptosis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Dr Swati Choksi for reading the manuscript.
Funding
Our research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
References
- 1. Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev 2006; 20:1-15; PMID:16391229; http://dx.doi.org/ 10.1101/gad.1376506 [DOI] [PubMed] [Google Scholar]
- 2. Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 2010; 11:700-14; PMID:20823910; http://dx.doi.org/ 10.1038/nrm2970 [DOI] [PubMed] [Google Scholar]
- 3. Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta 2006; 1757:1371-87; PMID:16950166; http://dx.doi.org/ 10.1016/j.bbabio.2006.06.014 [DOI] [PubMed] [Google Scholar]
- 4. Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: a specific form of programmed cell death? Exp Cell Res 2003; 283:1-16; PMID:12565815; http://dx.doi.org/ 10.1016/S0014-4827(02)00027-7 [DOI] [PubMed] [Google Scholar]
- 5. Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 2000; 1:489-95; PMID:11101870; http://dx.doi.org/ 10.1038/82732 [DOI] [PubMed] [Google Scholar]
- 6. Lin Y, Choksi S, Shen HM, Yang QF, Hur GM, Kim YS, Tran JH, Nedospasov SA, Liu ZG. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem 2004; 279:10822-8; PMID:14701813; http://dx.doi.org/ 10.1074/jbc.M313141200 [DOI] [PubMed] [Google Scholar]
- 7. Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 2005; 1:112-9; PMID:16408008; http://dx.doi.org/ 10.1038/nchembio711 [DOI] [PubMed] [Google Scholar]
- 8. He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009; 137:1100-11; PMID:19524512; http://dx.doi.org/ 10.1016/j.cell.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 9. Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009; 137:1112-23; PMID:19524513; http://dx.doi.org/ 10.1016/j.cell.2009.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009; 325:332-6; PMID: 19498109; http://dx.doi.org/ 10.1126/science.1172308 [DOI] [PubMed] [Google Scholar]
- 11. Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem 2013; 288:31268-79; PMID:24019532; http://dx.doi.org/ 10.1074/jbc.M113.462341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 2012; 11:290-7; PMID:22423968; http://dx.doi.org/ 10.1016/j.chom.2012.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 2014; 15:135-47; PMID:24452471; http://dx.doi.org/ 10.1038/nrm3737 [DOI] [PubMed] [Google Scholar]
- 14. Linkermann A, Green DR. Necroptosis. N Engl J Med 2014; 370:455-65; PMID:24476434; http://dx.doi.org/ 10.1056/NEJMra1310050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Herreweghe F, Festjens N, Declercq W, Vandenabeele P. Tumor necrosis factor-mediated cell death: to break or to burst, that's the question. Cell Mol Life Sci 2010; 67:1567-79; PMID:20198502; http://dx.doi.org/ 10.1007/s00018-010-0283-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A 2011; 108:20054-9; PMID:22123964; http://dx.doi.org/ 10.1073/pnas.1116302108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol 2012; 13:954-62; PMID:22922364; http://dx.doi.org/ 10.1038/ni.2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, Rall GF, Degterev A, Balachandran S. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A 2013; 110:E3109-18; PMID:23898178; http://dx.doi.org/ 10.1073/pnas.1301218110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012; 148:213-27; PMID:22265413; http://dx.doi.org/ 10.1016/j.cell.2011.11.031 [DOI] [PubMed] [Google Scholar]
- 20. Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A 2012; 109:5322-7; PMID:22421439; http://dx.doi.org/ 10.1073/pnas.1200012109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science 2002; 296:1634-5; PMID:12040173; http://dx.doi.org/ 10.1126/science.1071924 [DOI] [PubMed] [Google Scholar]
- 22. Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ 2003; 10:45-65; PMID:12655295; http://dx.doi.org/ 10.1038/sj.cdd.4401189 [DOI] [PubMed] [Google Scholar]
