Abstract
During the initiation stage of pancreatic adenocarcinoma induced by oncogenic Kras, pancreatic cells are exposed to both a protumoral effect and an opposing tumor suppressive process known as oncogene-induced senescence. Pancreatitis disrupts this balance in favor of the transforming effect of oncogenes by lowering the tumor suppressive threshold of oncogene-induced senescence through expression of the stress protein Nupr1.
Keywords: pancreatic cancer, Kras, PanIN, senescence, Nupr1
Pancreatic adenocarcinoma (PDAC) is currently the fourth leading cause of cancer death with a median survival of 6 mo and a dismal 5-y survival rate of less than 5%, but could move up to second place as early as 2020 according to a new report from the Pancreatic Cancer Action Network.1 Moreover, information from the Surveillance Epidemiology and End Results database indicates that the number of patients with PDAC in 2030 will represent more than a 2-fold increase over the current prevalence in the occidental world.1 Based on these data, the number of deaths from PDAC will exceed those from breast and colorectal cancer, and will be surpassed only by the loss of life from lung cancer.
PDAC progresses from precursor lesions called pancreatic intraepithelial neoplasias (PanINs). It is firmly established that oncogenic mutations in Kras are among the earliest stimuli for the formation of PanINs.2 This is strongly supported by animal models such as the Pdx1-Cre;LSL-KrasG12D transgenic mouse, in which pancreas-specific expression of oncogenic Kras promotes the occurrence of PanINs.3 Thus, the role of Kras as an initiating cancer mutation is one of the best-established pathobiologic mechanisms in the development of pancreatic cancer. However, during the initiation stage of PDAC pancreatic cells not only undergo protumoral processes, but also cellular events that counteract transformation. One of these tumor suppressive processes is oncogene-induced senescence (OIS). In the pancreas, the induction of senescence underlies the resistance of exocrine cells to oncogenic Kras-mediated transformation4 as a mechanism to prevent tumor promotion by common diseases such as chronic pancreatitis.5,6 Tissue injury in the pancreatitis weakens the defense mechanism posed by senescence, leading to its bypass by exocrine cells that can then readily form PanINs.4 Therefore, the emerging conceptual framework is that pancreatitis triggers long-term transcriptional responses that lower the threshold for OIS and thereby favor the transforming effect of oncogenes.
Nuclear protein 1 (Nupr1), also known as p8 and Com1, is a small basic helix-loop-helix molecule that is strongly induced by pancreatitis.7 The role of Nupr1 in PDAC development in mice was clearly established by recent experiments showing that the oncogenic form of KrasG12D is unable to promote PanINs in the absence of this chromatin protein.8 We hypothesized that Nupr1 might be one of the pancreatitis-induced factors that modulates OIS. We therefore designed a study with the goal of testing the hypothesis that Nupr1 cooperates with oncogenic Kras to induce PanIN formation by modulating the expression of gene networks that are necessary for bypassing OIS. The result of these studies demonstrated that genetic inactivation of Nupr1 under a KrasG12D genetic background induces cellular senescence in exocrine pancreatic cells. At the molecular level we showed that this phenomenon is characterized by the upregulation of gene networks that are known mediators of this phenomenon and by the induction of β-galactosidase activity.9 Together, these results provide mechanistic insight into how Nupr1 cooperates with oncogenic Kras in response to pancreatitis to promote the development of pancreatic preneoplastic lesions through the characterization of a role for this pancreatitis-inducible protein in modulating OIS (Fig. 1). The new information emerging from this study has both mechanistic and biomedical implications through a better understanding of the pathobiology of pancreatic cancer.
