Abstract
In osteosarcoma, knockdown of the parathyroid hormone-related protein (PTHrP) receptor reduces activation through cyclic AMP-dependent protein kinase A (PKA) and substantially decreases tumor differentiation, invasion, and proliferation in vivo. These findings complement other evidence supporting a central role of the PKA pathway in osteosarcoma biology and pathogenesis.
Keywords: osteosarcoma, PTHR1, PTHrP, PKA
Osteosarcoma (OS) is the fifth most common cancer in children. Although cytotoxic chemotherapy and improved surgical approaches have increased the overall long-term survival rate to 70%, patients with metastatic disease have a survival rate lower than 20%. The biology of OS has become the focus of recent attention, and an improved understanding could lead to new pathways of treatment. The role of parathyroid hormone receptor (PTHR1) signaling in OS has never been defined, nor indeed has that of PTH-related protein (PTHrP). Our recent findings suggest that PTHR1 acts to promote tumor invasion and proliferation in OS.
Induction of OS in rats by radiophosphorus injection yielded tumors that were markedly PTH-responsive.1 In that study, removing the source of PTH by parathyroidectomy had no influence on any aspect of OS, but that was many years before the existence of PTHrP was appreciated. Subsequent studies in OS cell lines from several species have established PTH responsiveness as a common, if not universal, feature of OS.2 PTHR1, a G-protein coupled receptor linked to adenylyl cyclase, is activated by the N-terminal regions of both PTH and PTHrP. PTHrP was discovered as the factor responsible for the humoral hypercalcemia of cancer, and was found to act physiologically as a paracrine/autocrine factor in many tissues, including bone, where it is produced by cells in the osteoblast lineage.3 There are 3 main subtypes of OS: osteoblastic, fibroblastic, and chondroblastic. Genetically engineered mouse models of OS have been used to generate the fibroblastic subtype by deletion of p53 and pRb from the osteoblast lineage4, and the osteoblastic subtype by shRNA-mediated knockdown of p53 within the osteoblast lineage.5 Primary and metastatic tumors from either subtype express functional PTHR1. OS cultures of both subtypes responded to treatment with either PTH or PTHrP with the expected changes in gene expression5,6 and all expressed PTHrP.6
The functional relevance of PTHR1 to OS biology has not been clearly defined in vivo. Consistent with a role for PTHR1 in OS, higher expression of PTHR1 mRNA was detected in metastatic or relapsed samples of a human OS series than in primary sites, and overexpression of PTHR1 in an OS cell line increased proliferation and invasion.7 Knockdown of PTHR1 in murine fibroblastic OS resulted in reduced tumor cell invasion in vitro and changes in gene expression that reflected loss of activation of PTHR1 and cAMP-dependent protein kinase A (PKA).6 Notably, PTHR1 knockdown resulted in greatly reduced proliferation and increased mineralization of the tumor in vivo. PTHrP was present as an intracellular protein in OS cells, but its secretion was very low to undetectable. Interestingly, treatment with a neutralizing monoclonal antibody against PTHrP failed to modify OS cell proliferation in vitro or to influence tumor size in vivo. Although the latter findings, together with evidence for its nuclear localization in these and in other cells,3 suggest that PTHrP might be acting in an intracrine manner in OS, in such a situation the action of PTHrP on PTHR1 would be difficult to explain. It seems more likely that an autocrine/paracrine action of PTHrP is responsible and that the antibody is insufficiently effective in this context.
