Abstract
Retinoic acid inducible gene-I (RIG-I), named for the observation that its mRNA expression is highly upregulated in the progression of all-trans retinoic acid (ATRA)-induced maturation of acute promyelocytic leukemia (APL) cells, has been well documented as a pivotal virus-associated molecular pattern recognition receptor (PRR) responsible for triggering innate immunity. Upon recognizing viral RNA ligands, RIG-I experiences a series of programmed conformational changes and modifications that unleash its activity through the formation of complexes with various binding partners. Such partners include the mitochondria membrane-anchored protein IPS-1 (also named MAVS/VISA/Cardif) that activates both the IRF3/7 and NF-κB pathways. These partnerships and resulting pathway activations underlie the synthesis of type I interferon and other inflammatory factors. Recent studies have demonstrated that RIG-I is also involved in the regulation of basic cellular processes outside of innate immunity against viral infections, such as hematopoietic proliferation and differentiation, maintenance of leukemic stemness, and tumorigenesis of hepatocellular carcinoma. In this review, we will highlight recent studies leading up to the recognition that RIG-I performs an essential function as a tumor suppressor and try to reconcile this activity of RIG-I with its well-known role in protecting cells against viral infection.
Keywords: antiviral innate immunity, retinoic acid inducible gene-I (RIG-I), tumor suppressor, viral RNA priming
Abbreviations
- 3′UTR
3′untranslated regions
- APL
acute promyelocytic leukemia
- ATM-IRF1
ataxia telangiectasia mutated-interferon regulatory factor 1
- ATRA
all-trans retinoic acid
- CARD
caspase activation and recruitment domains
- CTD
C-terminal regulatory domain
- CXCL10
chemokine (C-X-C motif) ligand 10
- dsRNA
double stranded RNA
- HNSCC
human head and neck squamous cell carcinoma
- IFN
interferon
- IFNα
interferon alpha
- IPS-1
interferon-beta promoter stimulator 1
- IRF3/7
interferon regulatory factor 3/7
- ISG
interferon stimulatory gene
- JAK-STAT1/2
Janus kinase-signal transducer and the activator of transcription1/2
- LGP2
laboratory of genetics and physiology 2
- MAVS
mitochondrial antiviral signaling
- MDA5
melanoma differentiation-associated gene 5
- NF-κB
nuclear factor κB
- PRR
pattern recognition receptor
- RIG-I
retinoic acid in ducible gene-I
- RLR
RIG-I like receptor
- STAT1
signal transducers and activators of transcription 1
- TGF-β 1
transforming growth factor-beta 1
- TNFκ
tumor necrosis factor α
- VISA
virus-induced signaling adaptor
As its name suggests, retinoic acid inducible gene-I (RIG-I) is induced by retinoic acid signaling during the myeloid maturation of acute promyelocytic leukemia (APL) cells.1 RIG-I did not catch the attention of the broader research community until the discovery by Yoneyama et al. in 2004 that RIG-I recognizes double-stranded RNAs (dsRNA) to trigger the transcription of type I IFN via activation of IRF3 and NF-κB.2 RIG-I is regarded as the proband member of the so-called RIG-I like receptor (RLR) family that also includes 2 other homologous molecules, melanoma differentiation associated gene 5 (MDA5) and laboratory of genetics and physiology 2 (LGP2).3-5 All RLR member proteins are expressed in most cell types. Structurally, RIG-I protein features 2 N-terminal caspase activation and recruitment domains (CARD), a linker region, a DExD/H helicase core, a pincer, and a C-terminal regulatory domain (CTD).6 Evolutionary analysis of the 3 RLR genes in mammals indicates that all identified domains/regions are enriched with target sites for positive selection, indicating the essential role of each of these domains/regions for the biological functions of RIG-I. Subsequent studies have quickly accumulated, delineating the structure–function relationship of these individual domains and how they collaborate to fulfill the function of RIG-I as a cytosolic pattern recognition receptor (PRR) that senses viral RNA invasion.3,5-12 In the current model, host cells devoid of active viral infection express RIG-I in an autoinhibited conformational state, with its CARD2 locked by tethering with the Hel2i domain of the helicase core.6 Upon invasion of the cytoplasm by viral RNA, a programmed conformational change occurs that proceeds through the sequential recognition of a 5′-ppp extremity and a paired RNA duplex (which together form the cognate viral RNA ligands) and also requires ATP for the CTD and helicase core. Next, with the help of the flexible elbow-like pincer domain and activation of the ATPase of helicase core, the N-terminal CARDs are released for polyubiquitination, resulting in RIG-I polymerization and physical association with the mitochondria protein IPS-1 (also named MAVS/VISA/Cardif) or other partners. The physical association of RIG-I with such partner molecules transduces viral RNA sensing into activation signals that induce the production of type-I interferon (IFN) and proinflammatory factors by the infected cells.3
