Abstract
Statins are promising anticancer agents that target the mevalonate pathway. Tumor cells are sensitive to depletion of mevalonate-derived products but this activity triggers a homeostatic feedback loop that blunts statin efficacy. We showed that dipyridamole inhibits this feedback response and potentiates statin antitumor activity. This study identifies statins plus dypridamole as a preclinically effective combination of approved agents.
Keywords: Mevalonate, HMG-CoA Reductase, SREBP2, statins, dipyridamole
The need for novel therapeutics for hematologic malignancies such as multiple myeloma and acute myelogenous leukemia (AML) is underscored by the difficulty in achieving long-term disease-free survival. Therapeutic options for heavily pretreated and relapsed patients are limited. We and others have shown that statins, which have been widely used for decades to successfully treat hypercholesterolemia, may help fill this gap and serve as a novel class of anticancer agents. Evidence that statins harbor tumor-specific anti-proliferative activity stems from preclinical,1 clinical,2-4 and epidemiological studies.5 Additional advantages are that statins are well tolerated and the majority are off patent, making them readily available and more cost-effective than newly emergent targeted therapies. Thus, statins have the potential to immediately and affordably improve cancer patient care and outcome.
Statins inhibit the rate-limiting enzyme of the mevalonate (MVA) pathway, 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) (Fig. 1A). The MVA pathway is the de novo source of cholesterol and inhibiting HMGCR depletes intracellular cholesterol. Sterol depletion triggers a feedback loop to replenish intracellular cholesterol. Following sterol depletion, the endoplasmic reticulum-bound transcription factor sterol regulatory element binding protein 2 (SREBP2) is cleaved and translocates to the nucleus where it transcriptionally induces genes of the MVA pathway, including HMGCR, HMG-CoA synthase 1 (HMGCS1), and low-density lipoprotein receptor (LDLr) (Fig. 1B). This stimulates intracellular synthesis of sterols and uptake of low-density lipoprotein (LDL) cholesterol from the bloodstream,6 thus effectively lowering serum cholesterol levels.
Figure 1.
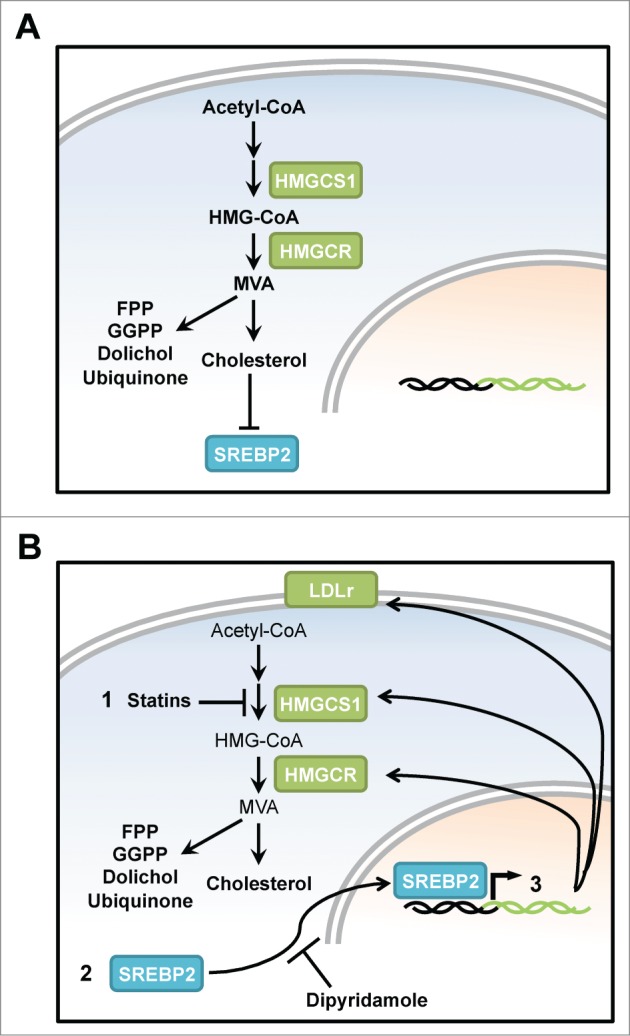
Targeting the mevalonate pathway using statins and its feedback loop using dipyridamole induces tumor cell apoptosis. (A) The mevalonate (MVA) pathway produces many critical cellular end products that are essential for cell proliferation and survival, including cholesterol, production of which is regulated by HMGCR, the rate limiting enzyme of the MVA pathway. The presence of cholesterol ensures that the transcription factor SREBP2 is maintained in the cytoplasm anchored to the endoplasmic reticulum. (B) Upon statin-mediated HMGCR inhibition (1), depletion of cholesterol and other sterol intermediates causes translocation of SREBP2 into the nucleus (2), and the ensuing transcription of sterol-responsive genes including HMGCR, HMGCS1, and LDLR, thereby blunting the anticancer efficacy of statins in tumor cells (3). When co-administered with statins, dipyridamole inhibits the feedback loop by preventing SREBP2 cleavage and thereby augmenting statin-induced apoptosis. MVA, mevalonate; HMGCR, 3-hydroxy-3-methylglutaryl coenzyme A reductase; HMGCS1, HMG-CoA synthase 1; SREBP2, sterol regulatory element binding transcription factor 2; FPP, farnesyl pyrophosphate; GGPP, geranaylgeranyl pyrophosphate; LDLr, low-density lipoprotein receptor.
