Abstract
Despite being a common feature of human cancer, the role of supernumerary centrosomes in tumourigenesis is still poorly understood. We have recently described a novel role for centrosome amplification in promoting cell invasion that could impact tumor progression.
Keywords: cancer, centrosomes, invasion, RAC1
In 1914, Theodor Boveri proposed that increased numbers of centrosomes caused cancer through the generation of aneuploidy.1 Indeed, a correlation between centrosome amplification and aneuploidy has long been recognized in human tumors and a mechanism linking extra centrosomes to chromosome missegregation and aneuploidy has recently been described.2 Importantly, extra centrosomes may also impact cell physiology independently of chromosome segregation. Previous work showed that defects in asymmetric cell division induced by extra centrosomes are associated with tumourigenesis in a fly transplantation model.3 However, many questions remain unanswered regarding the contribution of supernumerary centrosomes to tumor progression.
In several tumor types, centrosome amplification has been correlated with tumor grade, malignancy and lymph node metastasis, although the reason for this association is unknown. Using three-dimensional (3-D) culture models, we recently found that centrosome amplification is sufficient to confer invasive characteristics to non-transformed epithelial cells.4 This observation explains, at least in part, the observed correlation between centrosome amplification and increased malignancy in human tumors. Centrosome amplification, whether generated by transient overexpression of the centrosomal kinase Polo-like kinase 4 (PLK4) or induced by cytokinesis failure, promotes the formation of invasive protrusions in mammary epithelial cells, which requires the degradation of components of the extracellular matrix (ECM), such as collagen-I and laminin-V. Live-cell imaging demonstrated that these invasive structures are highly dynamic and that they provide a “track” through which cells can invade the surrounding microenvironment.4 This phenotype is reminiscent not only of oncogene-induced cell invasion,4 but also of the “collective cell invasion” observed in in vivo mouse models5 and in human tumor fragments grown in 3-D matrices.6 This suggests that extra centrosomes induce a bona fide invasive phenotype.
The invasive phenotype exhibited in cells with extra centrosomes is not due to aneuploidy. Depletion of the kinesin MCAK (Mitotic-Centromere Associated Kinesin), which induces aneuploidy, was not sufficient to promote invasion. Furthermore, single-nucleotide polymorphism (SNP) analysis of cells grown in 3-D culture did not detect a recurrent aneuploidy in cells with supernumerary centrosomes.4 Therefore, we conclude that aneuploidy per se cannot explain the invasive phenotype observed.
A clue about why cells with extra centrosomes are invasive came from observing their migratory properties. In contrast to normal epithelial cells, cells with supernumerary centrosomes display a “cell scattering” phenotype, which is often associated with defects in cell-cell adhesion. Using micropatterns to assess the integrity of cell-cell adhesion, we found that, similar to loss of the cell adhesion molecule p120 catenin, extra centrosomes compromise cell junction stability.4 Such defects have previously been linked to an increased activity of the small GTPase RAC1, which is associated with invasion and metastasis.7 Indeed, we found that cells with extra centrosomes had enhanced levels of active RAC1. Inhibition of RAC1 suppressed both cell-cell adhesion defects and the invasive phenotype of cells with extra centrosomes, further supporting a role for RAC1 activation in this process.4
In interphase, extra centrosomes are mostly clustered to form an enlarged centrosome. We found that these enlarged centrosomes have increased γ-tubulin levels and microtubule nucleation capacity.4 Because microtubule polymerization was previously shown to induce RAC1 activation,8 we hypothesized that in cells with extra centrosomes enhanced RAC1 activity occurs downstream of microtubules. Indeed, inhibition of microtubule dynamics with the microtubule stabilizing drug Taxol prevented RAC1 activation in cells with extra centrosomes. Likewise, knockdown of the centrosomal protein CEP192, which plays important roles in the recruitment of γ-tubulin to the centrosome in interphase, abolished RAC1 activation. CEP192 depletion also suppressed defects in cell-cell contacts and the invasive phenotype, strongly suggesting that increased microtubule nucleation downstream of extra centrosomes is essential to mediate invasion.4
The mechanism by which microtubules regulate RAC1 has not yet been determined. It is possible that microtubules coordinate the local delivery of RAC1 activators (Guanine Nucleotide Exchange Factors - GEFs) at the cell cortex to promote local RAC1 activation.9 We propose a model by which local RAC1 activation, mediated by microtubules, initiates the formation of the invasive structure (Fig. 1A). Local enhancement of RAC1 activity would trigger a cascade of events resulting in further polarization of the microtubule cytoskeleton and the formation of the invasive protrusion. In fact, active RAC1 can promote microtubule stabilization via inhibition of the microtubule destabiliser protein stathmin.9 This suggests that a positive feedback mechanism involving RAC1 activation could facilitate the initiation and extension of the invasive protrusion. Remarkably, multicellular 3-D structures with supernumerary centrosomes typically form one predominant protrusion (Fig. 1B),4 suggesting that mechanisms preventing the formation of multiple protrusions exist. Indeed, during border cell migration in Drosophila embryos, a leading cell with higher RAC1 activity generates directional movement, while inhibiting the formation of protrusions from other cells in the cluster to promote efficient migration.10 It would be interesting to determine if similar mechanisms are in place during cancer cell invasion.
