Abstract
Equal segregation of sister chromatids during mitosis requires that pairs of kinetochores establish proper attachment to microtubules emanating from opposite poles of the mitotic spindle. The spindle assembly checkpoint (SAC) protects against errors in segregation by delaying sister separation in response to improper kinetochore–microtubule interactions, and certain checkpoint proteins help to establish proper attachments. Anaphase entry is inhibited by the checkpoint through assembly of the mitotic checkpoint complex (MCC) composed of the 2 checkpoint proteins, Mad2 and BubR1, bound to Cdc20. The outer kinetochore acts as a catalyst for MCC production through the recruitment and proper positioning of checkpoint proteins and recently there has been remarkable progress in understanding how this is achieved. Here, we highlight recent advances in our understanding of kinetochore–checkpoint protein interactions and inhibition of the anaphase promoting complex by the MCC.
Keywords: APC/C, Kinetochore, Mitosis, SAC
Abbreviations
- APC/C
Anaphase Promoting Complex/Cyclosome
- CH
Calponin Homology
- MCC
Mitotic Checkpoint Complex
- MELT
Met-Glu-Leu-Thr sequence
- PP2A
Protein Phosphatase 2A
- SAC
Spindle Assembly Checkpoint
- TPR
Tetratricopeptide repeat
Introduction
The kinetochore is a large protein structure assembled at the centromere region. For accurate sister chromatid segregation it is essential that kinetochores bind microtubules in an end-on manner and that the sisters biorient in the center of the cell (Fig. 1).1 Incorrect attachments often occur, but luckily the cell has mechanisms in place that provide the time and tools to fix erroneous attachments. Time is provided by the spindle assembly checkpoint (SAC) that prevents anaphase entry until all kinetochores have made proper attachments whereas the tools are provided by the error correction machinery.2 Importantly, upstream components of the SAC are also part of the error correction machinery, thus providing a tight link between these activities. How the checkpoint discriminates proper from improper attachments is not clear but proper attachments generate tension that results in intrakinetochore stretching and this kinetochore state is unable to bind SAC proteins.3 A particular type of improper attachments that is not detected by the checkpoint is merotelic attachment, in which the same kinetochore binds to microtubules from both poles. Merotelic attachments generate tension and are therefore not sensed as erroneous.
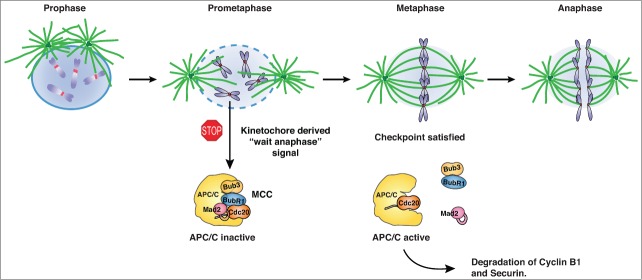
Figure 1.
Regulation of chromosome segregation by the spindle assembly checkpoint. Entry into mitosis is marked by nuclear envelope breakdown, at which stage the SAC becomes active because of the presence of unattached kinetochores (prometaphase state). The unattached kinetochores recruit SAC proteins resulting in the generation of the mitotic checkpoint complex (MCC), which can bind stably to the anaphase promoting complex (APC/C) and inhibit it. At metaphase, when all kinetochores have attached and are under tension, the checkpoint turns off and the MCC disassembles thus freeing Cdc20 for APC/C activation. The active APC/C-Cdc20 complex targets securin and cyclin B1 for degradation resulting in sister chromatid separation and mitotic exit, respectively.
Conserved components of the SAC that were originally identified by genetic screens in yeast are the Mad (mitotic-arrest deficient) proteins Mad1, Mad2, and Mad3 (BubR1 in humans; we will use the human name throughout) and the Bub (budding uninhibited by benzimidazole) proteins Bub1 and Bub3 (Fig. 2).2 Bub1 and BubR1 both exist in a stable complex with Bub3, which binds a conserved GLEBS motif in the proteins, whereas Mad1 and Mad2 form a stable tetrameric complex. In addition, a large fraction of Mad2 exists as a soluble unliganded form. In addition, the Aurora B and Mps1 kinases are essential for SAC signaling and their kinase activity is required for a functional SAC.
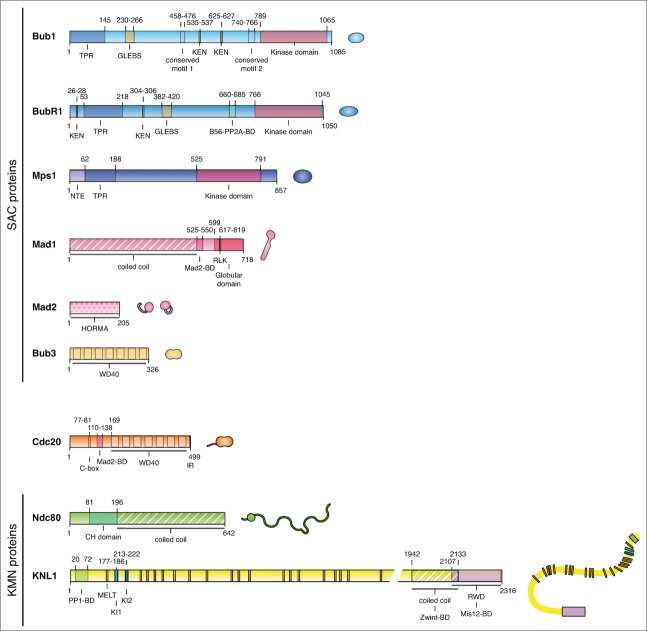
Figure 2.
Schematic of SAC proteins and KMN network components. A schematic of checkpoint proteins, Cdc20, and the KMN network components Ndc80 and KNL1. The primary structure is shown with important domains and motifs indicated. Next to the primary structure is an illustration of the schematic used for that protein in subsequent figures. BD, binding domain.
The final inhibitory complex consists of Mad2 and BubR1–Bub3 bound to Cdc20, the mitotic co-activator of the anaphase promoting complex/cyclosome (APC/C), and is referred to as the mitotic checkpoint complex (MCC). This complex can bind stably to the APC/C thereby inhibiting its E3 ubiquitin ligase activity (Fig. 1).4,5 The APC/C is a large ubiquitin ligase that targets multiple proteins for degradation through the attachment of ubiquitin chains.6 Its substrates contain short destruction motifs of different kinds, the most common being D-boxes and KEN-boxes. Binding of substrates to the APC/C depends on a combined binding pocket between Cdc20 and the APC10 subunit; in addition Cdc20 activates the APC/C.7 Thus, by inhibiting Cdc20, the SAC efficiently inhibits the APC/C and halts mitotic progression by preventing the degradation of 2 key substrates, securin and cyclin B1. Securin is an inhibitor of separase, a protease that cleaves a cohesin subunit allowing sister chromatid separation, and cyclin B1 is an activator of CDK1, the major mitotic kinase. Pioneering work by Conly Rieder showed that the SAC signal is generated by the kinetochore, and consistent with this all SAC components are recruited to unattached kinetochores where they exhibit rapid turnover.8-10 In addition, Cdc20 is recruited to kinetochores, strongly suggesting that Cdc20 is incorporated into inhibitory complexes at the kinetochore.9,11 Once all kinetochores are correctly attached to microtubules, the MCC disassembles and the APC/C–Cdc20 complex becomes active and triggers anaphase entry.
Given the central role of the kinetochore in the SAC there has been a strong interest in understanding how the checkpoint proteins interact with the kinetochore, the order in which they are recruited, and how this is regulated in response to microtubule attachment. With our increased understanding of kinetochore architecture and function it has become clear that the microtubule binding activity of the kinetochore constituted by the KNL1–Mis12–Ndc80 complex (KMN network) is the major binding site for SAC proteins at the outer kinetochore.12,13 There has recently been an explosion in our understanding of the interaction between checkpoint proteins and the KMN network and how these interactions are dynamically regulated. We will begin our tour of the SAC by introducing the KMN network before discussing how checkpoint proteins are recruited to assemble a platform for MCC production.