Anti-Tumor Potential of RIG-I Initiated by Foreign RNA Ligand
It has long been appreciated that programmed suicide of infected host cells represents a rational means of hindering the propagation of viral particles. Interestingly, it was then discovered that RIG-I activation by RNA ligands in melanoma cells also triggers apoptosis together with induction of the proapoptotic proteins Puma and Noxa, which depends on IPS-1 activation as well as TNFα or Trail induction but not type I IFN induction and the p53 pathway.13 Continuing this line of research, a recent study indicated that foreign RNA-primed RIG-I, but not unprimed RIG-I, associates with caspase 9, a potent initiator of apoptosis, in human head and neck squamous cell carcinoma (HNSCC) cells, most likely through a hemophilic CARD association similar to that mediating conventional RIG-I/IPS-1 association.14 These 2 studies provided a mechanistic basis for the notion that activation of the IPS-1 pathway by foreign RNA-primed RIG-I might be exploited to kill tumor cells. Nevertheless, the relative functional contribution of these 2 pathways remains to be clarified if any RIG-I-mediated antitumor treatments are to be successful. Some groups have attempted to develop an active cellular vaccine based on the idea that these apoptotic tumor cells harboring foreign RNA-activated RIG-I also secrete type I IFNs together with other types of immunostimulatory cytokines.3 A pioneering study was reported by Poeck et al., who delivered a 5′-ppp siRNA against the antiapoptotic protein Bcl-2 into melanoma cells in vitro and in vivo; this not only silenced Bcl-2 and stimulated the RIG-I/IPS-1 pathway leading to the apoptosis of tumor cells, but also created a para-micromilieu full of type I IFNs that helped turn an immunosuppressive microenvironment into an immunosupportive microenvironment.13 A similar study was performed by Ellermeier et al., who focused more on the regulation of tumor immunosurveillance. They employed a synthetic 5′-ppp siRNA against TGF-β1, which has been shown to play a critical role in the growth, invasion, and metastasis of pancreatic cancer.15,16 This bifunctional siRNA showed significant therapeutic efficacy in a murine model of pancreatic cancer, as evidenced by extensive apoptosis of tumor cells, induction of type I IFN and CXCL10, and recruitment of activated CD8+ T cells. As expected, the delivery of replication-incompetent virus (such as HVJ-E) or ordinary RIG-I ligands (such as Poly I:C or 5′-ppp RNA) drove potent induction of apoptosis in multiple types of human tumor cells including prostate cancer, mammary carcinoma, lung cancer, and glioblastoma cells.13-25 However, an important unresolved issue is how these apoptosis-inducing effects mediated through foreign RNA-primed RIG-I are seemingly able to spare nonmalignant cells. Taken together, these studies suggest the attractive possibility of exploiting the RIG-I–IPS-1 pathway within tumor cells in order to convert them into potent vaccines that can overcome the limitations of conventional therapeutic methods of immunization.17 Hypothetically, it may even be possible to use multiple types of RNA ligands in a combinatory manner in future studies, which may further optimize this RIG-I strategy and enhance the therapeutic benefits to patients.
An interesting, but not thoroughly examined, consideration is whether RIG-I can activate IPS-1 via a mechanism independent of foreign RNA stimulation. Notably, the study by Liu et al. indicated that induction of RIG-I by aging or certain stress conditions (such as irradiation, but not foreign RNA delivery or viral infection), most likely through activation of the ataxia telangiectasia mutated–interferon regulatory factor 1 (ATM–IRF1) pathway, resulted in the secretion of key inflammatory cytokines such as IL-6 and IL-8 in senescent cells. They further showed that secretion of these cytokines depends on the activation of IPS-1.26,27 Although this phenomenon cannot be completely explained by the current model of RIG-I activation,3 it is likely that certain unknown modifications of RIG-I imposed by stress-response pathways might be capable of unlocking the internal tethering forces between domains that prevent RIG-I from physically associating with and activating IPS-1.
