Abstract
Viruses are useful tools that often reveal previously unrecognized levels of control within a cell. By studying the oncogenic Kaposi's sarcoma-associated herpesvirus (KSHV), we discovered a new signaling axis in endothelial cells (ECs) that links actin cytoskeleton dynamics to post-transcriptional control of gene expression. Translational repression and rapid decay of mRNAs containing AU-rich elements (AREs) occurs in cytoplasmic RNA granules known as processing bodies (PBs). Rho-GTPase activity influences PB dynamics but mechanistic details remain obscure. We have previously shown that the KSHV Kaposin B protein blocks the degradation of ARE-mRNAs that encode potent cytokines and angiogenic factors, at least in part by preventing PB formation. Moreover, Kaposin B is sufficient to cause marked alterations in endothelial cell physiology including the formation of long parallel actin stress fibers and accelerated migration and angiogenic phenotypes. All of these phenotypes depend on Kaposin B-mediated activation of a non-canonical signaling pathway comprising the stress-inducible kinase MK2, hsp27, p115RhoGEF and RhoA. Accelerated endothelial cell migration and angiogenesis depends on the subsequent activation of the RhoA-dependent kinase ROCK, but PB disruption is ROCK-independent. In this Commentary, we discuss implications of the activation of this signaling axis, and propose mechanistic links between RhoA activation and PB dynamics.
Keywords: cytoskeleton, Kaposi's sarcoma-associated herpesvirus, MK2, p-body, Rho-GTPase
Introduction
Kaposi's sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus-8, is the infectious cause of the AIDS-related malignancy Kaposi's sarcoma (KS).1 KS lesions are predominantly comprised of KSHV-infected proliferating endothelial cells (ECs) that display a hallmark elongated or 'spindled' morphology (Fig. 1A). KS lesions have a strong inflammatory character, with elevated levels of pro-inflammatory cytokines and angiogenic factors, and marked lymphocyte infiltration.
Figure 2.
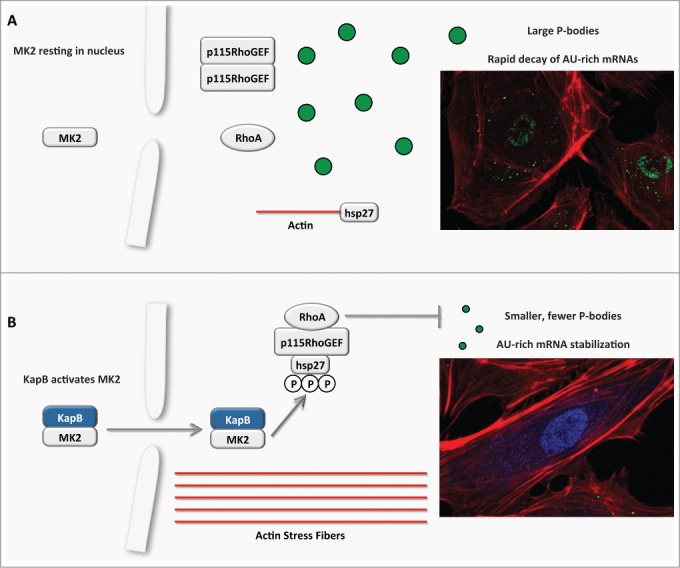
Model describing the effects of KapB in altering endothelial cell cytoskeleton and reprogramming gene expression by disrupting P-bodies and stabilizing ARE-mRNAs. (A) In the absence of environmental stress or infection, MK2 remains in the nucleus, hsp27 participates in capping actin filaments, p115RhoGEF is found in a cytosolic oligomer-induced inhibitory state, and RhoA is not active. In this resting state, p-bodies are clearly evident in the cytoplasm and participate in the decapping and degradation of labile AU-rich element (ARE) containing mRNAs. (B) KapB mimics the stress-induced MK2 activation by binding to MK2 and stimulating its kinase activity, resulting in hsp27 phosphorylation and the stimulation of p115RhoGEF activity, leading to RhoA activation. The consequences of the dual stimulation of RhoA and MK2 by KapB are as follows: 1. RhoA and ROCK-dependent formation of actin stress fibers, and migratory and angiogenic phenotype and 2. RhoA-dependent but ROCK-independent dispersal of PBs that correlates with the increased stability of ARE-containing mRNAs and the translation of their encoded proinflammatory and angiogenic protein products.
Figure 1.
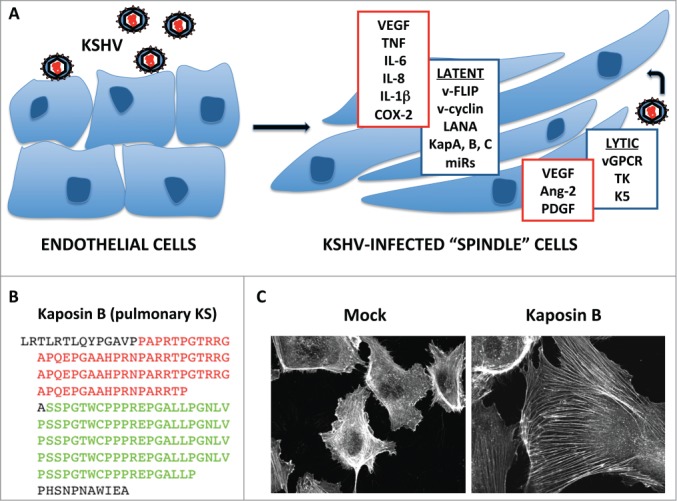
KSHV infection alters endothelial cell gene expression and physiology. (A) KSHV infection of endothelial cells (ECs) commonly results in the establishment of latency, wherein the viral genome is maintained as a circular episome in the nucleus, and gene expression is limited to products of the latency locus that include LANA, v-cyclin, v-FLIP, the Kaposins and 12 pre-miRNAs that can be processed into at least 25 mature miRNAs. In turn, these products modulate signal transduction pathways, altering the actin cytoskeleton and cell-cell contacts, which leads to the distinctive spindle shape. Reactivation from latency and expression of a wider range of viral gene products associated with lytic replication is commonly observed in KS tumors; lytic gene products include vGPCR, K5 and TK. Increased Kaposin mRNA transcription during lytic replication causes marked increases in production of Kaposin proteins. Together, KSHV latent and lytic gene products markedly alter the EC secretome, causing increased release of angiogenic factors and pro-inflammatory cytokines. (B) The Kaposin mRNA is largely comprised of 2 sets of GC-rich tandem repeats; the chief product of this transcript is Kaposin B, which comprises proline/arginine-rich repeats (red) fused to proline/leucine-rich repeats (green). (C) Kaposin B expression in primary ECs (HUVECs) is sufficient to cause the formation of actin stress fibers.