The KMN Network and Regulation of Kinetochore–Microtubule Interactions
The KMN network is the core microtubule binding activity of the outer kinetochore and is composed of 3 protein complexes, namely the large KNL1 protein in complex with Zwint (Zwint is only present in metazoans), the 4-subunit Mis12 complex (Mis12, Nnf1, Dsn1, Nsl1), and the 4-subunit Ndc80 complex (Ndc80, Nuf2, Spc24 and Spc25).1,14 The Mis12 complex links the KMN network to the inner kinetochore whereas the Ndc80 complex and KNL1 can directly bind to microtubules. As discussed below, the Ndc80 complex and KNL1 are docking sites for checkpoint proteins. The Ndc80 complex, and in particular the Ndc80 protein, is essential for end-on attachment of microtubules to kinetochores.15 The Ndc80 protein contains an N-terminal basic tail followed by a calponin homology (CH) domain that both contribute to microtubule binding (Fig. 2). The basic tail of Ndc80 contains numerous phosphorylation sites for the Aurora B kinase and these are phosphorylated in response to improper kinetochore–microtubule interactions, thereby destabilizing the interaction.16,17 As Aurora B is concentrated at the centromere region this establishes a gradient of Aurora B activity, and proper kinetochore–microtubule attachments move the KMN network away from Aurora B activity thus stabilizing the binding.16,18 In addition to this spatial regulation of kinetochore–microtubule interactions dictated by the Aurora B gradient, protein phosphatase 2A (PP2A) in complex with B56 regulatory subunits directly counteracts Aurora B at the outer kinetochore.19 Indeed, BubR1 recruits B56–PP2A to kinetochores, and the binding of BubR1 to B56–PP2A is stimulated by Cdk1- and Plk1-mediated phosphorylation of BubR1 specifically at kinetochores.20-23 A complex interplay between major mitotic kinases and phosphatases thus regulates microtubule binding to kinetochores.
Recruitment of Mps1 to Kinetochores
The Mps1 kinase is a master regulator of the SAC and its kinase activity is required for kinetochore recruitment of all downstream components. Mps1 and the Aurora B kinase constitute the most upstream components of the checkpoint.24-26 Aurora B is part of the chromosomal passenger complex (CPC) and is concentrated at the centromere; this concentration depends on interactions between the survivin and borealin CPC components and histone H3 phosphorylated on Thr3 and histone H2A phosphorylated on Thr120, respectively.27 Aurora B activity is required for the SAC and stimulates the rapid recruitment of Mps1 to the kinetochore, resulting in the recruitment of the Bub1 kinase that phosphorylates H2A on Thr120, thus creating a positive feedback loop stimulating further Mps1 recruitment.24-26,28 The major function of Aurora B is to stimulate the recruitment of Mps1 as artificial recruitment of Mps1 to the kinetochore bypasses the need for Aurora B in the checkpoint.24,26
The N-terminal 200 amino acids of Mps1 are critical for its kinetochore localization and contain an N-terminal extension (NTE) followed by a tetratricopeptide repeat (TPR) domain.29,30 TPR domains are found at the N-terminus of Mps1, Bub1, and BubR1 and are α-helical protein–protein interaction modules (Fig. 2). Both the NTE and TPR domains of Mps1 contribute to kinetochore localization, and the ability of Aurora B to stimulate Mps1 recruitment depends on this domain.29 Indeed, in the absence of the TPR domain Mps1 localization is no longer stimulated by Aurora B. Based on this finding, it was proposed that the TPR domain could inhibit the ability of the NTE to bind kinetochores and that this inhibition is relieved by Aurora B.29 Interestingly, autophosphorylated Mps1 accumulates to lower levels on kinetochores suggesting that Mps1 autophosphorylation might negatively regulate the NTE-TPR kinetochore-targeting module. Understanding the details of how Aurora B and Mps1 activity regulates Mps1 kinetochore localization will be important given that this is the initiating event in the SAC.
Mps1 kinetochore localization also depends on the Ndc80 complex. A direct interaction between Mps1 and the CH domain of the Ndc80 protein has been observed in budding yeast and appears to be conserved in humans.29,31 Moreover, the role of the Ndc80 CH domain in localizing Mps1 to kinetochores is in agreement with the observed requirement of this domain in the checkpoint.32 The fact that the Ndc80 CH domain directly contacts microtubules suggests that upon microtubule attachment the Mps1 binding site is blocked. Testing and validating this model requires that we understand the interaction between Ndc80 and Mps1 in more detail.
The KNL1 MELTing pot
Once Mps1 is located and active at kinetochores, it stimulates recruitment of the Bub1–Bub3 complex, which is needed for recruitment of BubR1–Bub3 and Mad1–Mad2. Bub1 kinase activity is not required for the SAC but is needed for proper chromosome segregation.2 Recent elegant work from a number of laboratories has clarified how Mps1 phosphorylation of the outer kinetochore protein KNL1 stimulates Bub1–Bub3 recruitment and thus BubR1–Bub3.33-35 KNL1 has previously been identified as the kinetochore receptor of Bub1 and BubR1, and earlier studies showed that 2 distinct KI motifs located in the N-terminal region of human KNL1 make contact specifically with the TPR domains of Bub1 or BubR1 (Fig. 3 box 1).36,37 The KI motifs are named after their consensus sequence (Lys-Ile-(Asp/Asn)-X-X-X-Phe-(Leu/Ile)-X-X-Leu-Lys) and bind a ridge on the convex side of the Bub1 and BubR1 TPR domains.38,39 It is, however, clear that the KI motif interactions are dispensable for Bub1 and BubR1 kinetochore localization, suggesting that additional mechanisms contribute.38-40 The breakthrough came when it was shown that Mps1 phosphorylates so-called Met-Glu-Leu-Thr (MELT) motifs in KNL1 and that this event is required for Bub1 kinetochore localization (Fig. 3).33-35 Binding to phosphorylated MELT motifs (MELTp) depends on Bub3, explaining why Bub1 and BubR1 need to bind to Bub3 in order to localize.41 The role of Bub3 became crystal clear when the structure of a ternary complex composed of budding yeast Bub1–Bub3 in complex with a phosphorylated form of the second MELT motif of Spc105 (the budding yeast homolog of KNL1) was solved.42 This structure showed that the residues of the MELTp motif interact almost exclusively with Bub3 and that the phospho-threonine residue of the MELTp motif directly faces the positive charge of 2 arginine residues of Bub3, thus explaining the increased affinity for phosphorylated MELT motifs. However, even though the interaction with the MELTp motif of KNL1 mainly relies on Bub3, Bub1 strongly increases the affinity of this interaction.33,42 The structure shows that this increase in affinity provided by Bub1 is due to stabilizing effects on the Bub3 MELTp binding region and direct contact between MELTp and Bub1.42
Figure 3.
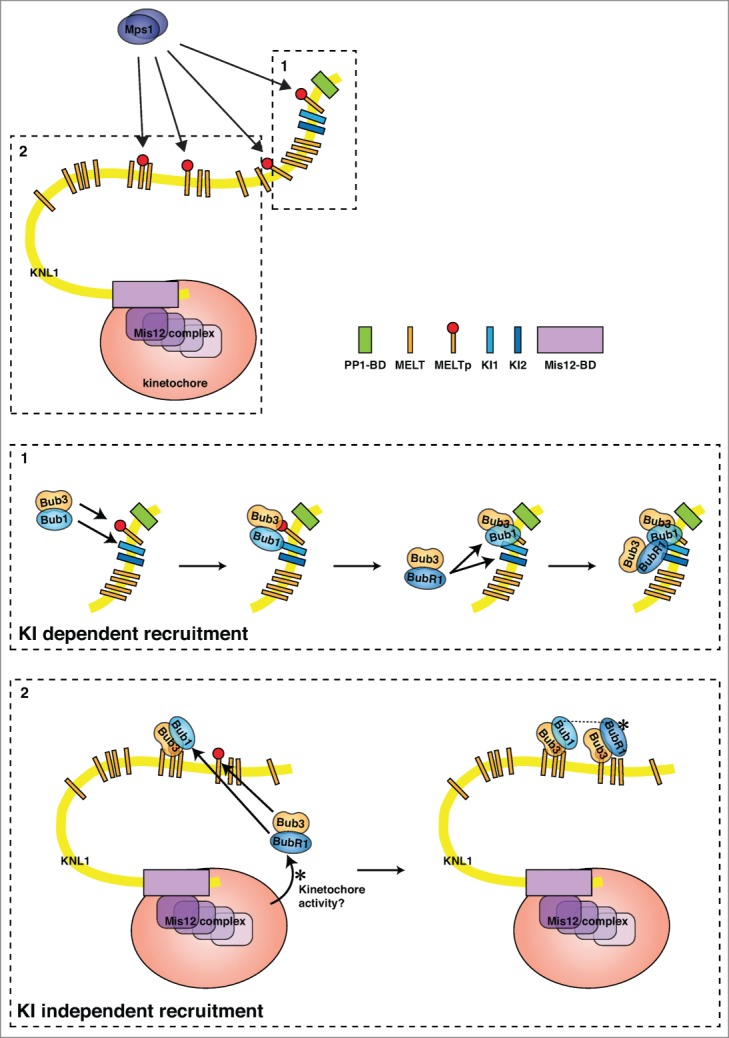
Bub protein interactions with KNL1. A schematic of the KNL1 protein showing the KI motifs, MELT motifs, and PP1 binding site (PP1-BD) as well as its domain for interaction with the Mis12 complex (Mis12-BD). Section 1 highlights the interactions centered on the N-terminal KI motifs and the phosphorylated MELT motif next to them. Bub1 interacts specifically with KI1, with Bub3 binding to the phosphorylated MELT motif; this facilitates BubR1-Bub3 binding through a BubR1–KI2 interaction. Section 2 highlights interactions involving phosphorylated MELT motifs that do not have neighboring KI motifs. Here, Bub1–Bub3 is recruited through an interaction between Bub3 and phosphorylated MELT motifs and similar interactions are also involved in BubR1–Bub3 recruitment. However, additional kinetochore-localized activities as well as Bub1 are required for kinetochore localization of BubR1.