The Recognition of Endogenous mRNAs by RIG-I
The nature of cognate RNA ligands recognized by RLR represents the most-explored aspect of RIG-I–related studies.3 In vitro biochemical studies and 3-dimensional structural analysis have documented that purified full-length RIG-I protein has an affinity for 5′-ppp blunt-ended dsRNA hundreds of times greater than that for the over-hanging or 5′-OH features characteristic of the endogenous RNA molecules. Although this observation explains how RIG-I is able to discriminate between self RNA and viral RNA, a recent study has raised the possibility that in cells devoid of active viral RNA production, RIG-I might also modulate the metabolism of certain endogenous RNAs via direct physical association, most likely through forming a complex with other partner proteins or RNA molecules.28 Using RNA immunoprecipitation of lysates of murine B lymphocytes, Zhang et al. found that His-tagged foreign Rig-I protein had binding affinity for hundreds of endogenous mRNA molecules, including those encoding many potential oncoproteins (such as NF-κB) and oncorepressors.28 Further analysis indicated that this type of physical association was mediated mainly by the recognition of conserved tandem motifs located within the 3′ UTR of NF-κB mRNA by Rig-I in complex with other proteins or with rRNA, which facilitates the translation of NF-κB mRNA. Compared with a control RNA moiety, these tandem motifs have an increased tendency to form RNA duplexes, and 3-dimensional structure prediction suggested that they might also tightly associate with the helicase domains and CTD of Rig-I protein. Nevertheless, it remains unclear whether the affinity of RIG-I for tandem motifs is comparable to that between RIG-I and 5′-ppp blunt dsRNA. Since the physical association between RIG-I and tandem motifs did not trigger IPS-1 activation, it is possible that the binding between tandem motifs and RIG-I does not displace the tethering lock that exists between the CARD2 and Hel2i domains.
Viral RNA-Unprimed RIG-I Acts as a Tumor Repressor
Given that RIG-I mRNA is highly upregulated during ATRA-induced differentiation of APL cells,1 studies were performed to test whether the expression of RIG-I functionally contributes to the myeloid differentiation of normal hematopoietic or leukemic progenitor cells. RIG-I was shown to play an essential role in ATRA-induced inhibition of leukemic cell proliferation and promotion of leukemic cell differentiation.29,30 Moreover, phenotypic analysis of Rig-I-targeted mice revealed that Rig-I also plays an essential role in negatively regulating myelopoiesis, as a high rate of myeloproliferative disorders developed in Rig-I−/− mice and could be rescued by the restoration of expression of Irf8, a classic interferon stimulatory gene (ISG).31 As the ATRA-induced myeloid differentiation model and the breeding conditions of Rig-I+/+ or Rig-I−/− mice are ideally devoid of any active viral infection and IPS-1 activation, it is interesting to speculate whether, in addition to functioning as a viral RNA sensor, RIG-I possesses other basic physiological or pathological functions when maintained in the dormant state for triggering IPS-1 activation. In line with this notion, a recent independent study provided evidence that Rig-I deficiency predisposed hepatic tissue to the development of hepatocellular carcinoma, as induced by the chemical carcinogen DEN in the absence of hepatitis viral infection.32 Other studies corroborated this finding, showing that RIG-I plays important roles in phagocytosis of bacteria and migration of immune cells, neither of which involve active viral RNA production. This particular function is most likely mediated via interactions of Rig-I with cytoskeletal proteins.33-35
In the context of the differentiation course for normal or leukemic myeloid progenitors, as well as the carcinogenesis of hepatocellular carcinoma and its treatment by type I IFN, 2 relatively independent RIG-I-related mechanisms have been documented (Fig. 1). First, comparison analysis of Rig-I+/+ and Rig-I−/− murine bone marrow cells indicated that the expression of ISGs is significantly affected by Rig-I deficiency.29,31 Further analysis showed that induction of Rig-I amplifies STAT1 activation, a key event regulating the signaling strength after the ligation of type I or type II IFNs with their cognate receptor. Expression of RIG-I itself, as an authentic ISG, is highly induced by type I or type II IFN via the activation of STAT1.29 Interestingly, Hou et al. noticed that hepatic RIG-I level predicted survival and the therapeutic response of hepatocellular carcinoma to IFNα.32 Mechanistic studies showed that IFNα signaling, in the absence of any introduced foreign RNA ligands, induced a RIG-I/STAT1 physical association via tethering of the RIG-I CARDs with the STAT1 SH2-transactivation (SH2-TA) domain, thereby competitively impeding the dephosphorylation and inactivation of STAT1 by the phosphatase SHP1, which requires prior recognition of the STAT1 SH2-TA domain by SHP1.32 Taken together, these results indicate that in the absence of priming by foreign RNA ligands the CARDs of RIG-I are not completely buried by the helicase core as previously assumed. This would allow certain parts of the RIG-I CARDs to remain accessible to recognition by activated STAT1.
Figure 1.