KSHV establishes persistent, life-long infection of its human host, and displays 2 modes of infection; a relatively quiescent 'latent' phase, and a 'lytic' phase marked by viral replication and release of infectious progeny. KSHV research has been greatly aided by primary EC infection models that support both latent and lytic phases of viral replication, while faithfully recapitulating many of the features of KS tumors. In the majority of KS spindle cells, or in vitro-infected primary ECs, the virus remains latent and gene expression is limited to 6 consensus protein products (LANA, v-cyclin, v-FLIP, Kaposins A, B, and C) and 12 pre-miRNAs that are processed into as many as 25 mature miRNAs.2-4 Several latent gene products have been shown to contribute to dramatic alterations in EC physiology.5-8 Meanwhile, KSHV lytic replication is thought to contribute to KS by promoting viral dissemination and the secretion of pathogenetically-important cytokines and growth factors9,10 (Fig. 1A).
Both latent and lytic KSHV gene products have been shown to modulate RhoA activity, cytoskeleton dynamics and cell morphology. RhoA is activated in the earliest stages of infection, and is required for viral entry and capsid trafficking to the nucleus.11-14 Upon establishment of latency, the latent gene product viral FLICE inhibitory protein (v-FLIP) causes potent NF-κΒ activation and contributes to cytoskeletal remodeling and spindling of ECs.6,15 Reactivation from latency causes expression of the full range of viral gene products, including a potent cell surface localized signaling protein, viral G protein-coupled receptor (vGPCR), and a viral tyrosine kinase (TK), that activate RhoA and remodel the actin cytoskeleton.16,17 Meanwhile, EC morphology and adherens junction integrity is compromised during lytic replication at least in part through the action of K5, a homolog of the MARCH family of human E3 ubiquitin ligases, which disrupts adherens junctions by proteasomal destruction of vascular endothelial (VE) cadherin18 (Fig. 1A). Thus, viral gene products elicit dramatic EC morphology changes in both latent and lytic phases of KSHV infection.
Kaposin B Activates the Stress-Responsive MK2-RhoA Signaling Axis
During latent KSHV infection, a complex translational program involving initiation at non-canonical start codons on the kaposin transcript, and decoding multiple GC-rich repeats, results in the generation of several kaposin protein products, including Kaposin B (KapB). KapB is a highly repetitive, proline-rich protein comprised largely of 2 sets of reiterated 23-amino acid direct repeats, known as DR1 and DR219,20 (Fig. 1B). A genetic screen revealed that KapB binds the host cell kinase mitogen-activated protein kinase (MAPK)-associated protein kinase 2 (MK2),20 an important downstream effector kinase in the stress-activated p38 MAPK signaling pathway that responds to extracellular inflammatory signals or environmental stress. Activated MK2 phosphorylates a variety of nuclear and cytoplasmic target proteins, including the small heat shock protein hsp27. Phosphorylated hsp27 participates in actin remodeling by forming an active complex with p115RhoGEF and RhoA.21 By binding and activating MK2, KapB achieves constitutive activation of this non-canonical MK2/hsp27/p115RhoGEF/RhoA signaling axis.22 Many studies have linked either p38/MK2 or RhoA activation to a common set of EC phenotypes that include actin stress fibers, changes to cell migration, angiogenesis and permeability (Table 1). The emergence of this new non-canonical signaling pathway may reconcile some of these disparate observations into a unified model of stress-regulated cytoskeleton control, especially as it relates to EC physiology.
Table 1.
Functional overlap between the phenotypic consequences of p38/MK2/hsp27 and RhoA/ROCK pathway activation in the literature. Several studies over the last 2 decades pinpoint the important role of these 2 pathways in the control of actin stress fiber formation, cell morphology, migration and endothelial barrier integrity. These pathways have also been shown to control gene expression via the modulation of mRNA stability and PB dispersion. However, very few studies have linked MK2 to the downstream activation of RhoA. Our recent work highlights the important connection between these 2 regulatory pathways.22 Please note that the references within this table are a mere subset of the many excellent studies performed in this field (with a focus on work performed in endothelial cells) and the table is meant to illustrate the connection between phenotypes that now link these 2 fields. We apologize to the authors whose work was not cited here due to space limitations
| Phenotype | p38/MK2/hsp27 | RhoA/ROCK |
|---|---|---|
| Cell Morphology and Actin Stress Fibers | 22,64,65,66,67,68,69,70,71,72,73,74,75,92,99 | 17,22,65,66,69,76,77,78,79,80,81,82,83,84,85,86,87,88,108 |
| Cell Migration and Invasion | 22,65,70,71,72,74,75,89,90,91,92,99 | 22,41,42,65,79,86,93,94,95,96 |
| Endothelial Barrier Dysfunction | 64,69,73,97,98,99,100,101 | 69,77,80,81,82,84,85,101,102,103,104,105,106,107,108,109,110 |
| Processing Body Dynamics | 22,28 | 22,27,28 |
| Gene Expression (mRNA decay) | 22,28,71,72,111,112,113,114,115,116,117,118,119,120,121,122 | 22,27,28,117,121,123,124 |
We are actively investigating the role of the MK2-RhoA signaling axis in KSHV infection. We have shown that stimulation of this pathway by KapB has several significant outcomes. MK2 and RhoA are both major regulatory proteins of the actin cytoskeletal network; their activation by KapB has several predictable outcomes, including changes in cell shape, polymerization of actin and rearrangement of actin filaments (Fig. 1C), cell migration and angiogenesis. All of these processes have been previously linked to RhoA activity. However, by hijacking this key regulatory signaling axis, KapB also disperses cellular PBs, which correlates with stabilization of labile AU-rich element (ARE)-containing mRNAs. Previous studies linked activation of the p38/MK2 pathway with enhanced stability of ARE-mRNAs and increased expression of ARE-mRNA products including potent pro-inflammatory cytokines, angiogenic molecules, and EC barrier regulators.23,24 MK2 phosphorylates several ARE-binding proteins (ARE-BPs), thereby enhancing ARE-mRNA stability.20,25 PBs are a major site of ARE-mRNA decay;26 our work shows that KapB mediates PB dispersion in a RhoA-dependent manner that correlates with the increased stability of ARE-mRNAs.22,27,28 Notably, KapB is not alone in activating MK2 and RhoA; we and others have shown that the lytic gene product vGPCR, a constitutively active homolog of the human CXCR2 chemokine receptor, activates MK2 via Rac1-dependent MAPK activation, and independently activates RhoA by assembly of canonical cell surface G-protein-containing signaling complexes.28 Thus, vGPCR also affects PB dynamics, ARE-mRNA stability and actin polymerization, but by distinct means. The functional relevance of the convergence of KapB and vGPCR on these common phenotypes remains unclear, but suggests that manipulation of these central nodes of stress signaling may support efficient viral replication.
Processing Bodies Control Inflammatory Mediator Release by ECs
The precise coordination of mRNA (mRNA) turnover and translation is a central feature of eukaryotic gene expression. Processing bodies (PBs) are small ribonucleoprotein (RNP)-containing cytoplasmic granules that promote the decay or translational arrest of cytoplasmic mRNA molecules. PBs regulate the constitutive decay of ARE-mRNAs,26,29-32 a class of constitutively labile mRNAs that encode potent regulatory molecules such as growth factors, pro-inflammatory cytokines, and angiogenic factors. ARE-mRNA turnover can be prevented by specific signaling events, thereby providing a mechanism for the cell to rapidly increase pools of a specific class of 'early response' mRNAs and their protein products. Thus, precise control of PB assembly and disassembly can significantly impact the expression of potent regulatory molecules.