Human KNL1 contains 12 MELT motifs and at least 7 MELT-like sequences, all located in the N-terminal half of the protein.43-45 The fact that a defining feature of KNL1 proteins appears to be the presence of numerous MELT motifs immediately poses the question of why there are so many. Recent studies indicate that KNL1 can recruit multiple Bub1–Bub3 and BubR1–Bub3 complexes and that the number recruited correlates with the number of MELT motifs.44-46 This argues that several, if not all, of the MELT motifs are capable of binding Bub3-containing complexes. Surprisingly, there appears to be a large degree of redundancy and flexibility since a KNL1 protein with a limited number of MELT motifs or engineered MELT motifs is fully functional in supporting chromosome segregation and the SAC.44,46 Chromosome alignment appears to be much more sensitive to the level of Bub protein recruited than the SAC and thus very low levels of Bub1 and BubR1 have to be present at kinetochores to generate a functional SAC signal. Although the collective conclusion appears to be that KNL1 might not need all its binding sites for the Bub proteins, it remains to be determined whether the individual MELT motifs have distinct roles under certain conditions.
The Bub Balance
Given the fact that Bub1 stimulates the recruitment of Aurora B whereas BubR1 recruits the B56–PP2A phosphatase to counteract Aurora B activity, it is likely that the exact amount and ratio of Bub1 and BubR1 proteins have to be precisely controlled to fine-tune Aurora B activity.20-22,27,28 Regulating the ratio of Bub1 to BubR1 would be difficult if both proteins used exactly the same mechanism of kinetochore localization. However, BubR1 is subjected to a higher level of regulation, as it requires Bub1 for its localization to the kinetochore.47,48 The exact reason for this is unclear but it is possible that BubR1, in contrast to Bub1, does not contribute to MELTp binding, making the affinity for MELTp too low for kinetochore recruitment. BubR1 can bind to the N-terminal region of KNL1 containing the first MELT motif only if both KIs are present.44,45 However, its binding to the other MELT motifs of KNL1 does not seem to rely on the KI motifs but instead requires the kinetochore environment (Fig. 3).44,45 Given that only one MELT motif is located in proximity to the KI domains, it has been proposed that Bub1–Bub3 makes contact with the MELTp motif and Bub1 stabilizes this interaction by binding to KI1. The bound Bub1–Bub3 can in turn allow subsequent loading of the BubR1–Bub3 complex, with BubR1 interacting with KI2 and possibly Bub1.45 Whether BubR1 needs the priming loading of Bub1 to be able to interact with the other MELTp repeats is not clear although some insight into this is provided by the protein BuGZ, a recently described Bub3 chaperone that regulates Bub3 stability.49,50 Similar to Bub1 and BubR1, BuGZ can directly bind Bub3 through its GLEBS motif and its knockdown reduces Bub3 protein levels, resulting in less Bub3 at the kinetochore. Interestingly, this leads to a reduction in the level of Bub1, but not of BubR1, at kinetochores.49 As a consequence, cells with reduced levels of BuGZ show alignment defects as a result of the reduced Aurora B activity at the kinetochore but have a functional checkpoint. It will be important to clarify whether BuGZ differentially regulates Bub1 and BubR1 localization and whether there is an excess of Bub1 to BubR1 on kinetochores that allows BubR1 levels to be unaffected despite decreasing Bub1 levels. There is clearly more to be learned about the role of BuGZ and Bub1 in kinetochore recruitment of BubR1.
Getting the Mads on Board
The loading onto kinetochores of the tetrameric Mad1–Mad2 complex, composed of a stable Mad1 dimer with each member bound to a Mad2 molecule, is the event that finally engages the SAC. The kinetochore provides a unique environment for activation of the complex and indeed artificial re-recruitment of the complex to kinetochores after the SAC has been silenced is sufficient to re-engage the checkpoint.51-53 This re-engagement of the checkpoint, which could normally occur at anaphase when kinetochore-microtubule tension is lost, is prevented by making Mad1–Mad2 localization dependent on high levels of cyclin B1-Cdk1 activity.54,55 The exact role of cyclin B1-Cdk1 activity in the SAC is yet to be determined but it is possible that the complex phosphorylates kinetochore or checkpoint proteins to allow Mad1–Mad2 recruitment.
How the Mad1–Mad2 complex interacts with the kinetochore is still somewhat enigmatic and it appears that different organisms might use different recruitment mechanisms. Two recent studies have pointed to Bub1 as the direct receptor for the Mad1–Mad2 complex. This is in line with original observations from the Hardwick laboratory that a larger Mad1–Mad2–Bub1–Bub3 complex forms during an active checkpoint in budding yeast.56 Building on this, London and Biggins found that in budding yeast, the middle part of Bub1 is the direct kinetochore receptor for Mad1–Mad2 and that the interaction depends on Mps1 phosphorylation of this region of Bub1.57 This region of Bub1 encompasses the conserved motif 1 (cm1) that is required for Mad1 localization and the SAC in human cells and fission yeast.48,58 Whether the cm1 of Bub1 directly binds the Mad1–Mad2 complex, potentially by binding the conserved Arg-Leu-Lys (RLK) motif in the C-terminus of Mad1 that is required for Mad1 kinetochore localization, is not clear.56,59 Moreover, the interaction between Mad1–Mad2 and Bub1 depends on Mad2, raising the possibility that Bub1 might also contact and regulate Mad2.56,57 A direct role of Bub1 in Mad1–Mad2 recruitment is also supported by recent work from the Desai laboratory in Caenorhabditis elegans.60 Here, the interaction depends on the C-terminal region of Bub1 encompassing the kinase domain and residues in the central part of the coiled-coil region of Mad1. As C. elegans lacks an Mps1 homolog, it is not clear whether the interaction is regulated by a different kinase or a different mechanism.
The kinetochore localization of Mad1–Mad2 in human cells is stimulated by Bub1 but appears to be more complex than simply a direct interaction with Bub1.48,59 In human cells and Drosophila, the Rod–ZW10–Zwilch (RZZ) complex has been shown to be required for Mad1 kinetochore localization, but whether this is through direct binding to Mad1 is not clear (Fig. 4A).61,62 The RZZ complex localizes dynein, a minus-end directed microtubule motor, to kinetochores through the adaptor protein spindly. This allows dynein to remove Mad1–Mad2 from kinetochores once they have attached to microtubules (Fig. 4B).63 It is possible that a combined binding interface composed of Bub1 and the RZZ complex mediates the interaction with Mad1 in human cells. Recent work from the Stukenberg laboratory has linked the centromeric protein CENP-I to protection of the Mad1–Mad2 complex from premature stripping from kinetochores that have not established mature microtubule attachments.64 It will be interesting to understand how CENP-I regulates this and further clarify the interactions of Mad1 with the RZZ complex and Bub1.