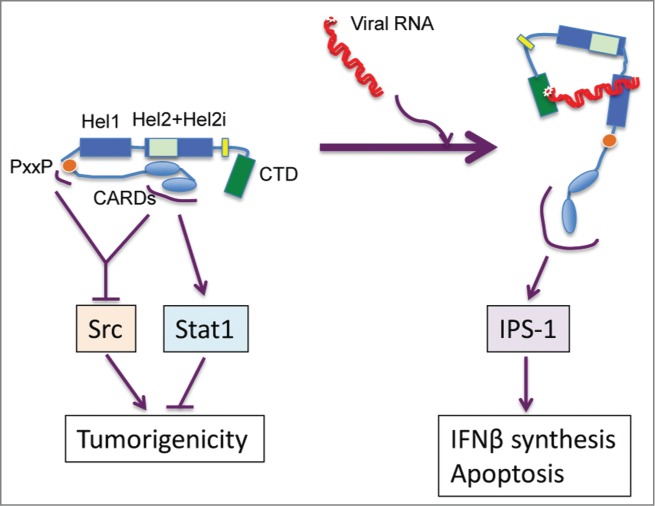
RIG-I stays between two different conformational and functional states. In a viral RNA-unprimed condition, the conformational state maintained by the physical association between the second CARD domain and hel2 domain prevents RIG-I CARD's association with IPS-1, but allows CARDs and PxxP motif of RIG-I accessible for the interaction with STAT1 or Src, by which RIG-I constrains the tumorigenicity of host cells. Conversely, once encountering viral RNA, RIG-I shifts to a different conformation state that unleashes the binding capacity of CARDs with IPS-1 while impedes their interaction with Src, which underlies the induction of type I IFN.
Second, mechanistic analysis of myeloid differentiation and proliferation of leukemia cell lines after doxycycline-induced upregulation of RIG-I led to the discovery that RIG-I suppresses the tyrosine phosphorylation and activation of AKT by blocking AKT recognition by Src, a well-known oncoprotein, in a STAT1 activation-independent manner.30 Consistent with this finding, the RIG-I expression level was negatively correlated with AKT phosphorylation at T308, whose level was as least partly facilitated by Src in primary human leukemic blasts and is an indicator of poor prognosis in leukemia patients. Domain mapping identified a classic well-conserved PxxP motif within the linker region between the CARDs and the helicase core. Further studies presented a working model of cooperation between RIG-I CARDs and the PxxP motif in disrupting the association of AKT and Src. This is mediated through physical association established initially by tethering RIG-I CARDs with the SH1 domain of active Src, whereupon the RIG-I PxxP motif effectively occupies the SH3 domain of Src thereby competitively preventing the association of Src SH3 with AKT PxxP that underlies the AKT/Src interaction. Importantly, this pathway was shown to contribute to negative regulation of oncogenic cytokine-stimulated proliferation of primary myeloid progenitors and in vivo maintenance of leukemic stemness. This RIG-I/Src association was actually inhibited by exposure to RIG-I RNA ligands both in vitro and in vivo, indicating that the active state of RIG-I associated with viral RNA is conformationally inaccessible to active Src. Taken together, these results indicate that cytosolic RIG-I may alternate between 2 alternative conformational and functional states depending upon whether it is primed by foreign RNA ligands (Fig. 1).
A Possible Contribution of Viral RNA-unprimed RIG-I to the Limitation of Viral Propagation
It is interesting to ask whether the regulatory activity of viral RNA-unprimed RIG-I on STAT1 activation or on AKT activation by Src (which is seemingly unrelated to the classic function of RIG-I in sensing viral invasion), would contribute to antiviral immunity.29,30 Here, we propose a model in which an active but viral RNA-unprimed RIG-I molecule in bystander tissue cells (in the vicinity of the virally-infected budding tissue cells), plays a critical role in the quarantine of viral propagation (Fig. 2). It is well accepted that type I IFNs secreted by the first batch of virus-infected host cells with an active RIG-I–IPS-1 pathway diffuse into the surrounding tissue and activate ISG induction in bystander cells through the JAK-STAT1/2 pathway. This paracrine signaling of IFNs contributes greatly to the restriction of infectious foci.36-38 We have previously shown that upregulation of RIG-I in normal cells via IFN signaling plays an essential positive feedback role in ISG induction.31 The capacity of upregulated RIG-I to amplify STAT1 activation without being primed by viral RNA allows the bystander cells to fully arm themselves prior to their encounter with invading viruses. The functional contribution of negative regulation of Src-AKT activation by the upregulated RIG-I is not clear. Nevertheless, it can be postulated that inactivation of the AKT-mTOR pathway in bystander cells may facilitate clearance of infective viral entities through the activation of autophagy.30 In support of this, a previous study showed that RIG-I physically associated with and facilitated the activation of Atg5-Atg12, major components of autophagic vesicle formation.10 We propose that the observed antiviral effect of RIG-I results from both its virus-sensing function that triggers type I IFN production and from the promotion of the effective phase of IFN signaling, especially within bystander cells.13,15,16,18,19,22,23,29 In addition to its antiviral role, the activities of RIG-I in the effective phase of antiviral immunity employ the same sets of mechanisms that underlie the regulation of proliferation and differentiation of leukemia or hepatocellular cells.