PBs form visible cytoplasmic foci that are constitutively present in most cells and contain the requisite enzymes for rapid mRNA deadenylation, decapping and 5′-3′ exonucleolytic degradation.29,30,32-35 PBs are extremely dynamic, changing in size and number in response to cell cycle stage, nutrient availability, and stresses such as ultraviolet light (UV), osmotic shock and other inhibitors of global translation.27,33,35,36 PBs can also transiently associate and exchange cargo with stress granules (SGs), cytoplasmic foci that triage stalled translationally-competent mRNPs.34 PB assembly and disassembly is influenced by changes in the degradative capacity of the cell; for example, when 5′-3′ exonucleolytic decay is prevented, the resulting accumulation of cytoplasmic mRNA awaiting destruction causes PB size and number to increase, whereas inhibiting de novo transcription or halting translation by trapping mRNA in polysomes has the opposite effect.37,38 PBs also maintain a dynamic relationship with the cytoskeleton; stationary PBs associate with actin bundles whereas mobile PBs connect to the microtubule network.36,39,40 Though PB formation was recently shown to be modified by the cytoskeletal regulator RhoA,22,27,28 the precise mechanism of action remains to be elucidated.
RhoA as a Regulator of Gene Expression
The Rho family of small GTPases are molecular switches that cycle between inactive GDP- and active GTP-bound forms and thereby control several fundamental cellular processes. RhoA regulates actin cytoskeleton dynamics to facilitate normal cell attachment, the formation of actin stress fibers, cell migration and angiogenesis (summarized in41-46 and Table 1 and references therein). RhoA activation also couples changes to the actin cytoskeleton with increased transcription and translation under certain circumstances (described below). Additional mechanisms for RhoA-mediated control of gene expression have recently emerged in the literature, including intriguing new cytoskeleton-independent modes of control. Considering the wealth of literature on RhoA and cytoskeletal dynamics, we are actively exploring several potential models for MK2/RhoA-dependent PB dissolution.
RhoA Regulates Transcription by Modifying the Status of the Actin Cytoskeleton
RhoA controls the transcription of genes containing serum-response elements (SREs) because it modifies the balance of monomeric globular actin (G-actin) and filamentous actin (F-actin) within the cell. As a general rule, in cultured cells the ratio of G-actin to F-actin is approximately 1:1.47 RhoA activation causes increased actin polymerization and formation of stress fibers. The subsequent loss of free G-actin leads to the dissociation of the transcriptional co-activator megakaryoblastic leukemia 1 (MKL1) that normally binds G-actin monomers in the cytoplasm. Free MKL1 translocates to the nucleus and collaborates with serum response factor (SRF) to induce transcription of SRE-regulated genes, including many cytoskeletal genes. In this way, RhoA couples changes in the actin cytoskeleton to transcription control.48-50
RhoA has also been linked to transcriptional regulation by the growth-regulating Hippo pathway. In the canonical Hippo tumor suppressor pathway the Mst1/2 and Lats1/2 kinases phosphorylate pro-growth YAP/TAZ transcription factors causing their nuclear exclusion and degradation. This pathway is exquisitely sensitive to changes in RhoA and the cytoskeleton. RhoA inhibition or F-actin disruption inhibits YAP/TAZ transcription.51-53 Conversely, stabilization of the actin cytoskeleton with jasplakinolide causes YAP/TAZ activation.54 Unlike the RhoA/actin/MKL1 pathway described above, the Hippo pathway is insensitive to changes in G-actin:F-actin ratio.51 Rather, emerging evidence indicates that F-actin structure and cell morphology regulate YAP/TAZ localization and activity.51-53,55
G-actin Regulates Translation Initiation in Times of Stress
Eukaryotic cells have mechanisms to arrest protein synthesis and promote cell survival in times of stress through the action of kinases that phosphorylate eukaryotic initiation factor-2-α (eIF2α). This is known as the integrated stress response (ISR).56 When stress is resolved, the resumption of protein synthesis requires eIF2α dephosphorylation by a phosphatase complex comprised of a catalytic domain (protein phosphatase 1 [PP1]) and a regulatory domain (PPP1R15).57 Two recent papers together indicate that G-actin associates with this complex and is required for efficient dephosphorylation of eIF2α.57,58 Depletion of the G-actin pool with jasplakinolide causes PPP1R15A-PP1 complex destabilization, thereby extending the period of translation arrest. Thus, proper orchestration of the ISR requires integration of signals from the actin cytoskeleton.
A Novel Mechanism of Post-transcriptional Control of Gene Expression: RhoA–Mediated Dispersion of Cytoplasmic Processing Bodies
Through our studies of a PB-regulating virus, we discovered new links between the non-canonical stress-responsive MK2/hsp27/p115RhoGEF/RhoA signaling pathway and the regulation of PB dynamics. Our detailed investigation of the mechanism of KSHV KapB-mediated control of this pathway revealed signal bifurcation downstream of RhoA activation, leading to 2 mechanistically distinct outcomes; (i) ROCK1/2-dependent alterations in cell shape, actin polymerization and stress fiber formation, and enhanced migratory and angiogenic capacity of primary ECs, (ii) ROCK1/2-independent dissolution of PBs that correlated with increased stability and translation of normally labile ARE-mRNAs. With so little currently known about the dynamic regulation of PBs, this represents an excellent opportunity for further mechanistic studies. Here, we consider 3 possible models for RhoA-mediated PB dissolution (Table 2).
Table 2.
Models describing potential interactions between Rho-GTPases, the actin cytoskeleton and p-bodies
| Model 1 | Model 2 | Model 3 |
|---|---|---|
| PB disruption due to alterations in G-actin:F-actin ratios | PB disruption by RhoA effector proteins | PB disruption due to interference with linkage to cytoskeleton |
| RhoA activation decreases ratio of G-actin:F-actin | RhoA activation stimulates specific downstream effector proteins. | RhoA activation stimulates specific downstream effector proteins and/or cytoskeleton alterations. |
| Decreased G-actin availability affects signal transduction and/or the ISR. | Disrupts PB Formation by causing post-translational modification or depletion of key PB scaffolding proteins | Mechanical disruption interfering with PB linkages to cytoskeleton, decreasing PB formation |
| actin dependent | actin independent | actin dependent or independent |
Model 1: PB disruption due to actin-mediated translation control. Similar to RhoA-mediated control of SRE transcription and the ISR, PB disruption may depend upon RhoA-induced stress fiber formation and reduced G-actin:F-actin ratio. If so, we predict that alteration of actin dynamics in a RhoA-independent manner will also affect PB size and number. These investigations are underway and employ 2 toxins: jasplakinolide, that causes aberrant F-actin bundles to form and thus depletes cytoplasmic G-actin, and latrunculin B, which depolymerizes actin to increase G-actin levels.59 In this model, PBs could be inhibited by ISR potentiation and extended periods of translation arrest that may disrupt the bulk flow of mRNAs to nascent PBs. Alternatively, PB formation could be affected by modulation of MKL1 or Hippo pathway signal transduction (described above) in a previously unappreciated fashion.
