Abstract
Asymmetric cell division (ACD) is a characteristic of cancer stem cells, which exhibit high malignant potential. However, the cellular mechanisms that regulate symmetric (self-renewal) and asymmetric cell divisions are mostly unknown. Using human neuroblastoma cells, we found that the oncosuppressor protein tripartite motif containing 32 (TRIM32) positively regulates ACD.
Keywords: asymmetric cell division, cancer stem cells, MYCN, TRIM32
Studies performed in recent years strongly suggest that small fractions of “cancer stem cells” or “cancer-initiating cells” exist in tumor cell populations and that these cells exhibit resistance to many anticancer drugs and produce many heterogeneous tumor cell populations, resulting in a high frequency of tumor recurrence and metastasis.1
It is now believed that cancer stem cells undergo asymmetric cell division (ACD),2 which is a physiological event of normal stem cells and occurs during development and tissue homeostasis in of a large variety of organisms. ACD is a very smart strategy because it maintains appropriate numbers of self-renewal stem cells and differentiated cells in a single division. Therefore, ACD keeps the balance between the stem cell pool and the progenitor cell pool. Recent studies showed that misregulation of the balance between self-renewal and differentiation by ACD in Drosophila melanogaster neuroblasts may lead to the emergence of abnormal stem cells, resulting in tumorigenesis.3,4 Thus, like normal stem cells, cancer stem cells also apply ACD as a strategy for producing both cancer stem cells and many differentiated cancer cells. However, whether human cancer stem cells conduct ACD in an evolutionarily conserved manner and the underlying mechanism are largely unknown.
We are analyzing the mechanism of ACD using a series of human neuroblastoma cultured cells as a model system. Neuroblastoma is one of the most common childhood solid tumors and has a broad spectrum of clinical behavior.5 Of the many genetic and biochemical features of neuroblastomas, MYCN oncogene amplification has been correlated with an aggressive phenotype and a poor outcome. Recent studies have revealed that MYCN oncoprotein not only shows oncogenic activity but also plays a central role in self-renewal of normal neural stem and precursor cells.5 In fact, neuroblastoma cells are derived from multipotent neural crest cells and have a cancer stem cell-like property due to aberrant MYCN expression. In addition, although cultured human neuroblastoma cells show unlimited growth, they differentiate to neuronal cells upon treatment with retinoids such as all-trans-retinoic acid (ATRA) and 13-cis-retinoic acid. Thus, cultured human neuroblastoma cells show cancer stem cell-like properties because they have both proliferation and differentiation capacity.5 We therefore supposed that human neuroblastoma cells might be a good model system for studying ACD.
ACD research is being actively performed by genetic analysis using model organisms such as Caenorhabditis elegans embryos and Drosophila melanogaster neuroblasts.3,4,6 These powerful genetic studies revealed that the machinery of ACD is highly evolutionarily conserved.4,6 Analogous to ACD studies using these lower organisms, we study ACD using human neuroblastoma cultured cells. In 2012, we found that one of the cell polarity cues, nuclear mitotic apparatus protein (NuMA), preferentially localizes to one side of the cell cortex during late mitosis.7 In addition, expression of the MYCN gene is involved in the regulation of cell division fate; cells with a single copy of MYCN show significantly higher percentages of ACD than those with MYCN amplification. Moreover, suppression of MYCN in MYCN-amplified cells causes ACD, whereas over expression of MYCN in MYCN non-amplified cells enhances symmetric cell division (self-renewal division). Moreover, transcriptional activity of MYCN is important for inducing self-renewal division in human neuroblastoma cells.7
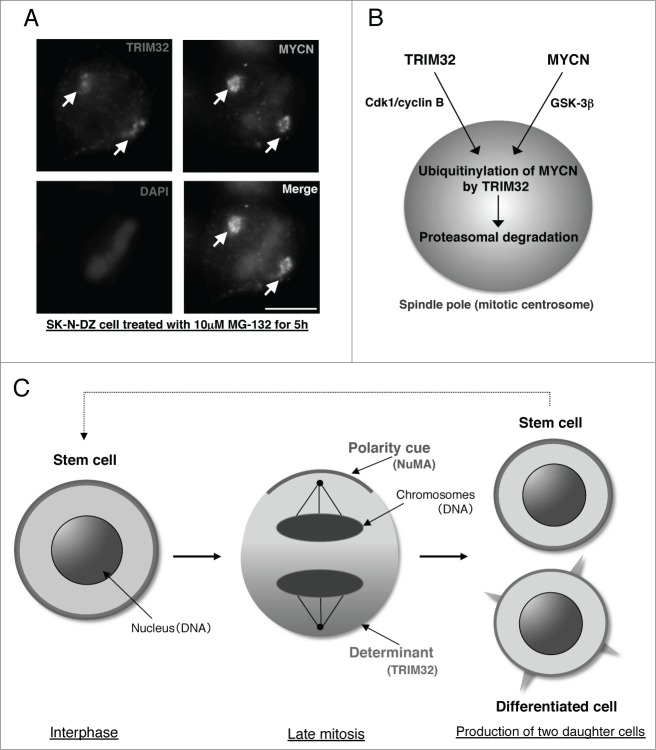
We subsequently focused on the functions of tripartite motif containing 32 (TRIM32), an ortholog of the Drosophila protein Brat, which participates in ACD as a neural determinant and inhibits Drosophila MYC (dMYC) function in fly neuroblasts.8 In addition, it is also reported that mouse TRIM32 has ubiquitin ligase activity, thus facilitating degradation of c-MYC oncoprotein in neurogenesis.9 Using human neuroblastoma cells, we recently discovered a new mechanism of ACD.10 Surprisingly, we found that MYCN accumulates at spindle poles as a result of phosphorylation by glycogen synthase kinase-3β (GSK-3β) during mitosis (Fig. 1A and B). In parallel, TRIM32 is recruited to spindle poles by cyclin-dependent kinase 1 (CDK1)/cyclin B-mediated phosphorylation (Fig. 1A and B). Thus, the interaction of TRIM32 with MYCN at spindle poles during mitosis facilitates proteasomal degradation of MYCN and induces ACD in human neuroblastoma cells (Fig. 1B). Importantly, c-MYC does not accumulate at spindle poles in c-MYC gene-amplified (SK-BR-3) and c-MYC-overexpressing cell lines (HeLa and U251-MG).10
Figure 1.