Figure 4.
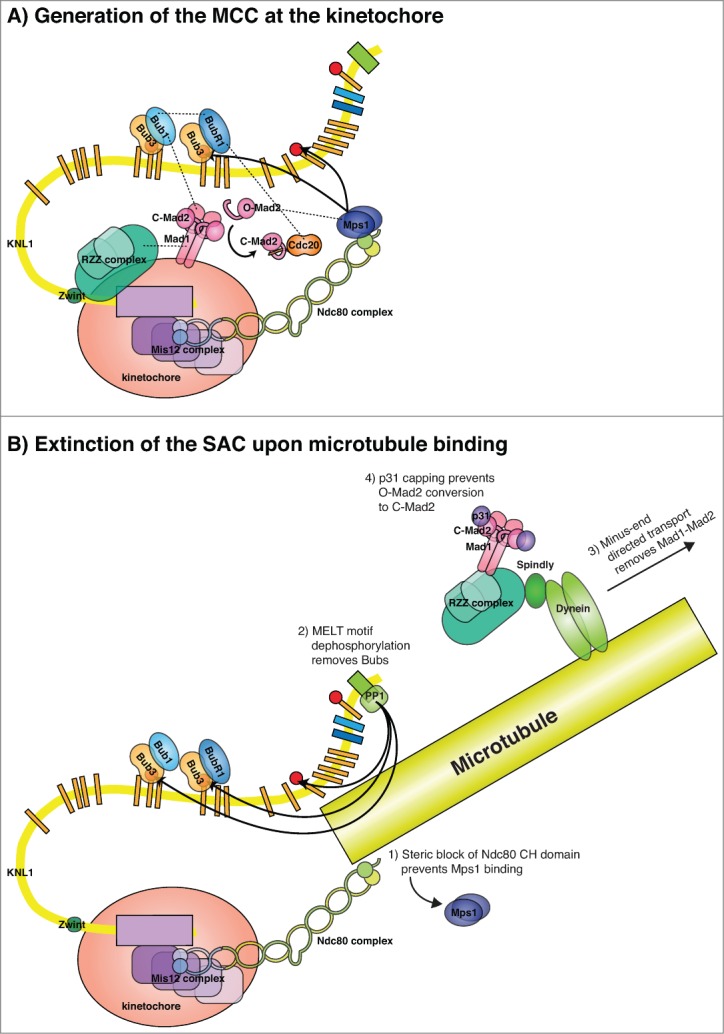
Generation of the MCC at kinetochores and silencing of this signal. (A) A schematic of the KMN network and the interaction of checkpoint proteins with this. Interactions between the RZZ complex and Bub1 could facilitate the localization of Mad1–Mad2 in human cells and an interaction between Cdc20 and BubR1 could position Cdc20 close to MCC components. The proper positioning of all MCC components, potentially scaffolded by a Mad1–Bub1 interaction, catalyzes the loading of soluble O-Mad2 onto Cdc20, locking Mad2 in its closed conformation (C-Mad2), and BubR1 subsequently binds. Kinetochore localized Mps1 might also directly regulate Mad2. (B) Several mechanisms might contribute to the removal of SAC proteins from the kinetochores once they have bound microtubules including (1) blocking of the Mps1 binding site on Ndc80, (2) dephosphorylation of the MELT motifs by PP1, (3) removal of the Mad1–Mad2 complex by dynein-mediated transport, and (4) capping of C-Mad2 by p31.
Generation of the MCC at Kinetochores
As previously mentioned, the kinetochore localization of the Mad1–Mad2 complex is essential for its activity in the SAC and recent work has shown that SAC strength correlates with the number of Mad2-positive kinetochores.65,66 Although we do not fully understand the molecular events that lead to MCC generation at kinetochores, the rate-limiting step is the binding of Mad2 to Cdc20. Mad2 exists in at least 2 extreme conformations, open (O-Mad2) and closed (C-Mad2), the latter being able to bind Mad1 and Cdc20. The “template model” for Mad2 activation suggests that the kinetochore localized Mad1–Mad2 complex recruits O-Mad2 to kinetochores through dimerization with C-Mad2 bound to Mad1 and this stimulates the conversion of soluble O-Mad2 into soluble C-Mad2 that can then bind Cdc20 (Fig. 4A).67 These interactions might be directly regulated by Mps1 at kinetochores as Mps1 stimulates O-Mad2 recruitment in human cells and directly phosphorylates Mad2 to allow its incorporation into checkpoint complexes in fission yeast.68,69 Indeed, O-Mad2 activation has been reconstituted with purified proteins but the low rates measured in vitro might suggest that kinetochores provide additional layers of catalysis.70,71 One clue to this is the recent observation that Mad1 plays a role in the SAC in addition to recruiting C-Mad2, and that this role depends on its C-terminal domain and the RLK motif.53,58,72 This suggests that Mad1 coordinates the assembly of larger checkpoint complex assemblies at kinetochores and that these are essential for efficient SAC signaling. Indeed, even when Mad1 is artificially tethered to kinetochores there is still a need for Bub1, the cm1 domain of Bub1, and Mps1 to mount an efficient checkpoint.51,58,72 As BubR1 kinetochore localization is also needed for efficient SAC signaling and BubR1 helps to recruit Cdc20, it is possible that a Mad1–Bub1 interaction precisely coordinates the positioning of all 3 MCC components at kinetochores to allow efficient complex formation (Fig. 4A).40,73 Ultimate testing of this will require biochemical reconstitution and biosensors that can monitor MCC formation spatially and temporally within cells.
MCC Interactions and Inhibition of the APC/C
Binding of Mad2 to Cdc20 stimulates the binding of BubR1 by several mechanisms. First, the crystal structure of the fission yeast MCC reveals that a contact between the Mad2 dimerization surface and BubR1 helps to position the N-terminal KEN box of BubR1 to allow Cdc20 binding.74 KEN boxes are destruction motifs that are recognized by Cdc20, and the N-terminal BubR1 KEN box is fully conserved and essential for MCC formation and the SAC.75,76 Second, Mad2 prevents binding of the N-terminal tail of Cdc20 to its own WD40 domain, thus exposing the BubR1 binding site.77 Despite these important functions of Mad2 in stabilizing the BubR1–Cdc20 interaction, it appears that once this interaction is established Mad2 can leave the MCC.77 This was elegantly demonstrated by the Cleveland laboratory who showed that controlled removal of Mad2 once the MCC had formed still resulted in robust APC/C inhibition, arguing that the Cdc20–BubR1–Bub3 complex is a potent inhibitor.77 These in vivo experiments were performed in the absence of the p31comet protein that acts to continually remove Mad2 from the MCC, thus under conditions where p31comet is present there would likely be a continual need for Mad2 to counterbalance the continual dissociation of the MCC.
The MCC exists both in a free state and stably bound to the APC/C, and stable interaction of the MCC with the APC/C is important for a functional SAC.5,78 BubR1 and the IR motif of Cdc20 contribute to stable MCC binding to the APC/C whereas Mad2 opposes this as the Mad2 binding site of Cdc20 is also required for APC/C interaction and activation.78-80 The exact details of MCC interaction with the APC/C are still unclear but with recent advancements in APC/C and MCC structures and their docking into the cryo-EM map of the APC/C-MCC complex we now have a good understanding of how the MCC inhibits the APC/C.5,7,74 One surprising observation is that MCC binding actually induces the active conformation of the APC/C; however, a number of mechanisms prevent APC/C–MCC activity. Within the MCC, Cdc20 is displaced from its normal position so that it no longer forms a combined binding site for D-boxes and therefore cannot bind substrates.5,7 The second KEN-box of BubR1 further prevents substrate binding by an unknown mechanism.40 In addition, the MCC contacts the catalytic APC2–APC11 module, potentially preventing interaction with the E2-ubiquitin complex and providing further inhibition.7 Given the strong inhibition of the APC/C by the MCC, it is puzzling why apo-APC/C also exists in cells and it will be important to determine whether distinct forms of the APC/C exist and whether only some of these have to be inhibited.
SAC Silencing
The rapid activation of APC/C–Cdc20 in response to attachment of the last kinetochore suggests that Cdc20 is quickly liberated from inhibition. This is because MCC disassembly is constantly occurring even during an active checkpoint, and as soon as MCC production ceases free Cdc20 accumulates.81,82 The generation of active Cdc20 consists of at least 2 steps, namely killing of the kinetochore signal and disassembly of existing MCC complexes.