Model 2: PB disruption by RhoA effector proteins. PB disruption could depend on downstream actions of known RhoA effector proteins, including Rho-associated kinases and Dia proteins. Recent studies have pinpointed phosphorylation of PB resident proteins as key to the control of their assembly.60-62 For example, the direct phosphorylation of PB scaffolding protein Pat1 by cAMP-dependent protein kinase PKA causes PB dissolution and reduces cell survival in times of stress.60 Similarly, several viruses have been shown to accelerate the decay of PB resident proteins, or recruit PB resident proteins to viral replication compartments to enhance replication.63 We are actively investigating whether individual PB resident proteins are degraded or modified to alter the structural scaffolds required for PB assembly.
Model 3: PB disruption due to interference with linkage to cytoskeleton. Stationary PBs associate with actin bundles whereas mobile PBs connect to the microtubule network,36,39,40 and these contact sites may be disrupted by active RhoA signaling and activation of specific downstream effector proteins. Alternatively, the formation of particular F-actin structures such as stress fibers may place a mechanical strain on the linkage between PB and cytoskeletal structures, mediating their dissociation. Time-lapse live confocal microscopy will permit precise measurements of PB dynamics in live cells, tracking the changes that occur to PBs, the actin cytoskeleton, and microtubules immediately after RhoA activation.
Viruses are excellent teachers, and the study of KSHV has shed new light on a poorly characterized MK2/hsp27/p115RhoGEF/RhoA signaling pathway, providing links to important aspects of EC physiology. The most important questions now facing us relate to links between this stress-regulated signaling pathway and control of PB formation. Elucidating these mechanistic details will expand our understanding of RhoA-mediated control of gene expression.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 1994; 266:1865-9; PMID:7997879; http://dx.doi.org/ 10.1126/science.7997879 [DOI] [PubMed] [Google Scholar]
- 2.Speck SH, Ganem D. Viral Latency and Its Regulation: Lessons from the γ-Herpesviruses. Cell Host and Microbe 2010; 8:100-15; PMID:20638646; http://dx.doi.org/ 10.1016/j.chom.2010.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arias C, Weisburd B, Stern-Ginossar N, Mercier A, Bellare P, Madrid AS, Holdorf M, Weissman JS, Ganem D. KSHV 2.0: A Comprehensive Annotation of the Kaposi's Sarcoma-Associated Herpesvirus Genome Using Next- Generation Sequencing Reveals Novel Genomic and Functional Features. PLoS Pathog 2014; 10:1-23; http://dx.doi.org/ 10.1371/journal.ppat.1003847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umbach JL, Cullen BR. In-depth analysis of Kaposi's sarcoma-associated herpesvirus microRNA expression provides insights into the mammalian microRNA-processing machinery. J Virol 2010; 84:695-703; PMID:19889781; http://dx.doi.org/ 10.1128/JVI.02013-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ojala PM, Schulz TF. Manipulation of endothelial cells by KSHV: Implications for angiogenesis and aberrant vascular differentiation. Semin Cancer Biol 2014; 26C:69-77; http://dx.doi.org/ 10.1016/j.semcancer.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 6.Grossmann C, Podgrabinska S, Skobe M, Ganem D. Activation of NF- B by the Latent vFLIP Gene of Kaposi's Sarcoma-Associated Herpesvirus Is Required for the Spindle Shape of Virus-Infected Endothelial Cells and Contributes to Their Proinflammatory Phenotype. J Virol 2006; 80:7179-85; PMID:16809323; http://dx.doi.org/ 10.1128/JVI.01603-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naranatt PP, Akula SM, Zien CA, Krishnan HH, Chandran B. Kaposi's Sarcoma-Associated Herpesvirus Induces the Phosphatidylinositol 3-Kinase-PKC- -MEK-ERK Signaling Pathway in Target Cells Early during Infection: Implications for Infectivity. J Virol 2003; 77:1524-39; PMID:12502866; http://dx.doi.org/ 10.1128/JVI.77.2.1524-1539.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciufo DM, Cannon JS, Poole LJ, Wu FY, Murray P, Ambinder RF, Hayward GS. Spindle cell conversion by Kaposi's sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J Virol 2001; 75:5614-26; PMID:11356969; http://dx.doi.org/ 10.1128/JVI.75.12.5614-5626.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montaner S, Sodhi A, Molinolo A, Bugge TH, Sawai ET, He Y, Li Y, Ray PE, Gutkind JS. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 2003; 3:23-36; PMID:12559173; http://dx.doi.org/ 10.1016/S1535-6108(02)00237-4 [DOI] [PubMed] [Google Scholar]
- 10.Montaner S. The Kaposi"s Sarcoma-Associated Herpesvirus G Protein-Coupled Receptor as a Therapeutic Target for the Treatment of Kaposi"s Sarcoma. Cancer Res 2006; 66:168-74; PMID:16397229; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-1026 [DOI] [PubMed] [Google Scholar]
- 11.Naranatt PP, Krishnan HH, Smith MS, Chandran B. Kaposi's sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J Virol 2005; 79:1191-206; PMID:15613346; http://dx.doi.org/ 10.1128/JVI.79.2.1191-1206.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veettil MV, Sharma-Walia N, Sadagopan S, Raghu H, Sivakumar R, Naranatt PP, Chandran B. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner. J Virol 2006; 80:11432-46; PMID:17005646; http://dx.doi.org/ 10.1128/JVI.01342-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma-Walia N, Naranatt PP, Krishnan HH, Zeng L, Chandran B. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J Virol 2004; 78:4207-23; PMID:15047836; http://dx.doi.org/ 10.1128/JVI.78.8.4207-4223.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Caballero A, Sivakumar R, Varga L, Bottero V, Chandran B. Lipid rafts of primary endothelial cells are essential for Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8-induced phosphatidylinositol 3-kinase and RhoA-GTPases critical for microtubule dynamics and nuclear delivery of viral DNA but dispensable for binding and entry. J Virol 2007; 81:7941-59; PMID:17507466; http://dx.doi.org/ 10.1128/JVI.02848-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballon G, Akar G, Cesarman E. Systemic Expression of Kaposi Sarcoma Herpesvirus (KSHV) Vflip in Endothelial Cells Leads to a Profound Proinflammatory Phenotype and Myeloid Lineage Remodeling In Vivo. PLoS Pathog 2015; 11:e1004581; PMID:25607954; http://dx.doi.org/ 10.1371/journal.ppat.1004581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shepard LW. Constitutive Activation of NF-kappa B and Secretion of Interleukin-8 Induced by the G Protein-coupled Receptor of Kaposi's Sarcoma-associated Herpesvirus Involve Galpha 13 and RhoA. J Biol Chem 2001; 276:45979-87; PMID:11590141; http://dx.doi.org/ 10.1074/jbc.M104783200 [DOI] [PubMed] [Google Scholar]