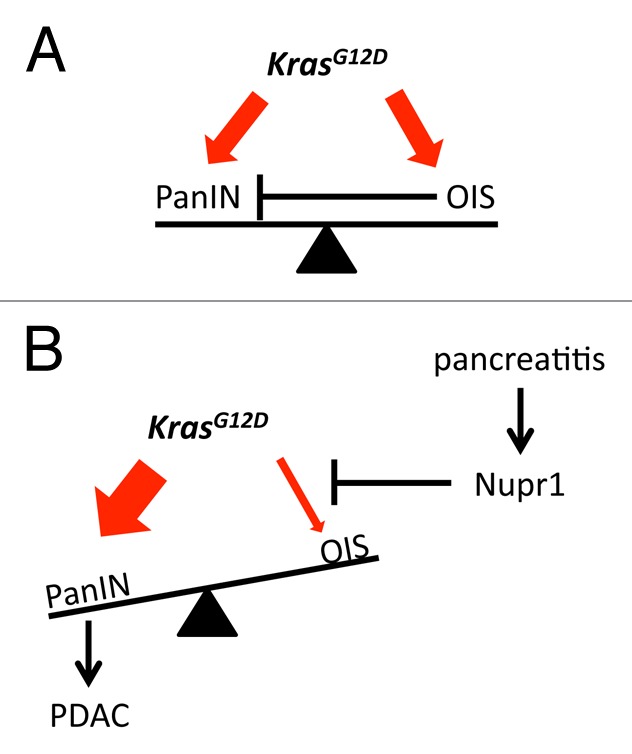
Figure 1. Opposing pathways in the initiation of pancreatic cancer by oncogenic KrasG12D. (A)During the initiation stage of pancreatic adenocarcinoma induced by oncogenic Kras, pancreatic cells are exposed to both a protumoral effect leading to pancreatic intraepithelial neoplasia (PanIN) and an opposing tumor suppressive process known as oncogene-induced senescence (OIS). (B) Pancreatitis induces Nupr1 expression, which modulates OIS and tips the balance in favor of transformation and development of pancreatic adenocarcinoma (PDAC).
Acknowledgments
This work was supported by grants from La Ligue Contre le Cancer, INCa, Canceropole PACA, DGOS (labelisation SIRIC) and INSERM to JLI; National Institutes of Health (grant DK52913), the Mayo Clinic Center for Cell Signaling in Gastroenterology (P30DK084567), and the Mayo Foundation to RU; and by the Fraternal Order of Eagles Cancer Award to GL.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. . Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9 - 29; http://dx.doi.org/ 10.3322/caac.21208; PMID: 24399786 [DOI] [PubMed] [Google Scholar]
- 2.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. . Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 2006; 20:1218 - 49; http://dx.doi.org/ 10.1101/gad.1415606; PMID: 16702400 [DOI] [PubMed] [Google Scholar]
- 3.Schubbert S, Shannon K, Bollag G. . Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer 2007; 7:295 - 308; http://dx.doi.org/ 10.1038/nrc2109; PMID: 17384584 [DOI] [PubMed] [Google Scholar]
- 4.Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernández-Porras I, Cañamero M, Rodriguez-Justo M, Serrano M, Barbacid M. . Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell 2011; 19:728 - 39; http://dx.doi.org/ 10.1016/j.ccr.2011.05.011; PMID: 21665147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. . Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol 2010; 24:349 - 58; http://dx.doi.org/ 10.1016/j.bpg.2010.02.007; PMID: 20510834 [DOI] [PubMed] [Google Scholar]
- 6.Guerra C, Schuhmacher AJ, Cañamero M, Grippo PJ, Verdaguer L, Pérez-Gallego L, Dubus P, Sandgren EP, Barbacid M. . Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 2007; 11:291 - 302; http://dx.doi.org/ 10.1016/j.ccr.2007.01.012; PMID: 17349585 [DOI] [PubMed] [Google Scholar]
- 7.Mallo GV, Fiedler F, Calvo EL, Ortiz EM, Vasseur S, Keim V, Morisset J, Iovanna JL. . Cloning and expression of the rat p8 cDNA, a new gene activated in pancreas during the acute phase of pancreatitis, pancreatic development, and regeneration, and which promotes cellular growth. J Biol Chem 1997; 272:32360 - 9; http://dx.doi.org/ 10.1074/jbc.272.51.32360; PMID: 9405444 [DOI] [PubMed] [Google Scholar]
- 8.Hamidi T, Algül H, Cano CE, Sandi MJ, Molejon MI, Riemann M, Calvo EL, Lomberk G, Dagorn JC, Weih F, et al. . Nuclear protein 1 promotes pancreatic cancer development and protects cells from stress by inhibiting apoptosis. J Clin Invest 2012; 122:2092 - 103; http://dx.doi.org/ 10.1172/JCI60144; PMID: 22565310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grasso D, Garcia MN, Hamidi T, Cano C, Calvo E, Lomberk G, Urrutia R, Iovanna JL. . Genetic inactivation of the pancreatitis-inducible gene Nupr1 impairs PanIN formation by modulating Kras(G12D)-induced senescence. Cell Death Differ 2014; In press http://dx.doi.org/ 10.1038/cdd.2014.74; PMID: 24902898 [DOI] [PMC free article] [PubMed] [Google Scholar]