Silencing the kinetochore signal requires the removal of the checkpoint proteins from the kinetochore. As discussed, one mechanism is the dynein-mediated removal of Mad1–Mad2, but the Mad1–Mad2 complex can also be removed from kinetochores in the absence of kinetochore–microtubule interactions. In yeast there is no RZZ complex and Mad1–Mad2 removal is coupled with Bub1 removal.43 The Mad1–Mad2 complex is also inhibited by “capping” of C-Mad2 by p31comet, which prevents binding of O-Mad2 to the complex once it is removed from kinetochores.83 In addition, protein phosphatase 1 (PP1) is required for SAC silencing, and this activity requires its interaction with kinetochores. KNL1 contains a conserved PP1 binding site close to the region of KNL1 that interacts with microtubules, and binding of PP1 to KNL1 contributes to SAC silencing.35,46,84-87 An obvious target of KNL1-bound PP1 is the phosphorylated MELT motifs and indeed increased kinetochore levels of Bub1 and BubR1 are observed when the PP1 binding site is removed.46 A model for PP1-mediated SAC silencing is thus dephosphorylation of MELT motifs to remove Bub1 and BubR1 from kinetochores (Fig. 4B). The binding of PP1 to KNL1 appears to be tightly regulated so that PP1 only strongly accumulates at kinetochores in metaphase.85 The PP1 binding site on KNL1 contains a phosphorylation site for Aurora B, which when phosphorylated prevents PP1 binding.85 Thus, PP1 and Aurora B antagonize each other on the outer kinetochore and when microtubules bind the balance tips toward PP1 binding and SAC silencing. Furthermore, as microtubule binding might prevent Mps1–Ndc80 interactions this would further favor removal of Bub1 and BubR1 from KNL1. In humans and C. elegans, preventing PP1 binding to KNL1 has a mild effect on SAC silencing, which might suggest that additional phosphatases play an important role in these organisms.46,87
MCC and APC/C–MCC are stable complexes and their disassembly is an active process. The exact mechanism of disassembly, or indeed whether free MCC and APC/C-bound MCC disassemble by the same mechanism, is not clear. At least 2 distinct pathways have been suggested to remove Mad2 from Cdc20: a p31comet-catalyzed mechanism and APC/C-mediated ubiquitination of Cdc20. p31comet binds specifically to C-Mad2 at the same dimerization surface as O-Mad2 and BubR1, therefore p31comet could facilitate MCC disassembly by preventing Mad2–BubR1 interactions within the MCC and destabilizing the entire complex.74,82,88 To facilitate efficient C-Mad2 removal p31comet might collaborate with the AAA-ATPase TRIP13.89,90 Why this then leads to selective removal of Mad2 and not BubR1 from the MCC is unclear, but as discussed above it appears that once the BubR1–Cdc20 interaction is established Mad2 is no longer needed. An interesting observation is that p31comet acts very inefficiently on APC/C-bound MCC, suggesting that the entire MCC might have to dissociate from the APC/C before p31comet can act on it.82 A second proposed mechanism of MCC dissociation, which would be specific for APC/C bound MCC, is ubiquitination of Cdc20 by the APC/C, a process regulated by APC15.79,91-93 APC15 is a small APC/C subunit close to the region of APC8 that likely engages the C-box of co-activators, and removal of APC15 leads to elevated levels of Cdc20 and MCC on the APC/C. Similar observations have been obtained with the budding yeast homolog Mnd2.7,79,91,92 Although different studies on APC15 and Mnd2 agree on a role in SAC silencing, they differ over whether this involves Cdc20 ubiquitination. One cautionary note is that since APC15 RNAi or Mnd2 deletion results in elevated total levels of Cdc20 and thus MCC, it is difficult to compare these to the wild-type situation. It might be that the cell has to reduce MCC levels below a certain threshold before anaphase is allowed and so even similar rates of MCC disassembly would result in slower mitotic exit if the initial MCC levels were higher. It will be important to clarify whether there is a threshold level of MCC that prevents anaphase and how this relates to free Cdc20 levels.
Concluding Remarks
Since the discovery of the SAC and its tight link to kinetochore status we now have a very detailed picture of the molecular events leading to APC/C inhibition. However, the more we understand, the more we realize how complex the system is and how much there still is to learn. Given the complexity of the kinetochore and the fact that that it might scaffold large checkpoint complex assemblies in a unique manner, it will be a challenge to study checkpoint protein interactions using traditional biochemical approaches. It might necessary to design novel quantitative tools to look at interactions in vivo before we can fully understand this checkpoint.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
We want to thank members of the laboratory for fruitful discussions and Dan Hayward and Marie Larsen for comments on the manuscript. We apologize to the people whose work we could not cite due to space constraints.
Funding
Work in the lab is funded by The Novo Nordisk Foundation, The Lundbeck Foundation, The Research Council for Independent Research, and the Danish Cancer Society.
References
- 1. Cheeseman IM. The kinetochore. Cold Spring Harb Perspect Biol 2014; 6:a015826;PMID:24984773; http://dx.doi.org/ 10.1101/cshperspect.a015826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lara-Gonzalez P, Westhorpe FG, Taylor SS. The spindle assembly checkpoint. Curr Biol 2012; 22:R966–80; PMID:23174302; http://dx.doi.org/ 10.1016/j.cub.2012.10.006 [DOI] [PubMed] [Google Scholar]
- 3. Maresca TJ, Salmon ED. Welcome to a new kind of tension: translating kinetochore mechanics into a wait-anaphase signal. J Cell Sci 2010; 123:825–35; PMID:20200228; http://dx.doi.org/ 10.1242/jcs.064790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sudakin V, Chan GK, Yen TJ. Checkpoint inhibition of the APCC in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J Cell Biol 2001; 154:925–36; PMID:11535616; http://dx.doi.org/ 10.1083/jcb.200102093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herzog F, Primorac I, Dube P, Lénárt P, Sander B, Mechtler K, Stark H, Peters J-M. Structure of the anaphase-promoting complexcyclosome interacting with a mitotic checkpoint complex. Science 2009; 323:1477–81; PMID:19286556; http://dx.doi.org/ 10.1126/science.1163300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pines J. Cubism and the cell cycle: the many faces of the APCC. Nat Rev Mol Cell Biol 2011; 12:427–38; PMID:21633387; http://dx.doi.org/ 10.1038/nrm3132 [DOI] [PubMed] [Google Scholar]
- 7. Chang L, Zhang Z, Yang J, McLaughlin SH, Barford D. Molecular architecture and mechanism of the anaphase-promoting complex. Nature 2014; 513:388-93; PMID:25043029; http://dx.doi.org/ 10.1038/nature13543 [Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rieder CL, Cole RW, Khodjakov A, Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J Cell Biol 1995; 130:941-8; PMID:7642709; http://dx.doi.org/ 10.1083/jcb.130.4.941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Howell BJ, Moree B, Farrar EM, Stewart S, Fang G, Salmon ED. Spindle checkpoint protein dynamics at kinetochores in living cells. Curr Biol 2004; 14:953-64; PMID:15182668; http://dx.doi.org/ 10.1016/j.cub.2004.05.053 [DOI] [PubMed] [Google Scholar]
- 10. Shah JV, Botvinick E, Bonday Z, Furnari F, Berns M, Cleveland DW. Dynamics of centromere and kinetochore proteins; implications for checkpoint signaling and silencing. Curr Biol 2004; 14:942-52; PMID:15182667 [DOI] [PubMed] [Google Scholar]