Figure 2.
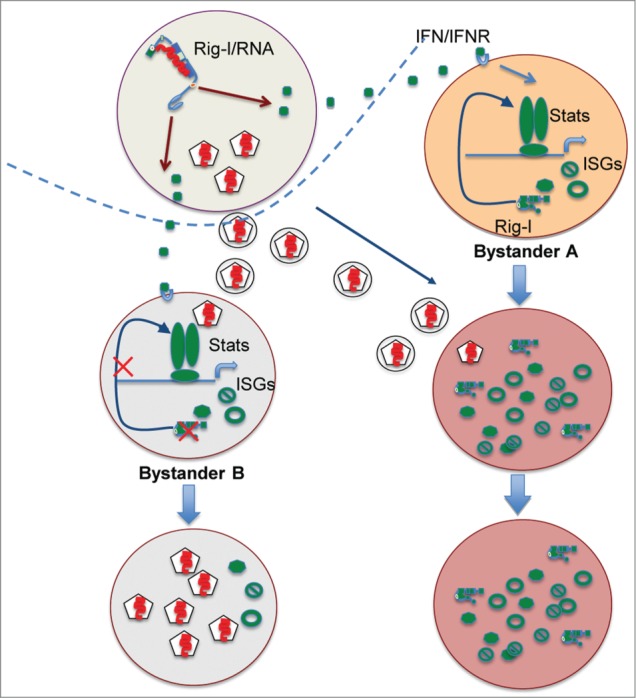
The amplifying role of viral RNA-unprimed RIG-I strengthens the paracrine IFN signaling to prepare the bystander cells for resisting viral infection. The upregulated RIG-Is by type I IFN signaling in bystander A cells, when staying at viral-RNA unprimed configuration, plays an essential role for the full induction of ISGs that ensures the quick termination of the replication cycle of the invading viral particles. In bystander B cells harboring a possible RIG-I deficiency that fails a full ISG induction after IFN signaling, the amplification of viral particle is established without being strongly resisted.
Future Perspectives
Almost 10 years of intensive research represent continued attempts of the scientific community, mainly but not exclusively immunologists, to understand the biological activities associated with RIG-I and the other two RLR members.4-6,11,20,22,40,41 However, compared with the general understanding of RIG-I's role as a viral RNA sensor, the functions and the underlying mechanisms of RIG-I in other biological settings are much less well explored.23,25,26,29-32,38,39,42-45 It is likely that more molecular features and functions of RIG-I will be uncovered in the following years. In light of the important roles of RIG-I in a variety of basic biological processes, understanding how these novel RIG-I activities are regulated will represent an important direction for the field. A more comprehensive and detailed understanding of the molecular features of RIG-I will most likely lead to the development of new therapeutic strategies, not only for infectious and immunological diseases but also for cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Liu TX, Zhang JW, Tao J, Zhang RB, Zhang QH, Zhao CJ, Tong JH, Lanotte M, Waxman S, Chen SJ, et al. Gene expression networks underlying retinoic acid-induced differentiation of acute promyelocytic leukemia cells. Blood 2000; 96:1496-504; PMID:10942397 [PubMed] [Google Scholar]
- 2. Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 2004; 5:730-7; PMID:15208624; http://dx.doi.org/ 10.1038/ni1087 [DOI] [PubMed] [Google Scholar]
- 3. Loo YM, Gale M, Jr. Immune signaling by RIG-I-like receptors. Immunity 2011; 34:680-92; PMID:21616437; http://dx.doi.org/ 10.1016/j.immuni.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Widau RC, Parekh AD, Ranck MC, Golden DW, Kumar KA, Sood RF, Pitroda SP, Liao Z, Huang X, Darga TE, et al. RIG-I-like receptor LGP2 protects tumor cells from ionizing radiation. Proc Natl Acad Sci U S A 2014; 111:E484-91; PMID:24434553; http://dx.doi.org/ 10.1073/pnas.1323253111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, Foy E, Loo YM, Gale M, Akira S, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol 2005; 175:2851-8; PMID: 16116171; http://dx.doi.org/ 10.4049/jimmunol.175.5.2851 [DOI] [PubMed] [Google Scholar]
- 6. Peisley A, Wu B, Xu H, Chen ZJ, Hur S. Structural basis for ubiquitin-mediated antiviral signal activation by RIG-I. Nature 2014; 509:110-4; PMID:24590070; http://dx.doi.org/ 10.1038/nature13140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arimoto K, Takahashi H, Hishiki T, Konishi H, Fujita T, Shimotohno K. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc Natl Acad Sci U S A 2007; 104:7500-5; PMID:17460044; http://dx.doi.org/ 10.1073/pnas.0611551104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cui J, Zhu L, Xia X, Wang HY, Legras X, Hong J, Ji J, Shen P, Zheng S, Chen ZJ, et al. NLRC5 negatively regulates the NF-kappaB and type I interferon signaling pathways. Cell 2010; 141:483-96; PMID:20434986; http://dx.doi.org/ 10.1016/j.cell.2010.