Some tumor cells have altered metabolism that culminates in an increased dependence on MVA-derived which products and sensitizes them to MVA pathway inhibition.1 For example, the MVA pathway contributes to oncogenic progression7 and is upregulated by mutant p53,8 supporting the concept that tumor cells can be dependent upon the MVA pathway. Tumor cell-specific apoptosis following statin-induced MVA pathway inhibition has been linked to depletion of the MVA end products farnesyl and geranylgeranyl pyrophosphate. These isoprenoid moieties are essential for the post-translational prenylation and activation of critical signaling proteins such as Ras. However, cholesterol depletion within both the tumor and the extracellular milieu may also contribute to tumor-specific apoptosis, which is triggered in response to statins.3 We anticipate that the mechanism of statin antitumor effects likely involves multiple aspects of statin activity on the MVA pathway. In our research7,9,10 we have focused our attention on the direct antiproliferative effects of statins on tumor cells and how best to use statins as anticancer agents.
Clinical trials across a wide spectrum of cancers have demonstrated that, like most cancer therapeutics, statin treatment triggers remarkable responses in some, but not all patients, suggesting that maximal efficacy will be achieved when statins are combined with other agents. In an effort to expand treatment options for hematologic malignancies, we recently carried out a screen to identify potentiators of statin-induced apoptosis.9 This screen was composed of Food and Drug Administration (FDA)-approved drugs, many of which are off patent, and identified dipyridamole, which is clinically used as an antiplatelet agent. Dipyridamole strongly synergized with and potentiated the proapoptotic effects of atorvastatin and fluvastatin in AML and multiple myeloma cell lines. Fluvastatin and atorvastatin are lipophilic statins that have been used at cholesterol-lowering doses to demonstrate anticancer activity in recent window-of-opportunity studies.2,4 We have also shown that the statin-dipyridamole combination demonstrated in vivo efficacy and induced apoptosis in primary AML patient samples but not normal cells.
The statin-insensitive LP1 multiple myeloma cell line underwent apoptosis when treated with the statin-dipyridamole combination. We previously linked the statin insensitivity of LP1 to a robust stimulation of the statin-induced sterol feedback loop and consequential upregulation of HMGCR.10 We now show that dipyridamole prevents the statin-induced upregulation of HMGCR and other sterol-responsive genes in AML and multiple myeloma cells by inhibiting the cleavage of SREBP2, which is essential for its translocation into the nucleus (Fig. 1B). Taken together, our data demonstrate that blocking the MVA pathway using statins and the ensuing feedback loop using dipyridamole is an effective novel therapeutic strategy for the treatment of hematologic malignancies.
This combination could potentially broaden the applicability of statins to more tumor types in which sterol feedback impedes statin efficacy, and future studies will assess this. Although dipyridamole blunted SREBP2 cleavage, the mechanism and specificity of this effect, as well as the functional consequence of SREBP2 knockdown in the context of statin sensitization, remain unknown. The statin-dipyridamole combination also retained the tumor-normal therapeutic index critical for effective treatment regimens. This is consistent with these drugs being safely co-prescribed for patients with cardiovascular indications. The preferential tumor cell death in response to statin-dipyridamole exposure also remains an area for further investigation.
