Abstract
Transcription in the vicinity of DNA double-strand breaks (DSBs) is suppressed via a process involving ataxia telangiectasia mutated protein (ATM) and H2AK119 ubiquitylation.1 We discuss recent findings that components of the Polybromo and Brahma-related gene 1 (BRG1)-associated factor (PBAF) remodeling complex and the polycomb repressive complex (PRC1/2) are also required.2 Failure to activate transcriptional suppression impedes a rapid DSB repair process.
Transcription necessitates changes to the surrounding chromatin and relaxation of DNA supercoiling. Chromatin remodeling complexes and topoisomerases are required for transcription, with distinct remodelers functioning at distinct genes.1 It is likely, therefore, that transcription might impede DNA repair and/or that repair processes might specifically target damage in actively transcribed genes. Such is the case for UV damage, where there is preferential repair of UV photoproducts.2 How DNA double-strand breaks (DSBs) affect transcription and conversely how transcription influences DSB repair is less clear. RNA polymerase II-dependent transcription is not globally shut down by exposure to ionizing radiation (IR), which efficiently induces DSBs; in fact, a healthy response to IR involves upregulation of multiple genes.
The DNA damage response (DDR) encompasses DSB repair pathways and a signal transduction response regulated by the ataxia telangiectasia mutated (ATM) protein. DNA non-homologous end joining (NHEJ) is the major pathway of DSB repair. ATM signaling is not a pre-requisite for NHEJ, although ATM is required for NHEJ-dependent repair of a subset of DSBs located in heterochromatin (HC), where ATM promotes localized HC relaxation. ATM signaling is initiated by phosphorylation of H2AX at the DSB site, promoting the orchestrated assembly of DDR proteins including the ubiquitin ligases Ring Finger Proteins 8 and 168 (RNF8/RNF168), which ubiquitylate H2A in the vicinity of the DSB. The mediator protein, p53-binding protein 1 (53BP1), lies toward the apex of ATM signaling.
The Greenberg laboratory developed a system to generate Fok1-endonuclease induced DSBs in proximity to a reporter gene whose transcription is regulated by doxycycline.3 Transcriptional activity was assessed by production of a transcript encoding a yellow fluorescent protein (YFP). Fok1-dependent DSBs cause YPF signal loss in an ATM-dependent manner. This transcriptional arrest occurs in cis to the DSB and requires ubiquitylation on H2AK119 (H2AK119ub). RNF8, and to a lesser extent RNF168, are also required, but 53BP1 is dispensable. Recently, we consolidated and extended these findings by showing that the process also involves the remodeling complex Polybromo and Brahma-related gene 1 (BRG1)-associated factor complex (PBAF) and polycomb repressive complex 1 and 2 (PRC1/2), subunits of the Polycomb group (PcG) complex. We additionally revealed an interface between damage-induced transcriptional silencing and DSB repair.4
Two remodeling complexes have been described in mammalian cells: SWI/SNF-B (or PBAF) and SWI/SNF-A (or BAF). Although these complexes share subunit composition—for example the ATPase subunit BRG1 is common to both complexes—BAF180, BAF200, and BRD7 are specific to PBAF.5 We found that DSB-induced transcriptional suppression requires BAF180 and BRG1 ATPase activity.4 Phosphorylation of BAF180 on S963 by ATM appears to be required since expression of S963A BAF180 impedes transcriptional repression whereas expression of S963E BAF180, a phosphomimic change, partly relieves the need for ATM. These findings are consistent with a model whereby enhancer of zeste homolog 2 (EZH2), a methyl transferase subunit of PRC2, promotes H3K27me3, facilitating recruitment of the ubiquitin ligase PRC1 and subsequent generation of H2AK119ub. Consistent with this model, induction of H2K119ub by damage also requires PRC2/PRC1.