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gack MU, Nistal-Villan E, Inn KS, Garcia-Sastre A, Jung JU. Phosphorylation-mediated negative regulation of RIG-I antiviral activity. J Virol 2010; 84:3220-9; PMID: 20071582; http://dx.doi.org/ 10.1128/JVI.02241-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jounai N, Takeshita F, Kobiyama K, Sawano A, Miyawaki A, Xin KQ, Ishii KJ, Kawai T, Akira S, Suzuki K, et al. The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proc Natl Acad Sci U S A 2007; 104:14050-5; PMID:17709747; http://dx.doi.org/ 10.1073/pnas.0704014104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt A, Endres S, Rothenfusser S. Pattern recognition of viral nucleic acids by RIG-I-like helicases. J Mol medicine 2011; 89:5-12; PMID:20820752; http://dx.doi.org/ 10.1007/s00109-010-0672-8 [DOI] [PubMed] [Google Scholar]
- 12. Sun Z, Ren H, Liu Y, Teeling JL, Gu J. Phosphorylation of RIG-I by casein kinase II inhibits its antiviral response. J Virol 2011; 85:1036-47; PMID:21068236; http://dx.doi.org/ 10.1128/JVI.01734-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poeck H, Besch R, Maihoefer C, Renn M, Tormo D, Morskaya SS, Kirschnek S, Gaffal E, Landsberg J, Hellmuth J, et al. 5'-Triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma. Nat Med 2008; 14:1256-63; PMID:18978796; http://dx.doi.org/ 10.1038/nm.1887 [DOI] [PubMed] [Google Scholar]
- 14. Hu J, He Y, Yan M, Zhu C, Ye W, Zhu H, Chen W, Zhang C, Zhang Z. Dose dependent activation of retinoic acid-inducible gene-I promotes both proliferation and apoptosis signals in human head and neck squamous cell carcinoma. PloS One 2013; 8:e58273; PMID:23484008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ellermeier J, Wei J, Duewell P, Hoves S, Stieg MR, Adunka T, Noerenberg D, Anders HJ, Mayr D, Poeck H, et al. Therapeutic efficacy of bifunctional siRNA combining TGF-beta1 silencing with RIG-I activation in pancreatic cancer. Cancer Res 2013; 73:1709-20; PMID:23338611; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-3850 [DOI] [PubMed] [Google Scholar]
- 16. Schnurr M, Duewell P. Breaking tumor-induced immunosuppression with 5'-triphosphate siRNA silencing TGFbeta and activating RIG-I. Oncoimmunology 2013; 2:e24170; PMID:23762798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glas M, Coch C, Trageser D, Dassler J, Simon M, Koch P, Mertens J, Quandel T, Gorris R, Reinartz R, et al. Targeting the cytosolic innate immune receptors RIG-I and MDA5 effectively counteracts cancer cell heterogeneity in glioblastoma. Stem Cells 2013; 31:1064-74; PMID:23390110; http://dx.doi.org/ 10.1002/stem.1350 [DOI] [PubMed] [Google Scholar]
- 18. Kaneda Y. The RIG-I/MAVS signaling pathway in cancer cell-selective apoptosis. Oncoimmunology 2013; 2:e23566; PMID:23734313; http://dx.doi.org/ 10.4161/onci.23566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kubler K, Gehrke N, Riemann S, Bohnert V, Zillinger T, Hartmann E, Polcher M, Rudlowski C, Kuhn W, Hartmann G, et al. Targeted activation of RNA helicase retinoic acid-inducible gene-I induces proimmunogenic apoptosis of human ovarian cancer cells. Cancer Res 2010; 70:5293-304; PMID:20551064; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-0825 [DOI] [PubMed] [Google Scholar]
- 20. Petrocca F, Lieberman J. RIG-ing an antitumor response. Nat Med 2008; 14:1152-3; PMID:18989278; http://dx.doi.org/ 10.1038/nm1108-1152 [DOI] [PubMed] [Google Scholar]