- 17.Gill MB, Turner R, Stevenson PG, Way M. KSHV-TK is a tyrosine kinase that disrupts focal adhesions and induces Rho-mediated cell contraction. EMBO J 2015; 34:448-65; PMID:25471072; http://dx.doi.org/ 10.15252/embj.201490358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansouri M, Rose PP, Moses AV, Fruh K. Remodeling of Endothelial Adherens Junctions by Kaposi's Sarcoma-Associated Herpesvirus. J Virol 2008; 82:9615-28; PMID:18667499; http://dx.doi.org/ 10.1128/JVI.02633-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadler R, Wu L, Forghani B, Renne R, Zhong W, Herndier B, Ganem D. A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposi's sarcoma-associated herpesvirus. J Virol 1999; 73:5722-30; PMID:10364323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCormick C. The Kaposin B Protein of KSHV Activates the p38/MK2 Pathway and Stabilizes Cytokine mRNAs. Science 2005; 307:739-41; PMID:15692053; http://dx.doi.org/ 10.1126/science.1105779 [DOI] [PubMed] [Google Scholar]
- 21.Garcia MC, Ray DM, Lackford B, Rubino M, Olden K, Roberts JD. Arachidonic Acid Stimulates Cell Adhesion through a Novel p38 MAPK-RhoA Signaling Pathway That Involves Heat Shock Protein 27. J Biol Chem 2009; 284:20936-45; PMID:19506078; http://dx.doi.org/ 10.1074/jbc.M109.020271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corcoran JA, Johnston BP, McCormick C. Viral Activation of MK2-hsp27-p115RhoGEF-RhoA Signaling Axis Causes Cytoskeletal Rearrangements, P-body Disruption and ARE-mRNA Stabilization. PLoS Pathog 2015; 11:e1004597; PMID:25569678; http://dx.doi.org/ 10.1371/journal.ppat.1004597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoecklin G, Stubbs T, Kedersha N, Wax S, Rigby WFC, Blackwell TK, Anderson P. MK2-induced tristetraprolin:14-3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J 2004; 23:1313-24; PMID:15014438; http://dx.doi.org/ 10.1038/sj.emboj.7600163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakheet T, Williams BRG, Khabar KSA. ARED 3.0: the large and diverse AU-rich transcriptome. Nucleic Acids Res 2006; 34:D111-4; PMID:16381826; http://dx.doi.org/ 10.1093/nar/gkj052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanduja S, Blanco FF, Dixon DA. The roles of TTP and BRF proteins in regulated mRNA decay. WIREs RNA 2010; 2:42-57; http://dx.doi.org/ 10.1002/wrna.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoecklin G, Mayo T, Anderson P. ARE-mRNA degradation requires the 5"-3" decay pathway. EMBO Rep 2006; 7:72-7; PMID:16299471; http://dx.doi.org/ 10.1038/sj.embor.7400572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi S, Sakurai K, Ebihara A, Kajiho H, Saito K, Kontani K, Nishina H, Katada T. RhoA activation participates in rearrangement of processing bodies and release of nucleated AU-rich mRNAs. Nucleic Acids Res 2011; 39:3446-57; PMID:21212127; http://dx.doi.org/ 10.1093/nar/gkq1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corcoran JA, Khaperskyy DA, Johnston BP, King CA, Cyr DP, Olsthoorn AV, McCormick C. Kaposi's Sarcoma-Associated Herpesvirus G-Protein-Coupled Receptor Prevents AU-Rich-Element-Mediated mRNA Decay. J Virol 2012; 86:8859-71; PMID:22696654; http://dx.doi.org/ 10.1128/JVI.00597-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker R, Sheth U. P Bodies and the Control of mRNA Translation and Degradation. Mol Cell 2007; 25:635-46; PMID:17349952; http://dx.doi.org/ 10.1016/j.molcel.2007.02.011 [DOI] [PubMed] [Google Scholar]
- 30.Fenger-Grøn M, Fillman C, Norrild B, Lykke-Andersen J. Multiple Processing Body Factors and the ARE Binding Protein TTP Activate mRNA Decapping. Mol Cell 2005; 20:905-15; PMID:16364915; http://dx.doi.org/ 10.1016/j.molcel.2005.10.031 [DOI] [PubMed] [Google Scholar]
- 31.Lykke-Andersen J. Recruitment and activation of mRNA decay enzymes by two ARE-mediated decay activation domains in the proteins TTP and BRF-1. Genes Dev 2005; 19:351-61; PMID:15687258; http://dx.doi.org/ 10.1101/gad.1282305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franks TM, Lykke-Andersen J. TTP and BRF proteins nucleate processing body formation to silence mRNAs with AU-rich elements. Genes Dev 2007; 21:719-35; PMID:17369404; http://dx.doi.org/ 10.1101/gad.1494707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheth U, Parker R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 2003; 300:805-8; PMID:12730603; http://dx.doi.org/ 10.1126/science.1082320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol 2005; 169:871-84; PMID:15967811; http://dx.doi.org/ 10.1083/jcb.200502088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kedersha N, Anderson P. Mammalian Stress Granules and Processing Bodies. In: Translation Initiation: Cell Biology, High‐Throughput Methods, and Chemical‐Based Approaches. Elsevier; 2007. pages 61-81 [DOI] [PubMed] [Google Scholar]
- 36.Aizer A, Brody Y, Ler LW, Sonenberg N, Singer RH, Shav-Tal Y. The Dynamics of Mammalian P Body Transport, Assembly, and Disassembly In Vivo. Mol Biol Cell 2008; 19:4154-66; PMID:18653466; http://dx.doi.org/ 10.1091/mbc.E08-05-0513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cougot N, Babajko S, Séraphin B. Cytoplasmic foci are sites of mRNA decay in human cells. J Cell Biol 2004; 165:31-40; PMID:15067023; http://dx.doi.org/ 10.1083/jcb.200309008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eulalio A, Behm-Ansmant I, Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat Rev Mol Cell Biol 2007; 8:9-22; PMID:17183357; http://dx.doi.org/ 10.1038/nrm2080 [DOI] [PubMed] [Google Scholar]
- 39.Kulkarni M, Ozgur S, Stoecklin G. On track with P-bodies. Biochem Soc Trans 2010; 38:242; PMID:20074068; http://dx.doi.org/ 10.1042/BST0380242 [DOI] [PubMed] [Google Scholar]