- 11. Kallio M, Weinstein J, Daum JR, Burke DJ, Gorbsky GJ. Mammalian p55CDC mediates association of the spindle checkpoint protein Mad2 with the cyclosomeanaphase-promoting complex, and is involved in regulating anaphase onset and late mitotic events. J Cell Biol 1998; 141:1393-406; PMID:9628895; http://dx.doi.org/ 10.1083/jcb.141.6.1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Varma D, Wan X, Cheerambathur D, Gassmann R, Suzuki A, Lawrimore J, Desai A, Salmon ED. Spindle assembly checkpoint proteins are positioned close to core microtubule attachment sites at kinetochores. J Cell Biol 2013; 202:735-46; PMID:23979716; http://dx.doi.org/ 10.1083/jcb.201304197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wan X, O’Quinn RP, Pierce HL, Joglekar AP, Gall WE, Deluca JG, Carroll CW, Liu S-T, Yen TJ, McEwen BF, et al. Protein architecture of the human kinetochore microtubule attachment site. Cell 2009; 137:672-84; PMID:19450515; http://dx.doi.org/ 10.1016/j.cell.2009.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 2006; 127:983-97; PMID:17129783; http://dx.doi.org/ 10.1016/j.cell.2006.09.039 [DOI] [PubMed] [Google Scholar]
- 15. Nilsson J. Looping in on Ndc80 - how does a protein loop at the kinetochore control chromosome segregation? Bioessays 2012; 34:1070-7; PMID:23154893; http://dx.doi.org/ 10.1002/bies.201200096 [DOI] [PubMed] [Google Scholar]
- 16. Welburn JPI, Vleugel M, Liu D, Yates JR, Lampson MA, Fukagawa T, Cheeseman IM. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol Cell 2010; 38:383-92; PMID:20471944; http://dx.doi.org/ 10.1016/j.molcel.2010.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deluca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 2006; 127:969-82; PMID:17129782; http://dx.doi.org/ 10.1016/j.cell.2006.09.047 [DOI] [PubMed] [Google Scholar]
- 18. Liu D, Vader G, Vromans MJM, Lampson MA, Lens SMA. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science 2009; 323:1350-3; PMID:19150808; http://dx.doi.org/ 10.1126/science.1167000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foley EA, Maldonado M, Kapoor TM. Formation of stable attachments between kinetochores and microtubules depends on the B56-PP2A phosphatase. Nat Cell Biol 2011; 13:1265-71; PMID:21874008; http://dx.doi.org/ 10.1038/ncb2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu P, Raetz EA, Kitagawa M, Virshup DM, Lee SH. BUBR1 recruits PP2A via the B56 family of targeting subunits to promote chromosome congression. Biol Open 2013; 2:479-86; PMID:23789096; http://dx.doi.org/ 10.1242/bio.20134051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kruse T, Zhang G, Larsen MSY, Lischetti T, Streicher W, Nielsen TK, Bjørn SP, Nilsson J. Direct binding between BubR1 and B56-PP2A phosphatase complexes regulate mitotic progression. J Cell Sci 2013; 126: 1086-92; PMID:23345399; http://dx.doi.org/ 10.1242/jcs.122481 [DOI] [PubMed] [Google Scholar]
- 22. Suijkerbuijk SJE, Vleugel M, Teixeira A, Kops GJPL. Integration of kinase and phosphatase activities by BUBR1 ensures formation of stable kinetochore-microtubule attachments. Dev Cell 2012; 23:745-55; PMID:23079597; http://dx.doi.org/ 10.1016/j.devcel.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 23. Elowe S, Dulla K, Uldschmid A, Li X, Dou Z, Nigg EA. Uncoupling of the spindle-checkpoint and chromosome-congression functions of BubR1. J Cell Sci 2010; 123:84-94; PMID:20016069; http://dx.doi.org/ 10.1242/jcs.056507 [DOI] [PubMed] [Google Scholar]
- 24. Saurin AT, van der Waal MS, Medema RH, Lens SMA, Kops GJPL. Aurora B potentiates Mps1 activation to ensure rapid checkpoint establishment at the onset of mitosis. Nat Commun 2011; 2:316; PMID:21587233; http://dx.doi.org/ 10.1038/ncomms1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Santaguida S, Vernieri C, Villa F, Ciliberto A, Musacchio A. Evidence that Aurora B is implicated in spindle checkpoint signalling independently of error correction. EMBO J 2011; 30:1508-19; PMID:21407176; http://dx.doi.org/ 10.1038/emboj.2011.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heinrich S, Windecker H, Hustedt N, Hauf S. Mph1 kinetochore localization is crucial and upstream in the hierarchy of spindle assembly checkpoint protein recruitment to kinetochores. J Cell Sci 2012; 125:4720-7; PMID:22825872; http://dx.doi.org/ 10.1242/jcs.110387 [DOI] [PubMed] [Google Scholar]
- 27. van der Horst A, Lens SMA. Cell division: control of the chromosomal passenger complex in time and space. Chromosoma 2014; 123:25-42; PMID:24091645; http://dx.doi.org/ 10.1007/s00412-013-0437-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Waal MS, Saurin AT, Vromans MJM, Vleugel M, Wurzenberger C, Gerlich DW, Medema RH, Kops GJPL, Lens SMA. Mps1 promotes rapid centromere accumulation of Aurora B. EMBO Rep 2012; 13: 847-54; PMID:22732840; http://dx.doi.org/ 10.1038/embor.2012.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nijenhuis W, Castelmur von E, Littler D, De Marco V, Tromer E, Vleugel M, van Osch MHJ, Snel B, Perrakis A, Kops GJPL. A TPR domain-containing N-terminal module of MPS1 is required for its kinetochore localization by Aurora B. J Cell Biol 2013; 201:217-31; PMID:23569217; http://dx.doi.org/ 10.1083/jcb.201210033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thebault P, Chirgadze DY, Dou Z, Blundell TL, Elowe S, Bolanos-Garcia VM. Structural and functional insights into the role of the N-terminal Mps1 TPR domain in the SAC (spindle assembly checkpoint). Biochem J 2012; 448:321-8; PMID:23067341; http://dx.doi.org/ 10.1042/BJ20121448 [DOI] [PubMed] [Google Scholar]
- 31. Kemmler S, Stach M, Knapp M, Ortiz J, Pfannstiel J, Ruppert T, Lechner J. Mimicking Ndc80 phosphorylation triggers spindle assembly checkpoint signalling. EMBO J 2009; 28:1099-110; PMID:19300438; http://dx.doi.org/ 10.1038/emboj.2009.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guimaraes GJ, Dong Y, McEwen BF, Deluca JG. Kinetochore-microtubule attachment relies on the disordered N-terminal tail domain of Hec1. Curr Biol 2008; 18:1778-84; PMID:19026543; http://dx.doi.org/ 10.1016/j.cub.2008.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamagishi Y, Yang C-H, Tanno Y, Watanabe Y. MPS1Mph1 phosphorylates the kinetochore protein KNL1Spc7 to recruit SAC components. Nat Cell Biol 2012; 14:746-52; PMID:22660415; http://dx.doi.org/ 10.1038/ncb2515 [DOI] [PubMed] [Google Scholar]
- 34. Shepperd LA, Meadows JC, Sochaj AM, Lancaster TC, Zou J, Buttrick GJ, Rappsilber J, Hardwick KG, Millar JBA. Phosphodependent recruitment of Bub1 and Bub3 to Spc7KNL1 by Mph1 kinase maintains the spindle checkpoint. Curr Biol 2012; 22: 891-9; PMID:22521786; http://dx.doi.org/ 10.1016/j.cub.2012.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. London N, Ceto S, Ranish JA, Biggins S. Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr Biol 2012; 22: 900-6; PMID:22521787; http://dx.doi.org/ 10.1016/j.cub.2012.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kiyomitsu T, Obuse C, Yanagida M. Human BlinkinAF15q14 is required for chromosome alignment and the mitotic checkpoint through direct interaction with Bub1 and BubR1. Dev Cell 2007; 13:663-76; PMID:17981135; http://dx.doi.org/ 10.1016/j.devcel.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 37. Kiyomitsu T, Murakami H, Yanagida M. Protein interaction domain mapping of human kinetochore protein Blinkin reveals a consensus motif for binding of spindle assembly checkpoint proteins Bub1 and BubR1. Mol Cell Biol 2011; 31:998-1011; PMID:21199919; http://dx.doi.org/ 10.1128/MCB.00815-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krenn V, Wehenkel A, Li X, Santaguida S, Musacchio A. Structural analysis reveals features of the spindle checkpoint kinase Bub1-kinetochore subunit Knl1 interaction. J Cell Biol 2012; 196:451-67; PMID:22331848; http://dx.doi.org/ 10.1083/jcb.201110013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bolanos-Garcia VM, Lischetti T, Matak-Vinković D, Cota E, Simpson PJ, Chirgadze DY, Spring DR, Robinson CV, Nilsson J, Blundell TL. Structure of a Blinkin-BUBR1 complex reveals an interaction crucial for kinetochore-mitotic checkpoint regulation via an unanticipated binding site. Structure 2011; 19:1691-700; PMID:22000412; http://dx.doi.org/ 10.1016/j.str.