- 23. Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, Webb AI, Rickard JA, Anderton H, Wong WW, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature 2011; 471:591-6; PMID:21455173; http://dx.doi.org/ 10.1038/nature09816 [DOI] [PubMed] [Google Scholar]
- 24. Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003; 114:181-90; PMID:12887920; http://dx.doi.org/ 10.1016/S0092-8674(03)00521-X [DOI] [PubMed] [Google Scholar]
- 25. O'Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, Xavier R, Green DR, Ting AT. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol 2011; 13:1437-42; PMID:22037414; http://dx.doi.org/ 10.1038/ncb2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moquin DM, McQuade T, Chan FK. CYLD deubiquitinates RIP1 in the TNFalpha-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS One 2013; 8:e76841; PMID:24098568; http://dx.doi.org/ 10.1371/journal.pone.0076841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N, Castanares M, Wu M. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal 2007; 19:2056-67; PMID:17644308; http://dx.doi.org/ 10.1016/j.cellsig.2007.05.016 [DOI] [PubMed] [Google Scholar]
- 28. Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 2012; 150:339-50; PMID: 22817896; http://dx.doi.org/ 10.1016/j.cell.2012.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, Hulpiau P, Weber K, Sehon CA, Marquis RW, et al. MLKL Compromises Plasma Membrane Integrity by Binding to Phosphatidylinositol Phosphates. Cell Rep 2014; 7:971-81; PMID:24813885; http://dx.doi.org/ 10.1016/j.celrep.2014.04.026 [DOI] [PubMed] [Google Scholar]
- 30. Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 2014; 16:55-65; PMID:24316671; http://dx.doi.org/ 10.1038/ncb2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell 2014; 54:133-46; PMID:24703947; http://dx.doi.org/ 10.1016/j.molcel.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 32. Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, Zheng X, Chen P, Han J. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res 2014; 24:105-21; PMID:24366341; http://dx.doi.org/ 10.1038/cr.2013.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rickard JA, O'Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL, Anderton H, et al. RIPK1 Regulates RIPK3-MLKL-Driven Systemic Inflammation and Emergency Hematopoiesis. Cell 2014; 157:1175-88; PMID:24813849; http://dx.doi.org/ 10.1016/j.cell.2014.04.019 [DOI] [PubMed] [Google Scholar]
- 34. Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, et al. RIPK1 Blocks Early Postnatal Lethality Mediated by Caspase-8 and RIPK3. Cell 2014; 157:1189-202; PMID:24813850; http://dx.doi.org/ 10.1016/j.cell.2014.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 2011; 477:330-4; PMID: 21804564; http://dx.doi.org/ 10.1038/nature10273 [DOI] [PubMed] [Google Scholar]
- 36. Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 2011; 471:373-6; PMID:21368761; http://dx.doi.org/ 10.1038/nature09878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 2011; 471:363-7; PMID:21368763; http://dx.doi.org/ 10.1038/nature09852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 2013; 39:443-53; PMID:24012422; http://dx.doi.org/ 10.1016/j.immuni.2013.06.018 [DOI] [PubMed] [Google Scholar]
- 39. Sciacca MF, Milardi D, Messina GM, Marletta G, Brender JR, Ramamoorthy A, La Rosa C. Cations as switches of amyloid-mediated membrane disruption mechanisms: calcium and IAPP. Biophys J 2013; 104:173-84; PMID:23332070; http://dx.doi.org/ 10.1016/j.bpj.2012.11.3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sheng L, Leshchyns'ka I, Sytnyk V. Cell adhesion and intracellular calcium signaling in neurons. Cell Commun Signal 2013; 11:94; PMID:24330678; http://dx.doi.org/ 10.1186/1478-811X-11-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ramos I, Wessel GM. Calcium pathway machinery at fertilization in echinoderms. Cell Calcium 2013; 53:16-23; PMID:23218671; http://dx.doi.org/ 10.1016/j.ceca.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jiang H, Stephens NL. Calcium and smooth muscle contraction. Mol Cell Biochem 1994; 135:1-9; PMID:7816050; http://dx.doi.org/ 10.1007/BF00925956 [DOI] [PubMed] [Google Scholar]