- 40.Rajgor D, Mellad JA, Soong D, Rattner JB, Fritzler MJ, Shanahan CM. Mammalian microtubule P-body dynamics are mediated by nesprin-1. J Cell Biol 2014; 205:457-75; PMID:24862572; http://dx.doi.org/ 10.1083/jcb.201306076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadok A, Marshall CJ. Rho GTPases: masters of cell migration. Small GTPases 2014; 5:e29710-7; http://dx.doi.org/ 10.4161/sgtp.29710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parri M, Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell Commun Signal 2010; 8:23; PMID:20822528; http://dx.doi.org/ 10.1186/1478-811X-8-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall A. The cytoskeleton and cancer. Cancer Metastasis Rev 2009; 28:5-14; PMID:19153674; http://dx.doi.org/ 10.1007/s10555-008-9166-3 [DOI] [PubMed] [Google Scholar]
- 44.Ridley AJ. Rho GTPases and cell migration. J Cell Sci 2001; 114:2713-22; PMID:11683406 [DOI] [PubMed] [Google Scholar]
- 45.Siehler S. Regulation of RhoGEF proteins by G 12/13-coupled receptors. Br J Pharmacol 2009; 158:41-9; PMID:19226283; http://dx.doi.org/ 10.1111/j.1476-5381.2009.00121.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buchsbaum RJ. Rho activation at a glance. J Cell Sci 2007; 120:1149-52; PMID:17376960; http://dx.doi.org/ 10.1242/jcs.03428 [DOI] [PubMed] [Google Scholar]
- 47.Rajakylä EK, Vartiainen MK. Rho, nuclear actin, and actin-binding proteins in the regulation of transcription and gene expression. Small GTPases 2014; 5:e27539; http://dx.doi.org/ 10.4161/sgtp.27539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miralles F, Posern G, Zaromytidou A-I, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 2003; 113:329-42; PMID:12732141; http://dx.doi.org/ 10.1016/S0092-8674(03)00278-2 [DOI] [PubMed] [Google Scholar]
- 49.Treisman R, Alberts AS, Sahai E. Regulation of SRF activity by Rho family GTPases. Cold Spring Harb Symp Quant Biol 1998; 63:643-51; PMID:10384329; http://dx.doi.org/ 10.1101/sqb.1998.63.643 [DOI] [PubMed] [Google Scholar]
- 50.Mouilleron S, Langer CA, Guettler S, McDonald NQ, Treisman R. Structure of a pentavalent G-actin*MRTF-A complex reveals how G-actin controls nucleocytoplasmic shuttling of a transcriptional coactivator. Sci Signal 2011; 4:ra40-0; PMID:21673315; http://dx.doi.org/ 10.1126/scisignal.2001750 [DOI] [PubMed] [Google Scholar]
- 51.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al.. Role of YAP/TAZ in mechanotransduction. Nature 2011; 474:179-83; PMID:21654799; http://dx.doi.org/ 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- 52.Wada K-I, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development 2011; 138:3907-14; PMID:21831922; http://dx.doi.org/ 10.1242/dev.070987 [DOI] [PubMed] [Google Scholar]
- 53.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013; 154:1047-59; PMID:23954413; http://dx.doi.org/ 10.1016/j.cell.2013.07.042 [DOI] [PubMed] [Google Scholar]
- 54.Reddy P, Deguchi M, Cheng Y, Hsueh AJW. Actin cytoskeleton regulates Hippo signaling. PLoS ONE 2013; 8:e73763; PMID:24040060; http://dx.doi.org/ 10.1371/journal.pone.0073763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Visser-Grieve S, Zhou Z, She Y-M, Huang H, Cyr TD, Xu T, Yang X. LATS1 tumor suppressor is a novel actin-binding protein and negative regulator of actin polymerization. Cell Res 2011; 21:1513-6; PMID:21808298; http://dx.doi.org/ 10.1038/cr.2011.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al.. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 2003; 11:619-33; PMID:12667446; http://dx.doi.org/ 10.1016/S1097-2765(03)00105-9 [DOI] [PubMed] [Google Scholar]
- 57.He B, Gross M, Roizman B. The gamma134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J Biol Chem 1998; 273:20737-43; PMID:9694816; http://dx.doi.org/ 10.1074/jbc.273.33.20737 [DOI] [PubMed] [Google Scholar]
- 58.Chen R, Rato C, Yan Y, Crespillo-Casado A, Clarke HJ, Harding HP, Marciniak SJ, Read RJ, Ron D. G-actin provides substrate-specificity to eukaryotic initiation factor 2α holophosphatases. Elife 2015; 4:e04871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chambers JE, Dalton LE, Clarke HJ, Malzer E, Dominicus CS, Patel V, Moorhead G, Ron D, Marciniak SJ. Actin dynamics tune the integrated stress response by regulating eukaryotic initiation factor 2α dephosphorylation. Elife 2015; 4:e04872; PMID:25774599; http://dx.doi.org/ 10.7554/eLife.04872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramachandran V, Shah KH, Herman PK. The cAMP-dependent protein kinase signaling pathway is a key regulator of P body foci formation. Mol Cell 2011; 43:973-81; PMID:21925385; http://dx.doi.org/ 10.1016/j.molcel.2011.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoon J-H, Choi E-J, Parker R. Dcp2 phosphorylation by Ste20 modulates stress granule assembly and mRNA decay in Saccharomyces cerevisiae. J Cell Biol 2010; 189:813-27; PMID:20513766; http://dx.doi.org/ 10.1083/jcb.200912019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rzeczkowski K, Beuerlein K, Müller H, Dittrich-Breiholz O, Schneider H, Kettner-Buhrow D, Holtmann H, Kracht M. c-Jun N-terminal kinase phosphorylates DCP1a to control formation of P bodies. J Cell Biol 2011; 194:581-96; PMID:21859862; http://dx.doi.org/ 10.1083/jcb.201006089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reineke LC, Lloyd RE. Diversion of stress granules and P-bodies during viral infection. Virology 2013; 436:255-67; PMID:23290869; http://dx.doi.org/ 10.1016/j.virol.2012.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang D, Xie P, Guo S, Li H. Induction of MAPK phosphatase-1 by hypothermia inhibits TNF- -induced endothelial barrier dysfunction and apoptosis. Cardiovasc Res 2010; 85:520-9; PMID:19793766; http://dx.doi.org/ 10.1093/cvr/cvp323 [DOI] [PubMed] [Google Scholar]
- 65.Tangkijvanich P, Melton AC, Santiskulvong C, Yee HF Jr. Rho and p38 MAP Kinase Signaling Pathways Mediate LPA-Stimulated Hepatic Myofibroblast Migration. J Biomed Sci 2003; 10:352-8; PMID:12711863; http://dx.doi.org/ 10.1007/BF02256455 [DOI] [PubMed] [Google Scholar]
- 66.Garcia MC, Ray DM, Lackford B, Rubino M, Olden K, Roberts JD. Arachidonic Acid Stimulates Cell Adhesion through a Novel p38 MAPK-RhoA Signaling Pathway That Involves Heat ShockProtein 27. J Biol Chem 2009; 284:20936-45; PMID:19506078; http://dx.doi.org/ 10.1074/jbc.M109.020271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans IM, Britton G, Zachary IC. Vascular endothelial growth factor induces heat shock protein (HSP) 27 serine 82 phosphorylation and endothelial tubulogenesis via protein kinase D and independent of p38 kinase. Cell Signall 2008; 20:1375-84; PMID:18440775; http://dx.doi.org/ 10.1016/j.cellsig.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 68.Wu Y, Zhan L, Ai Y, Hannigan M, Gaestel M, Huang C-K, Madri JA. MAPKAPK2-mediated LSP1 phosphorylation and FMLP-induced neutrophil polarization. Biochem Biophys Res Commun 2007; 358:170-5; PMID:17481585; http://dx.doi.org/ 10.1016/j.bbrc.2007.04.104 [DOI] [PubMed] [Google Scholar]
