Abstract
p62 regulates key signaling pathways including those that control cell death and autophagy. Recently, we reported that p62 is upregulated during all-trans retinoic acid (ATRA)-induced terminal differentiation of acute myeloid leukemia (AML) cells. This response reduces levels of ubiquitinated protein aggregates in mature cells and protects these cells against ATRA treatment. Thus, p62 confers a survival advantage to mature AML cells.
Keywords: autophagy, cell survival, differentiation, leukemia, SQSTM1/p62
Abbreviations
- 8-CPT-cAMP
8-(4-chlorophenylthio) 5′-cyclic adenosine monophosphate
- AML
acute myeloid leukemia
- ATRA
all-trans retinoic acid
- BCR-ABL
breakpoint cluster region–Abelson
- hrG-CSF
human recombinant granulocyte colony stimulating factor
- MAPK
mitogen-activated protein kinase
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor-κB
- Nrf-2
NF-E2 related factor E2
- NBT
- PKC
protein kinase C
- PML
promyelocytic leukemia
- RAR-α
Retinoic acid receptor alpha
- RIP
receptor-interacting protein
- SQSTM1
sequestosome 1
- TRAF6
tumor necrosis factor receptor-associated factor 6
Sequestosome 1 (SQSTM1, best known as p62) is an adapter protein that regulates multiple signaling axes including those involved in tumorigenesis, such as nuclear factor-κB (NF-κB), mammalian target of rapamycin (mTOR), nuclear factor erythroid 2-related factor 2 (NRF-2), and mitogen-activated protein kinase (MAPK) pathways.1,2 p62 can also serve as a receptor for selective autophagic clearance of protein aggregates and damaged organelles.3 In the context of myeloid leukemias, p62 functions in the degradation of the leukemic fusion proteins breakpoint cluster region-Abelson (BCR–ABL) and promyelocytic leukemia-retinoic acid receptor α (PML–RARA).4 Additionally, overexpression of p62 is observed in various tumors and its inhibition limits cell proliferation and tumorigenesis in some tumor tissues.5 Nevertheless, the function of p62 during cancer treatment is poorly documented.
All-trans retinoic acid (ATRA), a well-established differentiating agent, is an effective anticancer agent that is currently used in the treatment of acute promyelocytic leukemia, also known as M3-type acute myeloid leukemia (AML), which is characterized by arrested differentiation at the stage of promyelocytes.6 We have recently investigated the role and regulation of p62 in the course of ATRA-induced granulocytic differentiation of AML cells.7 Our results indicate that ATRA induces upregulation of p62 protein and mRNA, with a concomitant increase in the autophagic flux in AML cell lines that undergo granulocytic differentiation. These responses are impaired in maturation-resistant AML cells but can be re-established once differentiation is restored by protein kinase A stimulation using 8-(4-chlorophenylthio), 5’-cyclic adenosine monophosphate (8-CPT-cAMP) in combination with ATRA. These findings reveal the tight link between p62 expression levels, autophagy, and the myeloid maturation phenotype. In support of these data obtained in established cell lines, immature blast cells of AML patients display lower p62 mRNA levels than healthy mature granulocytes.
The effects of p62 on cell death are complex, as p62 can regulate both positive and negative players of programmed cell death pathways.2,5 We found that short hairpin RNA-mediated inhibition of p62 expression increased cell death induced by ATRA, underscoring the critical role of p62 in the viability of mature AML cells that undergo granulocytic differentiation. These observations have important clinical relevance as they suggest that p62 is involved in ATRA resistance in refractory or relapsed AML patients. Notably, this pro-survival role of p62 may also contribute to development of the ATRA syndrome, a potentially lethal complication of ATRA treatment caused by the accumulation of mature cells in the blood of patients.8
We showed that p62 mitigates the accumulation of ubiquitinated protein aggregates that occurs during the granulocytic differentiation process. This suggests that p62 plays a protective role against proteotoxic stress, although this remains to be fully demonstrated. As an adapter protein, p62 can interact with key signaling components involved in the activation of NF-κB and NRF-2 transcription factors, thereby leading to cell survival.2,5,9 Additional studies are required to fully explore the role of p62 in regulating such cell survival pathways during granulocytic differentiation.