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lara-Gonzalez P, Scott MIF, Diez M, Sen O, Taylor SS. BubR1 blocks substrate recruitment to the APCC in a KEN-box-dependent manner. J Cell Sci 2011; 124:4332-45; PMID:22193957; http://dx.doi.org/ 10.1242/jcs.094763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taylor SS, Ha E, McKeon F. The human homologue of Bub3 is required for kinetochore localization of Bub1 and a Mad3Bub1-related protein kinase. J Cell Biol 1998; 142:1-11; PMID:9660858; http://dx.doi.org/ 10.1083/jcb.142.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Primorac I, Weir JR, Chiroli E, Gross F, Hoffmann I, van Gerwen S, Ciliberto A, Musacchio A. Bub3 reads phosphorylated MELT repeats to promote spindle assembly checkpoint signaling. Elife 2013; 2:e01030; PMID:24066227; http://dx.doi.org/ 10.7554/eLife.01030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vleugel M, Hoogendoorn E, Snel B, Kops GJPL. Evolution and function of the mitotic checkpoint. Dev Cell 2012; 23:239-50; PMID:22898774; http://dx.doi.org/ 10.1016/j.devcel.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 44. Vleugel M, Tromer E, Omerzu M, Groenewold V, Nijenhuis W, Snel B, Kops GJPL. Arrayed BUB recruitment modules in the kinetochore scaffold KNL1 promote accurate chromosome segregation. J Cell Biol 2013; 203:943-55; PMID:24344183; http://dx.doi.org/ 10.1083/jcb.201307016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krenn V, Overlack K, Primorac I, van Gerwen S, Musacchio A. KI motifs of human Knl1 enhance assembly of comprehensive spindle checkpoint complexes around MELT repeats. Curr Biol 2014; 24:29-39; PMID:24361068; http://dx.doi.org/ 10.1016/j.cub.2013.11.046 [DOI] [PubMed] [Google Scholar]
- 46. Zhang G, Lischetti T, Nilsson J. A minimal number of MELT repeats supports all functions of KNL1 in chromosome segregation. J Cell Sci 2013; 127:871-84; PMID:24363448; http://dx.doi.org/ 10.1242/jcs.139725 [DOI] [PubMed] [Google Scholar]
- 47. Meraldi P, Draviam VM, Sorger PK. Timing and checkpoints in the regulation of mitotic progression. Dev Cell 2004; 7:45-60; PMID:15239953; http://dx.doi.org/ 10.1016/j.devcel.2004.06.006 [DOI] [PubMed] [Google Scholar]
- 48. Klebig C, Korinth D, Meraldi P. Bub1 regulates chromosome segregation in a kinetochore-independent manner. J Cell Biol 2009; 185:841-58; PMID:19487456; http://dx.doi.org/ 10.1083/jcb.200902128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Toledo CM, Herman JA, Olsen JB, Ding Y, Corrin P, Girard EJ, Olson JM, Emili A, Deluca JG, Paddison PJ. BuGZ is required for Bub3 stability, Bub1 kinetochore function, and chromosome alignment. Dev Cell 2014; 28: 282-94; PMID:24462187; http://dx.doi.org/ 10.1016/j.devcel.2013.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jiang H, He X, Wang S, Jia J, Wan Y, Wang Y, Zeng R, Yates J, Zhu X, Zheng Y. A microtubule-associated zinc finger protein, BuGZ, regulates mitotic chromosome alignment by ensuring Bub3 stability and kinetochore targeting. Dev Cell 2014; 28: 268-81; PMID:24462186; http://dx.doi.org/ 10.1016/j.devcel.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maldonado M, Kapoor TM. Constitutive Mad1 targeting to kinetochores uncouples checkpoint signalling from chromosome biorientation. Nat Cell Biol 2011; 13:475-82; PMID:21394085; http://dx.doi.org/ 10.1038/ncb2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kuijt TEF, Omerzu M, Saurin AT, Kops GJPL. Conditional targeting of MAD1 to kinetochores is sufficient to reactivate the spindle assembly checkpoint in metaphase. Chromosoma 2014; 123:471-80; ahead of print; PMID:24695965; http://dx.doi.org/ 10.1007/s00412-014-0458-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ballister ER, Riegman M, Lampson MA. Recruitment of Mad1 to metaphase kinetochores is sufficient to reactivate the mitotic checkpoint. J Cell Biol 2014; 204:901-8; PMID:24637323; http://dx.doi.org/ 10.1083/jcb.201311113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rattani A, Vinod PK, Godwin J, Tachibana-Konwalski K, Wolna M, Malumbres M, Novak B, Nasmyth K. Dependency of the spindle assembly checkpoint on Cdk1 renders the anaphase transition irreversible. Curr Biol 2014; 24:630-7; PMID:24583015; http://dx.doi.org/ 10.1016/j.cub.2014.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vázquez-Novelle MD, Sansregret L, Dick AE, Smith CA, McAinsh AD, Gerlich DW, Petronczki M. Cdk1 inactivation terminates mitotic checkpoint surveillance and stabilizes kinetochore attachments in anaphase. Curr Biol 2014; 24: 638-45; PMID:24583019; http://dx.doi.org/ 10.1016/j.cub.2014.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brady DM, Hardwick KG. Complex formation between Mad1p, Bub1p and Bub3p is crucial for spindle checkpoint function. Curr Biol 2000; 10:675-8; PMID:10837255; http://dx.doi.org/ 10.1016/S0960-9822(00)00515-7 [DOI] [PubMed] [Google Scholar]
- 57. London N, Biggins S. Mad1 kinetochore recruitment by Mps1-mediated phosphorylation of Bub1 signals the spindle checkpoint. Genes Dev 2014; 28: 140-52; PMID:24402315; http://dx.doi.org/ 10.1101/gad.233700.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Heinrich S, Sewart K, Windecker H, Langegger M, Schmidt N, Hustedt N, Hauf S. Mad1 contribution to spindle assembly checkpoint signalling goes beyond presenting Mad2 at kinetochores. EMBO Rep 2014; 15: 291-8; PMID:24477934; http://dx.doi.org/ 10.1002/embr.201338114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim S, Sun H, Tomchick DR, Yu H, Luo X. Structure of human Mad1 C-terminal domain reveals its involvement in kinetochore targeting. Proc Natl Acad Sci USA 2012; 109: 6549-54; PMID:22493223; http://dx.doi.org/ 10.1073/pnas.1118210109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Moyle MW, Kim T, Hattersley N, Espeut J, Cheerambathur DK, Oegema K, Desai A. A Bub1-Mad1 interaction targets the Mad1-Mad2 complex to unattached kinetochores to initiate the spindle checkpoint. J Cell Biol 2014; 204: 647-57; PMID:24567362; http://dx.doi.org/ 10.1083/jcb.201311015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kops GJPL, Kim Y, Weaver BAA, Mao Y, McLeod I, Yates JR, Tagaya M, Cleveland DW. ZW10 links mitotic checkpoint signaling to the structural kinetochore. J Cell Biol 2005; 169:49-60; PMID:15824131; http://dx.doi.org/ 10.1083/jcb.200411118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Karess R. Rod-Zw10-Zwilch: a key player in the spindle checkpoint. Trends Cell Biol 2005; 15:386-92; PMID:15922598; http://dx.doi.org/ 10.1016/j.tcb.2005.05.003 [DOI] [PubMed] [Google Scholar]
- 63. Barisic M, Geley S. Spindly switch controls anaphase: spindly and RZZ functions in chromosome attachment and mitotic checkpoint control. Cell Cycle 2011; 10:449-56; PMID:21252629; http://dx.doi.org/ 10.4161/cc.10.3.14759 [DOI] [PubMed] [Google Scholar]