- 69.Bogatcheva NV, Adyshev D, Mambetsariev B, Moldobaeva N, Verin AD. Involvement of microtubules, p38, and Rho kinases pathway in 2-methoxyestradiol-induced lung vascular barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 2006; 292:L487-99 [DOI] [PubMed] [Google Scholar]
- 70.Kobayashi M, Nishita M, Mishima T, Ohashi K, Mizuno K. MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J 2006; 25:713-26; PMID:16456544; http://dx.doi.org/ 10.1038/sj.emboj.7600973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kotlyarov A, Gaestel M. Is MK2 (mitogen-activated protein kinase-activated protein kinase 2) the key for understanding post-transcriptional regulation of gene expression? Biochem Soc Trans 2002; 30:959-63; PMID:12440954; http://dx.doi.org/ 10.1042/bst0300959 [DOI] [PubMed] [Google Scholar]
- 72.Kotlyarov A, Yannoni Y, Fritz S, Laass K, Telliez J-B, Pitman D, Lin L-L, Gaestel M. Distinct cellular functions of MK2. Mol Cell Biol 2002; 22:4827-35; PMID:12052889; http://dx.doi.org/ 10.1128/MCB.22.13.4827-4835.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia JGN, Wang P, Schaphorst PM, Borbiev T, Liu F, Birukova AA, Jacobs K, Bogatcheva NV, Verin AD. Critical involvement of p38 MAP kinase in pertussis toxin-induced cytoskeletal reorganizationand lung permeability. FASEB J 2002; 16:1064-76; PMID:12087068; http://dx.doi.org/ 10.1096/fj.01-0895com [DOI] [PubMed] [Google Scholar]
- 74.Landry J, Huot J. Regulation of actin dynamics by stress-activated protein kinase 2 (SAPK2)-dependent phosphorylation of heat-shock protein of 27 kDa (Hsp27). Biochem Soc Symp 1999; 64:79-89; PMID:10207622 [PubMed] [Google Scholar]
- 75.Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 1997; 15:2169-77; PMID:9393975; http://dx.doi.org/ 10.1038/sj.onc.1201380 [DOI] [PubMed] [Google Scholar]
- 76.Aburima A, Wraith KS, Raslan Z, Law R, Magwenzi S, Naseem KM. cAMP signaling regulates platelet myosin light chain (MLC) phosphorylation and shape change through targeting the RhoA-Rho kinase-MLC phosphatase signaling pathway. Blood 2013; 122:3533-45; PMID:24100445; http://dx.doi.org/ 10.1182/blood-2013-03-487850 [DOI] [PubMed] [Google Scholar]
- 77.Szulcek R, Beckers CML, Hodzic J, de Wit J, Chen Z, Grob T, Musters RJP, Minshall RD, van Hinsbergh VWM, Van Nieuw Amerongen GP. Localized RhoA GTPase activity regulates dynamics of endothelial monolayer integrity. Cardiovasc Res 2013; 99:471-82; PMID:23536606; http://dx.doi.org/ 10.1093/cvr/cvt075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wojciak-Stothard B, Zhao L, Oliver E, Dubois O, Wu Y, Kardassis D, Vasilaki E, Huang M, Mitchell JA, Harrington LS, et al.. Role of RhoB in the regulation of pulmonary endothelial and smooth muscle cell responses to hypoxia. Circulation Res 2012; 110:1423-34; PMID:22539766; http://dx.doi.org/ 10.1161/CIRCRESAHA.112.264473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vega FM, Fruhwirth G, Ng T, Ridley AJ. RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J Cell Biol 2011; 193:655-65; PMID:21576392; http://dx.doi.org/ 10.1083/jcb.201011038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peng J, He F, Zhang C, Deng X, Yin F. Protein kinase C-a signals P115RhoGEF phosphorylation and RhoA activation in TNF-a- induced mouse brain microvascular endothelial cell barrier dysfunction. J Neuroinflammation 2011; 8:28; PMID:21473788; http://dx.doi.org/ 10.1186/1742-2094-8-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Venkatesh D, Fredette N, Rostama B, Tang Y, Vary CPH, Liaw L, Urs S. RhoA-mediated signaling in Notch-induced senescence-like growth arrest and endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol 2011; 31:876-82; PMID:21273559; http://dx.doi.org/ 10.1161/ATVBAHA.110.221945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McKenzie JAG, Ridley AJ. Roles of Rho/ROCK and MLCK in TNF-α-induced changes in endothelial morphology and permeability. J Cell Physiol 2007; 213:221-8; PMID:17476691; http://dx.doi.org/ 10.1002/jcp.21114 [DOI] [PubMed] [Google Scholar]
- 83.Campos SB, Ashworth SL, Wean S, Hosford M, Sandoval RM, Hallett MA, Atkinson SJ, Molitoris BA. Cytokine-induced F-actin reorganization in endothelial cells involves RhoA activation. Am J Physiol Renal Physiol 2008; 296:F487-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation 2006; 13:237-47; PMID:16627366; http://dx.doi.org/ 10.1080/10739680600556944 [DOI] [PubMed] [Google Scholar]
- 85.Wojciak-Stothard B, Tsang LYF, Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 2005; 288:L749-60 [DOI] [PubMed] [Google Scholar]
- 86.Soga N. Rho Family GTPases Regulate VEGF-Stimulated Endothelial Cell Motility. Exp Cell Res 2001; 269:73-87; PMID:11525641; http://dx.doi.org/ 10.1006/excr.2001.5295 [DOI] [PubMed] [Google Scholar]
- 87.Maekawa M. Signaling from Rho to the Actin Cytoskeleton Through Protein Kinases ROCK and LIM-kinase. Science 1999; 285:895-8; PMID:10436159; http://dx.doi.org/ 10.1126/science.285.5429.895 [DOI] [PubMed] [Google Scholar]
- 88.Sahai E, Alberts AS, Treisman R. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J 1998; 17:1350-61; PMID:9482732; http://dx.doi.org/ 10.1093/emboj/17.5.1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gamell C, Susperregui AG, Bernard O, Rosa JL, Ventura F. The p38/MK2/Hsp25 Pathway Is Required for BMP-2-Induced Cell Migration. PLoS ONE 2011; 6:e16477; PMID:21297993; http://dx.doi.org/ 10.1371/journal.pone.0016477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu L, Chen S, Bergan RC. MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene 2006; 25:2987-98; PMID:16407830; http://dx.doi.org/ 10.1038/sj.onc.1209337 [DOI] [PubMed] [Google Scholar]