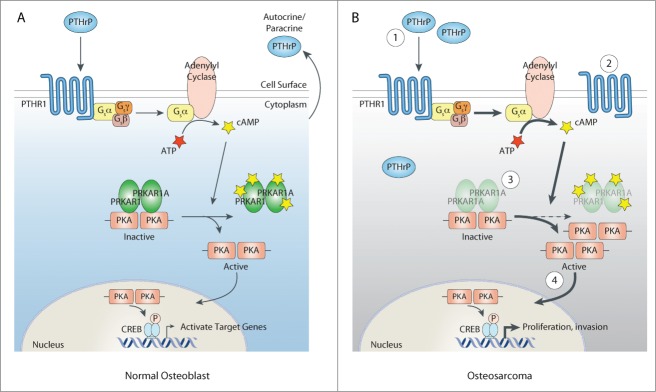
The role of PKA activation in endocrine tumors is well known and the subject of recent interest in OS. In mice with osteocalcin promoter-driven SV40T/t antigen-induced OS, a subset of OS with low expression of the α regulatory subunit of PKA type I (PRKAR1A) was identified.8 This PRKAR1A-low OS was highly invasive, leading to the conclusion that Prkar1a is an OS tumor suppressor. The functional consequence of reduced PRKAR1A is enhanced PKA activity. Consistent with a role of elevated PKA activity, an OS was identified with amplification of Prkaca, which encodes the catalytic component of PKA, and Prkaca RNA was shown to be overexpressed in that tumor. It seems that enhanced PKA signaling in OS might be mediated by later events in the PKA/CREB cascade, rather than strictly being ascribed to Prkar1a as a tumor suppressor. Figure 1 illustrates changes in the PKA pathway induced through these mechanism and through PTHrP/PTHR1.
Figure 1.
Alterations in the PTHrP→PTHR1→PKA pathway in osteosarcoma. (A) In normal osteoblasts, autocrine or paracrine parathyroid hormone-related protein (PTHrP) binds and activates its receptor, PTHR1. Activation of PTHR1 causes the generation of cyclic AMP (cAMP) from ATP through adenylyl cyclase. cAMP induces the dissociation of cAMP-dependent protein kinase (PKA) from its regulatory subunits, including the α regulatory subunit of PKA type I (PRKAR1A). Once activated, PKA is able to move to the nucleus, where it phosphorylates and activates CREB. This leads to the activation of target genes downstream of PTHR1 signaling. (B) In osteosarcoma cells, various aberrations in the PTHrP→PTHR1→PKA pathway that result in increased activation of the PKA pathway have been described. 1. Elevated production of PTHrP that can bind PTHR1 and stimulate cAMP formation. 2. Increased surface copy number of PTHR1. 3. Mutations in PRKAR1A that lead to increased PKA activity. 4. Amplification of Prkaca, which encodes the catalytic component of PKA.
Also of interest is the observation that loss of PRKAR1A increased the production of receptor activator of nuclear factor κB ligand (RANKL) in OS,8 whereas upon PTHR1 knockdown the expression of RANKL expression decreased and that of osteoprotegerin (OPG) increased.6 Two aspects of RANKL biology are significant in OS. It promotes both osteoclast formation, thereby favoring tumor establishment and proliferation, and the establishment and growth of metastatic cancers in bone. Importantly, however, there are also examples of osteoclastogenesis-independent effects of RANKL: blockade of RANKL/RANK can inhibit metastasis to bone by preventing cell migration, and RANKL can promote breast cancer metastasis to bone by a pro-migratory effect through its receptor, RANK, expressed on the cancer cells.9 Furthermore, in OS RANKL stimulated both invasion through matrigel and anchorage-independent growth, and each of these effects was prevented by blockade of the RANKL receptor.10
Taken together, these findings provide a compelling case for a role of the PTHrP→PTHR1→PKA axis in the maintenance of OS. If the driver through this axis is indeed PTHrP, manipulating this upstream target, for example through small-interfering RNA or neutralizing antibodies, could be used to regulate OS behavior.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author Contribution Statement
The content of this manuscript was discussed with all authors. The manuscript was written by T.J.M. and C.W. and reviewed by all authors.
Acknowledgments
Work from the authors’ laboratories is supported by the NHMRC (Australia), Cancer Council of Victoria, Zig Inge Foundation, 5 Point Foundation, and by the Victorian State Government Operational Infrastructure Support Program (to St. Vincent's Institute). C.W. is the Philip Desbrow Senior Research Fellow of the Leukaemia Foundation.