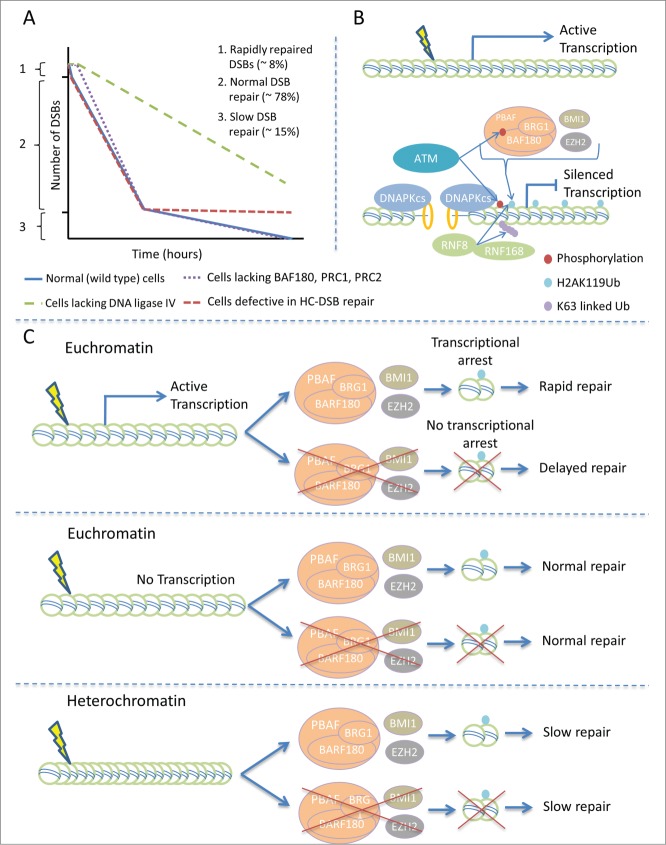
Enumeration of γ-H2AX foci represents a sensitive method to assess DSB formation and repair. This method and additional procedures have revealed that DSBs are repaired with two-component kinetics: approximately 85% of IR-induced DSBs are repaired by a fast process whereas DSBs within HC regions are predominantly repaired by a slow process.6 Intriguingly, we observed that the components required for DSB-induced transcriptional repression (i.e., BAF180, BRG1, PRC1, PRC2, and RNF8) as well as DNA ligase IV, the ligase essential for NHEJ, are required for a very rapid repair process that occurs at early times (15–30 minutes) post IR, representing a third component of repair (Fig. 1). Supporting this observation, increased chromosome breakage was observed using techniques involving premature chromosome condensation. Treatment with transcriptional inhibitors precluded this rapid repair. DSB repair was not abolished in the absence of transcriptional repression but was delayed. These findings suggest that DSBs in the vicinity of transcriptionally active sites are rapidly repaired by a process dependent upon transcriptional repression. This may not reflect a specific repair process per se but rather the ability to repair DSBs rapidly in DNA that has been “opened” for transcription. However, transcription needs to be silenced to allow rapid repair (see Fig. 1).
Figure 1.
(See previous page). DNA double-strand break (DSB)-induced transcriptional arrest and its impact on repair. (A) Depiction of the 3 components of DSB repair. (B) Factors required for transcriptional arrest and rapid DSB repair. A DSB activates ataxia telangiectasia mutated (ATM) signaling. ATM phosphorylates a range of substrates including γH2AX and S963 BAF180. γH2AX formation at the DSB enables the recruitment of DNA damage response (DDR) proteins including the ubiquitin ligases RNF8/RNF168, which promote the formation of K63-linked ubiquitin chains. BRG-1 associated factor (BAF180) and Polycomb repressive complex 1 and 2 (PRC1/PRC2) are required for ubiquitylation of H2AK119. All of these components are required for transcriptional arrest in the DSB vicinity. PBAF, Polybromo and BRG1 associated factor complex; BRG1, Brahma-related gene 1; BMI1 and EZH2, polycomb group (PcG) complex subunits; DNA-PKcs, DNA-dependent protein kinase catalytic subunit. The yellow ring on the DNA represents Ku, which, together with DNA-PKcs, forms the DNA-PK complex that initiates DNA non-homologous end joining (NHEJ). (C) Requirement for DDR proteins for repair in regions of distinct chromatin organization. DSBs in the vicinity of activate transcription are repaired by a rapid process involving the same proteins as required to arrest transcription. DSBs that do not localize to chromocenters undergo an intermediate (normal) rate of repair that does not need ATM signaling. DSBs localizing to chromocenters are repaired with slow kinetics via a process requiring ATM and Artemis, but not the factors required for transcriptional arrest.
The interface between chromatin remodeling and DSB repair is currently the subject of substantial study.7 This interface is complicated, with distinct complexes appearing to function in defined circumstances. Generally, homologous recombination, another DSB repair process that necessitates extensive end-processing and engagement with a sister chromatid, has a greater need for remodeling than NHEJ.8 In contrast, PBAF has a generalized role in transcriptional repression at DSBs, impacting upon NHEJ although PBAF is dispensable for NHEJ. This specificity reflects how remodelers appear to influence DSB repair.