A mechanism of asymmetric cell division in human neuroblastoma cells. (A) Immunostaining of SK-N-DZ cells treated with 10 μM MG-132 (a proteasome inhibitor) for 5 h. TRIM32 is red, MYCN is green, and DAPI (DNA) is blue. Scale bar shows 5 μm.10 (B) Recruitment of TRIM32 and MYCN to spindle poles during mitosis. TRIM32 is recruited to spindle poles (mitotic centrosomes) by Cdk1/cyclin B signaling. In parallel, MYCN is accumulated at spindle pole through GSK-3β signaling. (C) Schematic model of asymmetric cell division in human neuroblastoma cells with forced expression of TRIM32.
TRIM32 also suppresses sphere formation of neuroblastoma-initiating cells,10 suggesting that ACD produces differentiated neuroblastoma cells that will eventually die. Alternatively, the induction of differentiation potential in neuroblastoma-initiating cells by TRIM32 may lead to dormancy. Either way, TRIM32 functions as a positive regulator of ACD that acts against MYCN and should be considered as a tumor suppressor in human neuroblastoma cells (Fig. 1C).10 Thus, our findings offer novel insight into the mechanisms of ACD and clarify its contributions to human tumorigenesis. We believe that cultured human neuroblastoma cells are a very useful model system that provides clues to the mechanism of ACD.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank our laboratory members for their continuous encouragement.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan and the Kawano Masanori Memorial Foundation for the Promotion of Pediatrics, Japan.
References
- 1. Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature 2013; 501:328-37; PMID:24048065; http://dx.doi.org/ 10.1038/nature12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 2006; 441:1068-74; PMID:16810241; http://dx.doi.org/ 10.1038/nature04956 [DOI] [PubMed] [Google Scholar]
- 3. Gonzalez C. Spindle orientation, asymmetric division and tumour suppression in Drosophila stem cells. Nat Rev Genet 2007; 8:462-72; PMID:17510666; http://dx.doi.org/ 10.1038/nrg2103 [DOI] [PubMed] [Google Scholar]
- 4. Knoblich JA. Asymmetric cell division: recent developments and their implications for tumour biology. Nat Rev Mol Cell Biol 2010; 11:849-60; PMID:21102610; http://dx.doi.org/ 10.1038/nrm3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang M, Weiss WA. Neuroblastoma and MYCN. Cold Spring Harb Perspect Med 2013; 3:a014415; PMID:24086065; http://dx.doi.org/ 10.1101/cshperspect.a014415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gonczy P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nat Rev Mol Cell Biol 2008; 9:355-66; PMID:18431399; http://dx.doi.org/ 10.1038/nrm2388 [DOI] [PubMed] [Google Scholar]
- 7. Izumi H, Kaneko Y. Evidence of asymmetric cell division and centrosome inheritance in human neuroblastoma cells. Proc Natl Acad Sci U S A 2012; 109:18048-53; PMID:23064640; http://dx.doi.org/ 10.1073/pnas.1205525109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Betschinger J, Mechtler K, Knoblich JA. Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell 2006; 124:1241-53; PMID:16564014; http://dx.doi.org/ 10.1016/j.cell.2006.01.038 [DOI] [PubMed] [Google Scholar]
- 9. Schwamborn JC, Berezikov E, Knoblich JA. The TRIM-NHL protein TRIM32 activates microRNAs and prevents self-renewal in mouse neural progenitors. Cell 2009; 136:913-25; PMID:19269368; http://dx.doi.org/ 10.1016/j.cell.2008.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Izumi H, Kaneko Y. Trim32 facilitates degradation of MYCN on spindle poles and induces asymmetric cell division in human neuroblastoma cells. Cancer research 2014; 74:5620-30; PMID:25100564; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-0169 [DOI] [PubMed] [Google Scholar]