- 21. Qu J, Hou Z, Han Q, Zhang C, Tian Z, Zhang J. Poly(I:C) exhibits an anti-cancer effect in human gastric adenocarcinoma cells which is dependent on RLRs. Int Immunopharmacol 2013; 17:814-20; PMID:24029594; http://dx.doi.org/ 10.1016/j.intimp.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 22. Rehwinkel J, Reis e Sousa C. Targeting the viral Achilles' heel: recognition of 5'-triphosphate RNA in innate anti-viral defence. Curr Opin Microbiol 2013; 16:485-92; PMID:23707340; http://dx.doi.org/ 10.1016/j.mib.2013.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van den Boorn JG, Hartmann G. Turning tumors into vaccines: co-opting the innate immune system. Immunity 2013; 39:27-37; PMID:23890061; http://dx.doi.org/ 10.1016/j.immuni.2013.07.011 [DOI] [PubMed] [Google Scholar]
- 24. Wolf D, Heine A, Brossart P. Implementing combinatorial immunotherapeutic regimens against cancer: the concept of immunological conditioning. Oncoimmunology 2014; 3:e27588; PMID:24800168; http://dx.doi.org/ 10.4161/onci.27588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zitvogel L, Kroemer G. Anticancer immunochemotherapy using adjuvants with direct cytotoxic effects. J Clin Invest 2009; 119:2127-30; PMID:19620780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu F, Gu J. Retinoic acid inducible gene-I, more than a virus sensor. Protein Cell 2011; 2:351-7; PMID:21626268; http://dx.doi.org/ 10.1007/s13238-011-1045-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol 2011; 13:254-62; PMID:21336305; http://dx.doi.org/ 10.1038/ncb2167 [DOI] [PubMed] [Google Scholar]
- 28. Zhang HX, Liu ZX, Sun YP, Zhu J, Lu SY, Liu XS, Huang QH, Xie YY, Zhu HB, Dang SY, et al. Rig-I regulates NF-kappaB activity through binding to Nf-kappab1 3'-UTR mRNA. Proc Natl Acad Sci U S A 2013; 110:6459-64; PMID:23553835; http://dx.doi.org/ 10.1073/pnas.1304432110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang LJ, Zhang NN, Ding F, Li XY, Chen L, Zhang HX, Zhang W, Chen SJ, Wang ZG, Li JM, et al. RA-inducible gene-I induction augments STAT1 activation to inhibit leukemia cell proliferation. Proc Natl Acad Sci U S A 2011; 108:1897-902; PMID:21224412; http://dx.doi.org/ 10.1073/pnas.1019059108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li XY, Jiang LJ, Chen L, Ding ML, Guo HZ, Zhang W, Zhang HX, Ma XD, Liu XZ, Xi XD, et al. RIG-I modulates Src-mediated AKT activation to restrain leukemic stemness. Mol Cell 2014; 53:407-19; PMID:24412064; http://dx.doi.org/ 10.1016/j.molcel.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 31. Zhang NN, Shen SH, Jiang LJ, Zhang W, Zhang HX, Sun YP, Li XY, Huang QH, Ge BX, Chen SJ, et al. RIG-I plays a critical role in negatively regulating granulocytic proliferation. Proc Natl Acad Sci U S A 2008; 105:10553-8; PMID: 18650396; http://dx.doi.org/ 10.1073/pnas.0804895105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hou J, Zhou Y, Zheng Y, Fan J, Zhou W, Ng IO, Sun H, Qin L, Qiu S, Lee JM, et al. Hepatic RIG-I predicts survival and interferon-alpha therapeutic response in hepatocellular carcinoma. Cancer Cell 2014; 25:49-63; PMID: 24360797; http://dx.doi.org/ 10.1016/j.ccr.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 33. Kong L, Sun L, Zhang H, Liu Q, Liu Y, Qin L, Shi G, Hu JH, Xu A, Sun YP, et al. An essential role for RIG-I in toll-like receptor-stimulated phagocytosis. Cell Host Microbe 2009; 6:150-61; PMID:19683681; http://dx.doi.org/ 10.1016/j.chom.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 34. Mukherjee A, Morosky SA, Shen L, Weber CR, Turner JR, Kim KS, Wang T, Coyne CB. Retinoic acid-induced gene-1 (RIG-I) associates with the actin cytoskeleton via caspase activation and recruitment domain-dependent interactions. J Biol Chem 2009; 284:6486-94; PMID:19122199; http://dx.doi.org/ 10.1074/jbc.M807547200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohman T, Rintahaka J, Kalkkinen N, Matikainen S, Nyman TA. Actin and RIG-I/MAVS signaling components translocate to mitochondria upon influenza A virus infection of human primary macrophages. J Immunol 2009; 182:5682-92; PMID:19380815; http://dx.doi.org/ 10.4049/jimmunol.0803093 [DOI] [PubMed] [Google Scholar]