Our data indicate that NF-κB regulates p62 levels because inhibition of NF-κB activity impaired the increase in p62 observed upon ATRA treatment. It remains unclear, however, how NF-κB regulates p62 expression in the context of granulocytic differentiation and whether this involves additional regulatory mechanisms, such as the microRNA (miR)-17 family that targets p62 mRNA in myeloid progenitors cells.10 The impact of other transcription factors such as NFR-2 on p62 levels also warrants further investigation.2 Moreover, whether NF-κB–dependent p62 expression is a specific response to ATRA treatment or also occurs with other NF-κB activators remains to be explored. Of note, p62 has been shown to interact with several NF-κB signaling components including protein kinase C (PKC), receptor-interacting protein (RIP), and tumor necrosis factor receptor-associated factor 6 (TRAF6).1,2 However, whether p62 regulates NF-κB activity in the context of AML differentiation is still an open question.
Our finding that p62 is upregulated during human recombinant granulocyte colony-stimulating factor (hrG-CSF)-induced differentiation of non-leukemic primary CD34+ progenitors cells supports the idea that p62 has a physiological role during normal granulocytic development. Whether and how p62 protects mature granulocytes against cellular stresses that occur during normal differentiation as a result of massive changes in intracellular content remains unclear.
Our results have broader implications. Interestingly, we also showed that p62 upregulation occurs during differentiation of myeloid leukemia cells into either monocytes or megakaryocytes (7 and data not shown). Therefore, this phenomenon is not restricted to granulocytic differentiation but may represent a general response that occurs during terminal differentiation of myeloid leukemia cells toward a specific cell lineage. Further investigations are required to explore the role of p62 in leukemia cells that undergo lineage-specific differentiation programs.
In conclusion, our data clearly demonstrate the pro-survival role of p62 during granulocytic differentiation of AML cells and implicate p62 in ATRA resistance of refractory and relapsed AML patients. Of note, the physiological role of p62 in normal myeloid development must be clarified before targeting p62 as a therapeutic strategy for the treatment of leukemia. Figure 1.
Figure 1.
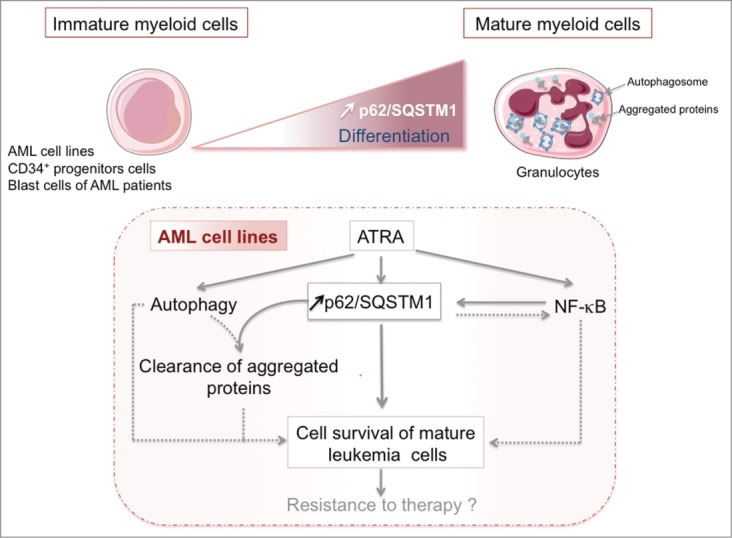
Pro-survival role of p62 during myeloid leukemia cell differentiation. Low levels of sequestosome 1 (SQSTM1, best known as p62) are a characteristic of cells that display an immature myeloid phenotype (i.e., acute myeloid leukemia [AML] cell lines, primary CD34+ progenitors cells, primary blast cells from AML patients) compared with terminally differentiated cells. All-trans retinoic acid (ATRA) induces granulocytic differentiation of AML cells, which is associated with p62 upregulation, an increase in autophagic flux, and the accumulation of aggregated proteins in mature AML cells. p62 upregulation is under the control of NF-κB activity; conversely, p62 can activate the NF-κB activity under certain conditions. p62 mediates survival of mature AML cells and alleviates the accumulation of protein aggregates that occurs during differentiation. This suggests a role of p62 in ATRA resistance observed in some patients with AML. Dashed lines show other pro-survival pathways that are stimulated in response to ATRA. Additional factors involved in the regulation and function of p62 remain to be identified (see text for more detailed information).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Acknowledgment
The authors would like to thank Servier Medical Art for images used in the illustration.