- 43. Clapham DE. Calcium signaling. Cell 2007; 131:1047-58; PMID:18083096; http://dx.doi.org/ 10.1016/j.cell.2007.11.028 [DOI] [PubMed] [Google Scholar]
- 44. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 2012; 298:229-317; PMID:22878108; http://dx.doi.org/ 10.1016/B978-0-12-394309-5.00006-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 2009; 1787:1395-401; PMID:19576166; http://dx.doi.org/ 10.1016/j.bbabio.2009.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nomura M, Ueno A, Saga K, Fukuzawa M, Kaneda Y. Accumulation of cytosolic calcium induces necroptotic cell death in human neuroblastoma. Cancer Res 2014; 74:1056-66; PMID:24371227; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-1283 [DOI] [PubMed] [Google Scholar]
- 47. Croall DE, Ersfeld K. The calpains: modular designs and functional diversity. Genome Biol 2007; 8:218; PMID:17608959; http://dx.doi.org/ 10.1186/gb-2007-8-6-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verkhratsky A. Calcium and cell death. Subcell Biochem 2007; 45:465-80; PMID:18193648; http://dx.doi.org/ 10.1007/978-1-4020-6191-2_17 [DOI] [PubMed] [Google Scholar]
- 49. Gwag BJ, Canzoniero LM, Sensi SL, Demaro JA, Koh JY, Goldberg MP, Jacquin M, Choi DW. Calcium ionophores can induce either apoptosis or necrosis in cultured cortical neurons. Neuroscience 1999; 90:1339-48; PMID:10338301; http://dx.doi.org/ 10.1016/S0306-4522(98)00508-9 [DOI] [PubMed] [Google Scholar]
- 50. Criddle DN, Gerasimenko JV, Baumgartner HK, Jaffar M, Voronina S, Sutton R, Petersen OH, Gerasimenko OV. Calcium signalling and pancreatic cell death: apoptosis or necrosis? Cell Death Differ 2007; 14:1285-94; PMID:17431416; http://dx.doi.org/ 10.1038/sj.cdd.4402150 [DOI] [PubMed] [Google Scholar]
- 51. McConkey DJ, Orrenius S. The role of calcium in the regulation of apoptosis. J Leukoc Biol 1996; 59:775-83; PMID:8691060 [PubMed] [Google Scholar]
- 52. Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol 2007; 7:39-47; PMID:17088105; http://dx.doi.org/ 10.1016/j.coph.2006.08.011 [DOI] [PubMed] [Google Scholar]
- 53. Bianchi L, Gerstbrein B, Frokjaer-Jensen C, Royal DC, Mukherjee G, Royal MA, Xue J, Schafer WR, Driscoll M. The neurotoxic MEC-4(d) DEG/ENaC sodium channel conducts calcium: implications for necrosis initiation. Nat Neurosci 2004; 7:1337-44; PMID: 15543143; http://dx.doi.org/ 10.1038/nn1347 [DOI] [PubMed] [Google Scholar]
- 54. Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci U S A 2004; 101:6752-7; PMID:15082829; http://dx.doi.org/ 10.1073/pnas.0308636100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 2009; 136:876-90; PMID:19249086; http://dx.doi.org/ 10.1016/j.cell.2009.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim KD, Srikanth S, Yee MK, Mock DC, Lawson GW, Gwack Y. ORAI1 deficiency impairs activated T cell death and enhances T cell survival. J Immunol 2011; 187:3620-30; PMID:21873530; http://dx.doi.org/ 10.4049/jimmunol.1100847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brustovetsky T, Bolshakov A, Brustovetsky N. Calpain activation and Na+/Ca2+ exchanger degradation occur downstream of calcium deregulation in hippocampal neurons exposed to excitotoxic glutamate. J Neurosci Res 2010; 88:1317-28; PMID:19937813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Samanta K, Kar P, Chakraborti T, Chakraborti S. Calcium-dependent cleavage of the Na(+)/Ca(2+) exchanger by m-calpain in isolated endoplasmic reticulum. J Biochem 2010; 147:225-35; PMID:19884190; http://dx.doi.org/ 10.1093/jb/mvp176 [DOI] [PubMed] [Google Scholar]