- 64. Matson DR, Stukenberg PT. CENP-I and Aurora B act as a molecular switch that ties RZZMad1 recruitment to kinetochore attachment status. J Cell Biol 2014; 205:541-54; PMID:24862574; http://dx.doi.org/ 10.1083/jcb.201307137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Collin P, Nashchekina O, Walker R, Pines J. The spindle assembly checkpoint works like a rheostat rather than a toggle switch. Nat Cell Biol 2013; 15:1378-85; PMID:24096242; http://dx.doi.org/ 10.1038/ncb2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dick AE, Gerlich DW. Kinetic framework of spindle assembly checkpoint signalling. Nat Cell Biol 2013; 15:1370-7; PMID:24096243; http://dx.doi.org/ 10.1038/ncb2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. De Antoni A, Pearson CG, Cimini D, Canman JC, Sala V, Nezi L, Mapelli M, Sironi L, Faretta M, Salmon ED, et al. The Mad1Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr Biol 2005; 15:214-25; PMID:15694304; http://dx.doi.org/ 10.1016/j.cub.2005.01.038 [DOI] [PubMed] [Google Scholar]
- 68. Hewitt L, Tighe A, Santaguida S, White AM, Jones CD, Musacchio A, Green S, Taylor SS. Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1-C-Mad2 core complex. J Cell Biol 2010; 190:25-34; PMID:20624899; http://dx.doi.org/ 10.1083/jcb.201002133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zich J, Sochaj AM, Syred HM, Milne L, Cook AG, Ohkura H, Rappsilber J, Hardwick KG. Kinase activity of fission yeast Mph1 is required for Mad2 and Mad3 to stably bind the anaphase promoting complex. Curr Biol 2012; 22:296-301; PMID:22281223; http://dx.doi.org/ 10.1016/j.cub.2011.12.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kulukian A, Han JS, Cleveland DW. Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev Cell 2009; 16:105-17; PMID:19154722; http://dx.doi.org/ 10.1016/j.devcel.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Simonetta M, Manzoni R, Mosca R, Mapelli M, Massimiliano L, Vink M, Novak B, Musacchio A, Ciliberto A. The influence of catalysis on mad2 activation dynamics. PLoS Biol 2009; 7:e10; PMID:19143472; http://dx.doi.org/ 10.1371/journal.pbio.1000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kruse T, Larsen MSY, Sedgwick GG, Sigurdsson JO, Streicher W, Olsen JV, Nilsson J. A direct role of Mad1 in the spindle assembly checkpoint beyond Mad2 kinetochore recruitment. EMBO Rep 2014; 15: 282-90; PMID:24477933; http://dx.doi.org/ 10.1002/embr.201338101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li D, Morley G, Whitaker M, Huang J-Y. Recruitment of Cdc20 to the kinetochore requires BubR1 but not Mad2 in Drosophila melanogaster. Mol Cell Biol 2010; 30:3384-95; PMID:20421417; http://dx.doi.org/ 10.1128/MCB.00258-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chao WCH, Kulkarni K, Zhang Z, Kong EH, Barford D. Structure of the mitotic checkpoint complex. Nature 2012; 484:208-13; PMID:22437499; http://dx.doi.org/ 10.1038/nature10896 [DOI] [PubMed] [Google Scholar]
- 75. King EMJ, van der Sar SJA, Hardwick KG. Mad3 KEN boxes mediate both Cdc20 and Mad3 turnover, and are critical for the spindle checkpoint. PLoS ONE 2007; 2:e342; PMID:17406666; http://dx.doi.org/ 10.1371/journal.pone.0000342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Burton JL, Solomon MJ. Mad3p, a pseudosubstrate inhibitor of APCCdc20 in the spindle assembly checkpoint. Genes Dev 2007; 21:655-67; PMID:17369399; http://dx.doi.org/ 10.1101/gad.1511107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Han JS, Holland AJ, Fachinetti D, Kulukian A, Cetin B, Cleveland DW. Catalytic assembly of the mitotic checkpoint inhibitor BubR1-Cdc20 by a Mad2-induced functional switch in Cdc20. Mol Cell 2013; 51: 92-104; PMID:23791783; http://dx.doi.org/ 10.1016/j.molcel.2013.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hein JB, Nilsson J. Stable MCC binding to the APCC is required for a functional spindle assembly checkpoint. EMBO Rep 2014; 15: 264-72; PMID; http://dx.doi.org/ 10.1002/embr.201337496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Foster SA, Morgan DO. The APCC subunit Mnd2Apc15 promotes Cdc20 autoubiquitination and spindle assembly checkpoint inactivation. Mol Cell 2012; 47:921-32; PMID:22940250; http://dx.doi.org/ 10.1016/j.molcel.2012.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Izawa D, Pines J. Mad2 and the APCC compete for the same site on Cdc20 to ensure proper chromosome segregation. J Cell Biol 2012; 199:27-37; PMID:23007648; http://dx.doi.org/ 10.1083/jcb.201205170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Varetti G, Guida C, Santaguida S, Chiroli E, Musacchio A. Homeostatic control of mitotic arrest. Mol Cell 2011; 44:710-20; PMID:22152475; http://dx.doi.org/ 10.1016/j.molcel.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 82. Westhorpe FG, Tighe A, Lara-Gonzalez P, Taylor SS. p31comet-mediated extraction of Mad2 from the MCC promotes efficient mitotic exit. J Cell Sci 2011; 124:3905-16; PMID:22100920; http://dx.doi.org/ 10.1242/jcs.093286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fava LL, Kaulich M, Nigg EA, Santamaria A. Probing the in vivo function of Mad1:C-Mad2 in the spindle assembly checkpoint. EMBO J 2011; 30:3322-36; PMID:21772247; http://dx.doi.org/ 10.1038/emboj.2011.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rosenberg JS, Cross FR, Funabiki H. KNL1Spc105 recruits PP1 to silence the spindle assembly checkpoint. Curr Biol 2011; 21:942-7; PMID:21640906; http://dx.doi.org/ 10.1016/j.cub.2011.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu D, Vleugel M, Backer CB, Hori T, Fukagawa T, Cheeseman IM, Lampson MA. Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J Cell Biol 2010; 188:809-20; PMID:20231380; http://dx.doi.org/ 10.1083/jcb.201001006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Meadows JC, Shepperd LA, Vanoosthuyse V, Lancaster TC, Sochaj AM, Buttrick GJ, Hardwick KG, Millar JBA. Spindle checkpoint silencing requires association of PP1 to both Spc7 and kinesin-8 motors. Dev Cell 2011; 20:739-50; PMID:21664573; http://dx.doi.org/ 10.1016/j.devcel.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Espeut J, Cheerambathur DK, Krenning L, Oegema K, Desai A. Microtubule binding by KNL-1 contributes to spindle checkpoint silencing at the kinetochore. J Cell Biol 2012; 196:469-82; PMID:22331849; http://dx.doi.org/ 10.1083/jcb.201111107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yang M, Li B, Tomchick DR, Machius M, Rizo J, Yu H, Luo X. p31comet blocks Mad2 activation through structural mimicry. Cell 2007; 131:744-55; PMID:18022368; http://dx.doi.org/ 10.1016/j.cell.2007.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Eytan E, Wang K, Miniowitz-Shemtov S, Sitry-Shevah D, Kaisari S, Yen TJ, Liu S-T, Hershko A. Disassembly of mitotic checkpoint complexes by the joint action of the AAA-ATPase TRIP13 and p31comet. Proc Natl Acad Sci USA 2014; 12901; PMID:25139987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang K, Sturt-Gillespie B, Hittle JC, Macdonald D, Chan GK, Yen TJ, Liu S-T. Thyroid hormone receptor interacting protein 13 (TRIP13) AAA-ATPase is a novel mitotic checkpoint-silencing protein. J Biol Chem 2014; 289:23928-37; PMID:25012665; http://dx.doi.org/ 10.1074/jbc.M114.585315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mansfeld J, Collin P, Collins MO, Choudhary JS, Pines J. APC15 drives the turnover of MCC-CDC20 to make the spindle assembly checkpoint responsive to kinetochore attachment. Nat Cell Biol 2011; 13:1234-43; PMID:21926987; http://dx.doi.org/ 10.1038/ncb2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Uzunova K, Dye BT, Schutz H, Ladurner R, Petzold G, Toyoda Y, Jarvis MA, Brown NG, Poser I, Novatchkova M, et al. APC15 mediates CDC20 autoubiquitylation by APCC(MCC) and disassembly of the mitotic checkpoint complex. Nat Struct Mol Biol 2012; 19: 1116-23; PMID:23007861; http://dx.doi.org/ 10.1038/nsmb.2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Reddy SK, Rape M, Margansky WA, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature 2007; 446:921-5; PMID:17443186; http://dx.doi.org/ 10.1038/nature05734 [DOI] [PubMed] [Google Scholar]