References
- 1. Martin TJ, Ingleton PM, Underwood JC, Michelangeli VP, Hunt NH, Melick RA. Parathyroid hormone-responsive adenylate cyclase in induced transplantable osteogenic rat sarcoma. Nature 1976; 260:436-8; PMID:1062678; http://dx.doi.org/ 10.1038/260436a0 [DOI] [PubMed] [Google Scholar]
- 2. Goerdeladze J, Jablonski G, Paulssen R, Mortensen B, Gautvik K, Haug E, Rian E, Jemtland R, Friedman E, Bruland O. G-protein coupled signaling in osteosarcoma cell lines. In: Novak JF, McMaster JH, eds. Frontiers in osteosarcoma research. Seattle, Toronto: Hogref and Huber, 1993:297-308. [Google Scholar]
- 3. McCauley LK, Martin TJ. Twenty-five years of PTHrP progress: from cancer hormone to multifunctional cytokine. J Bone Miner Res 2012; 27:1231-9; PMID:22549910; http://dx.doi.org/ 10.1002/jbmr.1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI, Rodda SJ, Snay E, Dunning P, Fahey FH, et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev 2008; 22:1662-76; PMID:18559481; http://dx.doi.org/ 10.1101/gad.1656808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mutsaers AJ, Ng AJ, Baker EK, Russell MR, Chalk AM, Wall M, Liddicoat BJ, Ho PW, Slavin JL, Goradia A, et al. Modeling distinct osteosarcoma subtypes in vivo using Cre:lox and lineage-restricted transgenic shRNA. Bone 2013; 55:166-78; PMID:23486187; http://dx.doi.org/ 10.1016/j.bone.2013.02.016 [DOI] [PubMed] [Google Scholar]
- 6. Ho PW, Goradia A, Russell MR, Chalk AM, Milley KM, Baker EK, Danks JA, Slavin JL, Walia M, Crimeen-Irwin B, et al. Knockdown of PTHR1 in osteosarcoma cells decreases invasion and growth and increases tumor differentiation in vivo. Oncogene 2014; PMID:25043296; http://dx.doi.org/doi:10:1038/onc.2014.217 [DOI] [PubMed] [Google Scholar]
- 7. Yang R, Hoang BH, Kubo T, Kawano H, Chou A, Sowers R, Huvos AG, Meyers PA, Healey JH, Gorlick R. Overexpression of parathyroid hormone Type 1 receptor confers an aggressive phenotype in osteosarcoma. Int J Cancer 2007; 121:943-54; PMID:17410535; http://dx.doi.org/ 10.1002/ijc.22749 [DOI] [PubMed] [Google Scholar]
- 8. Molyneux SD, Di Grappa MA, Beristain AG, McKee TD, Wai DH, Paderova J, Kashyap M, Hu P, Maiuri T, Narala SR, et al. Prkar1a is an osteosarcoma tumor suppressor that defines a molecular subclass in mice. J Clin Invest 2010; 120:3310-25; PMID:20697156; http://dx.doi.org/ 10.1172/JCI42391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campbell JP, Karolak MR, Ma Y, Perrien DS, Masood-Campbell SK, Penner NL, Munoz SA, Zijlstra A, Yang X, Sterling JA, et al. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol 2012; 10:e1001363; PMID:22815651; http://dx.doi.org/ 10.1371/journal.pbio.1001363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beristain AG, Narala SR, Di Grappa MA, Khokha R. Homotypic RANK signaling differentially regulates proliferation, motility and cell survival in osteosarcoma and mammary epithelial cells. J Cell Sci 2012; 125:943-55; PMID:22421365; http://dx.doi.org/ 10.1242/jcs.094029 [DOI] [PubMed] [Google Scholar]