- 59. Atherton J, Kurbatskaya K, Bondulich M, Croft CL, Garwood CJ, Chhabra R, Wray S, Jeromin A, Hanger DP, Noble W. Calpain cleavage and inactivation of the sodium calcium exchanger-3 occur downstream of Abeta in Alzheimer's disease. Aging Cell 2014; 13:49-59; PMID:23919677; http://dx.doi.org/ 10.1111/acel.12148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yamashima T. Ca2+-dependent proteases in ischemic neuronal death: a conserved 'calpain-cathepsin cascade' from nematodes to primates. Cell Calcium 2004; 36:285-93; PMID:15261484; http://dx.doi.org/ 10.1016/j.ceca.2004.03.001 [DOI] [PubMed] [Google Scholar]
- 61. Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell 2005; 120:275-85; PMID:15680332; http://dx.doi.org/ 10.1016/j.cell.2004.11.049 [DOI] [PubMed] [Google Scholar]
- 62. Polster BM, Basanez G, Etxebarria A, Hardwick JM, Nicholls DG. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J Biol Chem 2005; 280:6447-54; PMID:15590628; http://dx.doi.org/ 10.1074/jbc.M413269200 [DOI] [PubMed] [Google Scholar]
- 63. Boujrad H, Gubkina O, Robert N, Krantic S, Susin SA. AIF-mediated programmed necrosis: a highly regulated way to die. Cell Cycle 2007; 6:2612-9; PMID: 17912035; http://dx.doi.org/ 10.4161/cc.6.21.4842 [DOI] [PubMed] [Google Scholar]
- 64. Xu K, Tavernarakis N, Driscoll M. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca(2+) release from the endoplasmic reticulum. Neuron 2001; 31:957-71; PMID: 11580896; http://dx.doi.org/ 10.1016/S0896-6273(01)00432-9 [DOI] [PubMed] [Google Scholar]
- 65. Matsuda T, Takuma K, Nishiguchi E, Hashimoto H, Azuma J, Baba A. Involvement of Na+-Ca2+ exchanger in reperfusion-induced delayed cell death of cultured rat astrocytes. Eur J Neurosci 1996; 8:951-8; PMID: 8743743; http://dx.doi.org/ 10.1111/j.1460-9568.1996.tb01582.x [DOI] [PubMed] [Google Scholar]
- 66. McNulty S, Fonfria E. The role of TRPM channels in cell death. Pflugers Arch 2005; 451:235-42; PMID:16025303; http://dx.doi.org/ 10.1007/s00424-005-1440-4 [DOI] [PubMed] [Google Scholar]
- 67. Clausen MJ, Poulsen H. Sodium/Potassium homeostasis in the cell. Met Ions Life Sci 2013; 12:41-67; PMID:23595670; http://dx.doi.org/ 10.1007/978-94-007-5561-1_3 [DOI] [PubMed] [Google Scholar]
- 68. Probert AW, Borosky S, Marcoux FW, Taylor CP. Sodium channel modulators prevent oxygen and glucose deprivation injury and glutamate release in rat neocortical cultures. Neuropharmacology 1997; 36:1031-8; PMID:9294967; http://dx.doi.org/ 10.1016/S0028-3908(97)00072-5 [DOI] [PubMed] [Google Scholar]
- 69. Lalo U, Pankratov Y, Parpura V, Verkhratsky A. Ionotropic receptors in neuronal-astroglial signalling: what is the role of "excitable" molecules in non-excitable cells. Biochim Biophys Acta 2011; 1813:992-1002; PMID:20869992; http://dx.doi.org/ 10.1016/j.bbamcr.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 70. Koike T, Tanaka S, Oda T, Ninomiya T. Sodium overload through voltage-dependent Na(+) channels induces necrosis and apoptosis of rat superior cervical ganglion cells in vitro. Brain Res Bull 2000; 51:345-55; PMID:10704786; http://dx.doi.org/ 10.1016/S0361-9230(99)00246-4 [DOI] [PubMed] [Google Scholar]
- 71. Yamashita J, Kita S, Iwamoto T, Ogata M, Takaoka M, Tazawa N, Nishikawa M, Wakimoto K, Shigekawa M, Komuro I, et al. Attenuation of ischemia/reperfusion-induced renal injury in mice deficient in Na+/Ca2+ exchanger. J Pharmacol Exp Ther 2003; 304:284-93; PMID:12490603; http://dx.doi.org/ 10.1124/jpet.102.039024 [DOI] [PubMed] [Google Scholar]
- 72. Barros LF, Stutzin A, Calixto A, Catalan M, Castro J, Hetz C, Hermosilla T. Nonselective cation channels as effectors of free radical-induced rat liver cell necrosis. Hepatology 2001; 33:114-22; PMID:11124827; http://dx.doi.org/ 10.1053/jhep.2001.20530 [DOI] [PubMed] [Google Scholar]
- 73. Martinez-Sanchez M, Striggow F, Schroder UH, Kahlert S, Reymann KG, Reiser G. Na(+) and Ca(2+) homeostasis pathways, cell death and protection after oxygen-glucose-deprivation in organotypic hippocampal slice cultures. Neuroscience 2004; 128:729-40; PMID:15464281; http://dx.doi.org/ 10.1016/j.neuroscience.2004.06.074 [DOI] [PubMed] [Google Scholar]