Funding
This work was supported by funds from the Institut National de la Santé et de la Recherche Médicale (INSERM), the University of Bordeaux, the Bergonié Institute, the Conseil Regional d’Aquitaine, the Ligue contre le Cancer Comité de la Gironde (to MDM); the INCa-DGOS-Inserm 6046 (to MDM); the Ligue contre le Cancer Comité Ile de France (to E S-B); the Fondation de France (to E S-B); the Swiss National Science Foundation (31003A 143739); and the Werner and Hedy Berger-Janser Foundation of Cancer Research (to MPT).
References
- 1. Moscat J, Diaz-Meco MT. p62: a versatile multitasker takes on cancer. Trends Biochem Sci 2012; 37:230-6; PMID:22424619; http://dx.doi.org/ 10.1016/j.tibs.2012.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Komatsu M, Kageyama S, Ichimura Y. p62/SQSTM1/A170: physiology and pathology. Pharmacol Res 2012; 66:457-62; PMID:22841931; http://dx.doi.org/ 10.1016/j.phrs.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 3. Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy 2011; 7:279-96; PMID:21189453; http://dx.doi.org/ 10.4161/auto.7.3.14487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Orfali N, McKenna SL, Cahill MR, Gudas LJ, Mongan NP. Retinoid receptor signaling and autophagy in acute promyelocytic leukemia. Exp Cell Res 2014; 324:1-12; PMID:24694321; http://dx.doi.org/ 10.1016/j.yexcr.2014.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 2009; 137:1001-4; PMID:19524504; http://dx.doi.org/ 10.1016/j.cell.2009.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castaigne S, Chomienne C, Daniel MT, Ballerini P, Berger R, Fenaux P, Degos L. All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results. Blood 1990; 76:1704-9; PMID:2224119 [PubMed] [Google Scholar]
- 7. Trocoli A, Bensadoun P, Richard E, Labrunie G, Merhi F, Schlafli AM, Brigger D, Souquere S, Pierron G, Pasquet JM, et al. . p62/SQSTM1 upregulation constitutes a survival mechanism that occurs during granulocytic differentiation of acute myeloid leukemia cells. Cell Death Differ 2014; 21:1852-61; PMID:25034783; http://dx.doi.org/ 10.1038/cdd.2014.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Botton S, Dombret H, Sanz M, Miguel JS, Caillot D, Zittoun R, Gardembas M, Stamatoulas A, Condé E, Guerci A, et al. . Incidence, clinical features, and outcome of all trans-retinoic acid syndrome in 413 cases of newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood 1998; 92:2712-8; PMID:9763554 [PubMed] [Google Scholar]
- 9. Nezis IP, Stenmark H. p62 at the interface of autophagy, oxidative stress signaling, and cancer. Antioxid Redox Signal 2012; 17:786-93; PMID:22074114; http://dx.doi.org/ 10.1089/ars.2011.4394 [DOI] [PubMed] [Google Scholar]
- 10. Meenhuis A, van Veelen PA, de Looper H, van Boxtel N, van den Berge IJ, Sun SM, Taskesen E, Stern P, de Ru AH, van Adrichem AJ, et al. . MiR-17/20/93/106 promote hematopoietic cell expansion by targeting sequestosome 1-regulated pathways in mice. Blood 2011; 118:916-25; PMID:21628417; http://dx.doi.org/ 10.1182/blood-2011-02-336487 [DOI] [PMC free article] [PubMed] [Google Scholar]