Many questions arise from these findings. ATM appears to lie upstream, promoting PBAF phosphorylation and PRC1/2-dependent H2AK119ub. Potential models could involve PBAF-dependent chromatin remodeling to allow PRC2 access and/or PRC1-dependent K119ub. Alternatively, PBAF may serve to stabilize H2AK119ub by preventing deubiquitylation. BAF180 is mutated in many renal cancers and 2 cancer mutations impede transcriptional repression and rapid DSB repair. The contribution of this process to cancer suppression remains to be deduced. Additionally, DNA-PK has been reported to regulate repression of RNA polymerase II-dependent transcription at DSBs.9 Interplay between the 2 transcriptional silencing processes remains to be examined. Finally, topoisomerases, which relax supercoiling for transcription, create transient intermediates called cleavable complexes that can occasionally become trapped, likely impeding subsequent transcription with outcomes causal of human diseases.10 PBAF may also promote removal of such trapped complexes that arise as a result of DNA damage.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
PAJ and JAD were supported by the Medical Research Council for this work. JAD was additionally funded by the Cancer Research UK.
References
- 1. Papamichos-Chronakis M, Peterson CL. Chromatin and the genome integrity network. Nat Rev Genet 2013; 14:62-75; PMID:23247436; http://dx.doi.org/ 10.1038/nrg3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vermeulen W, Fousteri M. Mammalian transcription-coupled excision repair. Cold Spring Harb Perspect Biol 2013; 5:a012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shanbhag NM, Rafalska-Metcalf IU, Balane-Bolivar C, Janicki SM, Greenberg RA. ATM-dependent chromatin changes silence transcription in cis to DNA double-strand breaks. Cell 2010; 141:970-81; PMID:20550933; http://dx.doi.org/ 10.1016/j.cell.2010.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kakarougkas A, Ismail A, Chambers AL, Riballo E, Herbert AD, Kunzel J, Lobrich M, Jeggo PA, Downs JA. Requirement for PBAF in transcriptional repression and repair at DNA breaks in actively transcribed regions of chromatin. Mol Cell 2014; 55:723-32; PMID:25066234; http://dx.doi.org/ 10.1016/j.molcel.2014.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chambers AL, Downs JA. The RSC and INO80 chromatin-remodeling complexes in DNA double-strand break repair. Prog Mol Biol Transl Sci 2012; 110:229-61; PMID:22749148; http://dx.doi.org/ 10.1016/B978-0-12-387665-2.00009-2 [DOI] [PubMed] [Google Scholar]
- 6. Goodarzi AA, Noon AT, Deckbar D, Ziv Y, Shiloh Y, Lobrich M, Jeggo PA. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell 2008; 31:167-77; PMID:18657500; http://dx.doi.org/ 10.1016/j.molcel.2008.05.017 [DOI] [PubMed] [Google Scholar]
- 7. Seeber A, Hauer M, Gasser SM. Nucleosome remodelers in double-strand break repair. Curr Opin Genet Dev 2013; 23:174-84; PMID:23352131; http://dx.doi.org/ 10.1016/j.gde.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 8. Bennett G, Papamichos-Chronakis M, Peterson CL. DNA repair choice defines a common pathway for recruitment of chromatin regulators. Nat Commun 2013; 4:2084; PMID:23811932; http://dx.doi.org/ 10.1038/ncomms3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pankotai T, Bonhomme C, Chen D, Soutoglou E. DNAPKcs-dependent arrest of RNA polymerase II transcription in the presence of DNA breaks. Nat Struct Mol Biol 2012; 19:276-82; PMID:22343725; http://dx.doi.org/ 10.1038/nsmb.2224 [DOI] [PubMed] [Google Scholar]
- 10. Katyal S, Lee Y, Nitiss KC, Downing SM, Li Y, Shimada M, Zhao J, Russell HR, Petrini JH, Nitiss JL, et al. . Aberrant topoisomerase-1 DNA lesions are pathogenic in neurodegenerative genome instability syndromes. Nat Neurosci 2014; 17:813-21; PMID:24793032; http://dx.doi.org/ 10.1038/nn.3715 [DOI] [PMC free article] [PubMed] [Google Scholar]