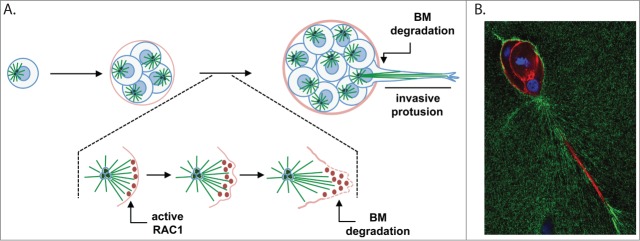
Figure 1.
Centrosome amplification induces the formation of invasive protrusions. (A) Schematic representation of mammary epithelial cells (MCF10A) with extra centrosomes growing in 3-D cultures. Single cells plated on top of a mix containing matrigel:collagen-I will divide and form small spheres, called acini. In cells with extra centrosomes, acini will form long, invasive protrusions that are able to degrade the basement membrane (BM). This process requires the activation of RAC1 downstream of microtubules in cells with extra centrosomes. We propose that localized activation of RAC1 is determined by the polarization of the microtubule cytoskeleton toward the cell cortex. After the initial activation of RAC1, changes in the cell cortex, such as Arp2/3-mediated actin polymerization and lamellipodia formation, will determine the formation of the invasive protrusion. (B) Invasive acini containing extra centrosomes. Cells were stained for F-actin (red), fibronectin (green) and DNA (blue). One predominant invasive protrusion can usually be observed in these acini. Increased accumulation of fibronectin surrounding the invasive protrusions can also be detected.
We have provided the first evidence for a role of supernumerary centrosomes in cell invasion. Our findings imply that, at least in certain tumor types, centrosome amplification could directly contribute to tumor malignancy by facilitating cell invasion. Our work also suggests that other cancer mutations associated with changes in the microtubule cytoskeleton could have similar effects on RAC1 activity and invasion. Thus, understanding the frequency of increased microtubule nucleation in cancer would be important. Developing drugs to specifically modulate microtubule nucleation could potentially diminish the harmful effects of centrosome amplification or other cancer mutations in promoting invasion in cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Boveri T. Concerning the origin of malignant tumours by Theodor Boveri. Translated and annotated by Henry Harris. J Cell Sci 2008; 121 Suppl 1:1-84; PMID:18089652; http://dx.doi.org/ 10.1242/jcs.025742 [DOI] [PubMed] [Google Scholar]
- 2. Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature 2009; 460:278-82; PMID:19506557; http://dx.doi.org/ 10.1038/nature08136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Basto R, Brunk K, Vinadogrova T, Peel N, Franz A, Khodjakov A, Raff JW. Centrosome amplification can initiate tumorigenesis in flies. Cell 2008; 133:1032-42; PMID:18555779; http://dx.doi.org/ 10.1016/j.cell.2008.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Godinho SA, Picone R, Burute M, Dagher R, Su Y, Leung CT, Polyak K, Brugge JS, Thery M, Pellman D. Oncogene-like induction of cellular invasion from centrosome amplification. Nature 2014; PMID:24739973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friedl P, Locker J, Sahai E, Segall JE. Classifying collective cancer cell invasion. Nat Cell Biol 2012; 14:777-83; PMID:22854810; http://dx.doi.org/ 10.1038/ncb2548 [DOI] [PubMed] [Google Scholar]
- 6. Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 2013; 155:1639-51; PMID:24332913; http://dx.doi.org/ 10.1016/j.cell.2013.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mack NA, Whalley HJ, Castillo-Lluva S, Malliri A. The diverse roles of Rac signaling in tumorigenesis. Cell Cycle 2011; 10:1571-81; PMID:21478669; http://dx.doi.org/ 10.4161/cc.10.10.15612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat Cell Biol 1999; 1:45-50; PMID:10559863; http://dx.doi.org/ 10.1038/9018 [DOI] [PubMed] [Google Scholar]
- 9. Siegrist SE, Doe CQ. Microtubule-induced cortical cell polarity. Genes Dev 2007; 21:483-96; PMID:17344411; http://dx.doi.org/ 10.1101/gad.1511207 [DOI] [PubMed] [Google Scholar]
- 10. Wang X, He L, Wu YI, Hahn KM, Montell DJ. Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nat Cell Biol 2010; 12:591-7; PMID:20473296; http://dx.doi.org/ 10.1038/ncb2061 [DOI] [PMC free article] [PubMed] [Google Scholar]