In summary, this combination of 2 FDA-approved drugs has the potential to be fast-tracked to cancer patient care. Mechanistically, targeted inhibition of the MVA pathway using statins and the restorative feedback loop using dipyridamole shows that 2 hits are better than one.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Dr. Pete Mullen and Rosemary Yu of the Penn laboratory for review of the manuscript.
Funding
This work was undertaken in part thanks to funding from the CRC Program (LZP), the OICR through funding provided by the Province of Ontario (LZP), the CBCF (AP) and the Ontario Ministry of Health and Long Term Care. The views expressed do not necessarily reflect those of the Ontario Ministry of Health and Long Term Care.
References
- 1. Clendening JW, Penn LZ. Targeting tumor cell metabolism with statins. Oncogene 2012; 31(48):4967-4978; PMID:22310279; http://dx.doi.org/ 10.1038/onc.2012.6 [DOI] [PubMed] [Google Scholar]
- 2. Garwood ER, Kumar AS, Baehner FL, Moore DH, Au A, Hylton N, Flowers CI, Garber J, Lesnikoski BA, Hwang ES, et al. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Res Treat 2010; 119(1):137-144; PMID:19728082; http://dx.doi.org/ 10.1007/s10549-009-0507-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Advani AS, McDonough S, Copelan E, Willman C, Mulford DA, List AF, Sekeres MA, Othus M, Appelbaum FR. SWOG0919: a Phase 2 study of idarubicin and cytarabine in combination with pravastatin for relapsed acute myeloid leukaemia. British J Haematol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bjarnadottir O, Romero Q, Bendahl PO, Jirstrom K, Ryden L, Loman N, Uhlén M, Johannesson H, Rose C, Grabau D, et al. Targeting HMG-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Res Treat 2013; 138(2):499-508; PMID:23471651; http://dx.doi.org/ 10.1007/s10549-013-2473-6 [DOI] [PubMed] [Google Scholar]
- 5. Ahern TP, Pedersen L, Tarp M, Cronin-Fenton DP, Garne JP, Silliman RA, Sørensen HT, Lash TL. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Institute 2011; 103(19):1461-1468; PMID:21813413; http://dx.doi.org/ 10.1093/jnci/djr291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kita T, Brown MS, Goldstein JL. Feedback regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in livers of mice treated with mevinolin, a competitive inhibitor of the reductase. J Clin Invest 1980; 66(5):1094-1100; PMID:6903572; http://dx.doi.org/ 10.1172/JCI109938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clendening JW, Pandyra A, Boutros PC, El Ghamrasni S, Khosravi F, Trentin GA, Martirosyan A, Hakem A, Hakem R, Jurisica I, et al. Dysregulation of the mevalonate pathway promotes transformation. Proc Natl Acad Sci U S A 2010; 107(34):15051-15056; PMID:20696928; http://dx.doi.org/ 10.1073/pnas.0910258107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Freed-Pastor WA, Mizuno H, Zhao X, Langerod A, Moon SH, Rodriguez-Barrueco R, Barsotti A, Chicas A, Li W, Polotskaia A, et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell 2012; 148(1-2):244-258; PMID:22265415; http://dx.doi.org/ 10.1016/j.cell.2011.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pandyra A, Mullen PJ, Kalkat M, Yu R, Pong JT, Li Z, Trudel S, Lang KS, Minden MD, Schimmer AD, et al. Immediate Utility of Two Approved Agents to Target both the Metabolic Mevalonate Pathway and its Restorative Feedback loop. Cancer Res 2014; 74(17):4772-82; PMID:24994712 [DOI] [PubMed] [Google Scholar]
- 10. Clendening JW, Pandyra A, Li Z, Boutros PC, Martirosyan A, Lehner R, Jurisica I, Trudel S, Penn LZ. Exploiting the mevalonate pathway to distinguish statin-sensitive multiple myeloma. Blood 2010; 115(23):4787-4797; PMID:20360469; http://dx.doi.org/ 10.1182/blood-2009-07-230508 [DOI] [PubMed] [Google Scholar]