- 36. Cui XF, Imaizumi T, Yoshida H, Borden EC, Satoh K. Retinoic acid-inducible gene-I is induced by interferon-gamma and regulates the expression of interferon-gamma stimulated gene 15 in MCF-7 cells. Biochem Cell Biol 2004; 82:401-5; PMID:15181474; http://dx.doi.org/ 10.1139/o04-041 [DOI] [PubMed] [Google Scholar]
- 37. Dempoya J, Matsumiya T, Imaizumi T, Hayakari R, Xing F, Yoshida H, Okumura K, Satoh K. Double-stranded RNA induces biphasic STAT1 phosphorylation by both type I interferon (IFN)-dependent and type I IFN-independent pathways. J Virol 2012; 86:12760-9; PMID: 22973045; http://dx.doi.org/ 10.1128/JVI.01881-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matsumiya T, Prescott SM, Stafforini DM. IFN-epsilon mediates TNF-alpha-induced STAT1 phosphorylation and induction of retinoic acid-inducible gene-I in human cervical cancer cells. J Immunol 2007; 179:4542-9; PMID: 17878351; http://dx.doi.org/ 10.4049/jimmunol.179.7.4542 [DOI] [PubMed] [Google Scholar]
- 39. Meng G, Xia M, Xu C, Yuan D, Schnurr M, Wei J. Multifunctional antitumor molecule 5'-triphosphate siRNA combining glutaminase silencing and RIG-I activation. Int J Cancer 2014; 134:1958-71; PMID:23921958; http://dx.doi.org/ 10.1002/ijc.28416 [DOI] [PubMed] [Google Scholar]
- 40. Besch R, Poeck H, Hohenauer T, Senft D, Hacker G, Berking C, Hornung V, Endres S, Ruzicka T, Rothenfusser S, et al. Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells. J Clin Invest 2009; 119:2399-411; PMID:19620789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seya T, Azuma M, Matsumoto M. Targeting TLR3 with no RIG-I/MDA5 activation is effective in immunotherapy for cancer. Expert Opin Ther Targets 2013; 17:533-44; PMID:23414438; http://dx.doi.org/ 10.1517/14728222.2013.765407 [DOI] [PubMed] [Google Scholar]
- 42. Imaizumi T, Yagihashi N, Hatakeyama M, Yamashita K, Ishikawa A, Taima K, Yoshida H, Yagihashi S, Satoh K. Upregulation of retinoic acid-inducible gene-I in T24 urinary bladder carcinoma cells stimulated with interferon-gamma. Tohoku J Exp Med 2004; 203:313-8; PMID:15297736; http://dx.doi.org/ 10.1620/tjem.203.313 [DOI] [PubMed] [Google Scholar]
- 43. Long TM, Chakrabarti A, Ezelle HJ, Brennan-Laun SE, Raufman JP, Polyakova I, Silverman RH, Hassel BA. RNase-L deficiency exacerbates experimental colitis and colitis-associated cancer. Inflamm Bowel Dis 2013; 19:1295-305; PMID:23567782; http://dx.doi.org/ 10.1097/MIB.0b013e318281f2fd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pan M, Geng S, Xiao S, Ren J, Liu Y, Li X, Li Z, Peng Z. Apoptosis induced by synthetic retinoic acid CD437 on human melanoma A375 cells involves RIG-I pathway. Arch Dermatol Res 2009; 301:15-20; PMID:18936944; http://dx.doi.org/ 10.1007/s00403-008-0902-x [DOI] [PubMed] [Google Scholar]
- 45. Wang Y, Zhang HX, Sun YP, Liu ZX, Liu XS, Wang L, Lu SY, Kong H, Liu QL, Li XH, et al. Rig-I-/- mice develop colitis associated with downregulation of G alpha i2. Cell Res 2007; 17:858-68; PMID:17893708; http://dx.doi.org/ 10.1038/cr.2007.81 [DOI] [PubMed] [Google Scholar]