- 74. Simard JM, Woo SK, Gerzanich V. Transient receptor potential melastatin 4 and cell death. Pflugers Arch 2012; 464:573-82; PMID:23065026; http://dx.doi.org/ 10.1007/s00424-012-1166-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schieber M, Chandel NS. ROS Function in Redox Signaling and Oxidative Stress. Curr Biol 2014; 24:R453-62; PMID:24845678; http://dx.doi.org/ 10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med 2009; 47:1239-53; PMID: 19628035; http://dx.doi.org/ 10.1016/j.freeradbiomed.2009.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jezek P, Hlavata L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int J Biochem Cell Biol 2005; 37:2478-503; PMID: 16103002; http://dx.doi.org/ 10.1016/j.biocel.2005.05.013 [DOI] [PubMed] [Google Scholar]
- 78. Quinn MT, Ammons MC, Deleo FR. The expanding role of NADPH oxidases in health and disease: no longer just agents of death and destruction. Clin Sci (Lond) 2006; 111:1-20; PMID:16764554 [DOI] [PubMed] [Google Scholar]
- 79. Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 2004; 4:181-9; PMID:15039755; http://dx.doi.org/ 10.1038/nri1312 [DOI] [PubMed] [Google Scholar]
- 80. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000; 408:239-47; PMID:11089981; http://dx.doi.org/ 10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- 81. Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal 2000; 2:811-20; PMID:11213485; http://dx.doi.org/ 10.1089/ars.2000.2.4-811 [DOI] [PubMed] [Google Scholar]
- 82. Rhee SG, Yang KS, Kang SW, Woo HA, Chang TS. Controlled elimination of intracellular H(2)O(2): regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid Redox Signal 2005; 7:619-26; PMID:15890005; http://dx.doi.org/ 10.1089/ars.2005.7.619 [DOI] [PubMed] [Google Scholar]
- 83. Schulze-Osthoff K, Bakker AC, Vanhaesebroeck B, Beyaert R, Jacob WA, Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem 1992; 267:5317-23; PMID:1312087 [PubMed] [Google Scholar]
- 84. Cauwels A, Janssen B, Waeytens A, Cuvelier C, Brouckaert P. Caspase inhibition causes hyperacute tumor necrosis factor-induced shock via oxidative stress and phospholipase A2. Nat Immunol 2003; 4:387-93; PMID:12652297; http://dx.doi.org/ 10.1038/ni914 [DOI] [PubMed] [Google Scholar]
- 85. Festjens N, Kalai M, Smet J, Meeus A, Van Coster R, Saelens X, Vandenabeele P. Butylated hydroxyanisole is more than a reactive oxygen species scavenger. Cell Death Differ 2006; 13:166-9; PMID:16138110; http://dx.doi.org/ 10.1038/sj.cdd.4401746 [DOI] [PubMed] [Google Scholar]
- 86. Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell 2007; 26:675-87; PMID:17560373; http://dx.doi.org/ 10.1016/j.molcel.2007.04.021 [DOI] [PubMed] [Google Scholar]
- 87. Tait SW, Oberst A, Quarato G, Milasta S, Haller M, Wang R, Karvela M, Ichim G, Yatim N, Albert ML, et al. Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Rep 2013; 5:878-85; PMID:24268776; http://dx.doi.org/ 10.1016/j.celrep.2013.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 2012; 148:228-43; PMID:22265414; http://dx.doi.org/ 10.1016/j.cell.2011.11.030 [DOI] [PubMed] [Google Scholar]
- 89. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39:1-10; PMID:23890059; http://dx.doi.org/ 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 90. Moujalled DM, Cook WD, Murphy JM, Vaux DL. Necroptosis induced by RIPK3 requires MLKL but not Drp1. Cell Death Dis 2014; 5:e1086; PMID: 24577084; http://dx.doi.org/ 10.1038/cddis.2014.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005; 434:652-8; PMID:15800626; http://dx.doi.org/ 10.1038/nature03317 [DOI] [PubMed] [Google Scholar]
- 92. Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A 2013; 110: 12024-9; PMID:23818611; http://dx.doi.org/ 10.1073/pnas.1305538110 [DOI] [PMC free article] [PubMed] [Google Scholar]