- 91.Rousseau S, Dolado I, Beardmore V, Shpiro N, Marquez R, Nebreda AR, Arthur JSC, Case LM, Tessier-Lavigne M, Gaestel M, et al.. CXCL12 and C5a trigger cell migration via a PAK1/2-p38α MAPK-MAPKAP-K2-HSP27 pathway. Cell Signal 2006; 18:1897-905; PMID:16574378; http://dx.doi.org/ 10.1016/j.cellsig.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 92.Rousseau S, Houle F, Kotanides H, Witte L, Waltenberger J, Landry J, Huot J. Vascular endothelial growth factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and requires concerted activation of stress-activated protein kinase 2 (SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal adhesion kinase. J Biol Chem 2000; 275:10661-72; PMID:10744763; http://dx.doi.org/ 10.1074/jbc.275.14.10661 [DOI] [PubMed] [Google Scholar]
- 93.Chen M, Knifley T, Subramanian T, Spielmann HP, O'Connor KL. Use of synthetic isoprenoids to target protein prenylation and Rho GTPases in breast cancer invasion. PLoS ONE 2014; 9:e89892; PMID:24587105; http://dx.doi.org/ 10.1371/journal.pone.0089892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dachsel JC, Ngok SP, Lewis-Tuffin LJ, Kourtidis A, Geyer R, Johnston L, Feathers R, Anastasiadis PZ. The Rho guanine nucleotide exchange factor Syx regulates the balance of dia and ROCK activities to promote polarized-cancer-cell migration. Mol Cell Biol 2013; 33:4909-18; PMID:24126053; http://dx.doi.org/ 10.1128/MCB.00565-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roh-Johnson M, Bravo-Cordero JJ, Patsialou A, Sharma VP, Guo P, Liu H, Hodgson L, Condeelis J. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene 2013; 33:4203-12; PMID:24056963; http://dx.doi.org/ 10.1038/onc.2013.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reymond N, Riou P, Ridley AJ. Rho GTPases and cancer cell transendothelial migration. Methods Mol Biol 2012; 827:123-42; PMID:22144272; http://dx.doi.org/ 10.1007/978-1-61779-442-1_9 [DOI] [PubMed] [Google Scholar]
- 97.Wu W, Huang Q, Miao J, Xiao M, Liu H, Zhao K, Zhao M. MK2 plays an important role for the increased vascular permeability that follows thermal injury. Burns 2013; 39:923-34; PMID:23465795; http://dx.doi.org/ 10.1016/j.burns.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 98.Wolfson RK, Chiang ET, Garcia JGN. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc Res 2011; 81:189-97; PMID:21146549; http://dx.doi.org/ 10.1016/j.mvr.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang E, Heo KS, Woo CH, Lee H, Le NT, Thomas TN, Fujiwara K, Abe JI. MK2 SUMOylation regulates actin filament remodeling and subsequent migration in endothelial cells by inhibiting MK2 kinase and HSP27 phosphorylation. Blood 2011; 117:2527-37; PMID:21131586; http://dx.doi.org/ 10.1182/blood-2010-08-302281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shivanna M, Rajashekhar G, Srinivas SP. Barrier Dysfunction of the Corneal Endothelium in Response to TNF- : Role of p38 MAP Kinase. Invest Ophthalmol Vis Sci 2010; 51:1575-82; PMID:19797215; http://dx.doi.org/ 10.1167/iovs.09-4343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nwariaku FE, Rothenbach P, Liu Z, Zhu X, Turnage RH, Terada LS. Rho inhibition decreases TNF-induced endothelial MAPK activation and monolayer permeability. J Appl Physiol 2003; 95:1889-95; PMID:12844496; http://dx.doi.org/ 10.1152/japplphysiol.00225.2003 [DOI] [PubMed] [Google Scholar]
- 102.Marcos-Ramiro B, García-Weber D, Millán J. TNF-induced endothelial barrier disruption: beyond actin and Rho. Thromb Haemost 2014; 112:1088-102; PMID:25078148; http://dx.doi.org/ 10.1160/TH14-04-0299 [DOI] [PubMed] [Google Scholar]
- 103.Duluc L, Wojciak-Stothard B. Rho GTPases in the regulation of pulmonary vascular barrier function. Cell Tissue Res 2014; 355:675-85; PMID:24599334; http://dx.doi.org/ 10.1007/s00441-014-1805-0 [DOI] [PubMed] [Google Scholar]
- 104.Amado-Azevedo J, Valent ET, Van Nieuw Amerongen GP. Regulation of the endothelial barrier function: a filum granum of cellular forces, Rho-GTPase signaling and microenvironment. Cell Tissue Res 2014; 355:557-76; PMID:24633925; http://dx.doi.org/ 10.1007/s00441-014-1828-6 [DOI] [PubMed] [Google Scholar]
- 105.Di Lorenzo A, Lin MI, Murata T, Landskroner-Eiger S, Schleicher M, Kothiya M, Iwakiri Y, Yu J, Huang PL, Sessa WC. eNOS-derived nitric oxide regulates endothelial barrier function through VE-cadherin and Rho GTPases. J Cell Sci 2013; 126:5541-52; PMID:24046447; http://dx.doi.org/ 10.1242/jcs.115972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Breslin JW. ROCK and cAMP promote lymphatic endothelial cell barrier integrity and modulate histamine and thrombin-induced barrier dysfunction. Lymphat Res Biol 2011; 9:3-11; PMID:21417762; http://dx.doi.org/ 10.1089/lrb.2010.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gorovoy M, Han J, Pan H, Welch E, Neamu R, Jia Z, Predescu D, Vogel S, Minshall RD, Ye RD, et al.. LIM Kinase 1 Promotes Endothelial Barrier Disruption and Neutrophil Infiltration in Mouse Lungs. Circ Res 2009; 105:549-56; PMID:19679840; http://dx.doi.org/ 10.1161/CIRCRESAHA.109.195883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mong PY, Petrulio C, Kaufman HL, Wang Q. Activation of Rho kinase by TNF-α is required for JNK activation in human pulmonary microvascular endothelial cells. J Immunol 2008; 180:550-8; PMID:18097057; http://dx.doi.org/ 10.4049/jimmunol.180.1.550 [DOI] [PubMed] [Google Scholar]
- 109.Adamson RH, Curry FE, Adamson G, Liu B, Jiang Y, Aktories K, Barth H, Daigeler A, Golenhofen N, Ness W, et al.. Rho and rho kinase modulation of barrier properties: cultured endothelial cells and intact microvessels of rats and mice. J Physiol (Lond) 2002; 539:295-308; PMID:11850521; http://dx.doi.org/ 10.1113/jphysiol.2001.013117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci 2001; 114:1343-55; PMID:11257000 [DOI] [PubMed] [Google Scholar]
- 111.Ronkina N, Menon MB, Schwermann J, Arthur JSC, Legault H, Telliez JB, Kayyali US, Nebreda AR, Kotlyarov A, Gaestel M. Stress induced gene expression: a direct role for MAPKAP kinases in transcriptional activation of immediate early genes. Nucleic Acids Res 2011; 39:2503-18; PMID:21109534; http://dx.doi.org/ 10.1093/nar/gkq1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Knapinska AM, Gratacos FM, Krause CD, Hernandez K, Jensen AG, Bradley JJ, Wu X, Pestka S, Brewer G. Chaperone Hsp27 Modulates AUF1 Proteolysis and AU-Rich Element-Mediated mRNA Degradation. Mol Cell Biol 2011; 31:1419-31; PMID:21245386; http://dx.doi.org/ 10.1128/MCB.00907-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Clement SL, Scheckel C, Stoecklin G, Lykke-Andersen J. Phosphorylation of tristetraprolin by MK2 impairs AU-rich element mRNA decay by preventing deadenylase recruitment. Mol Cell Biol 2011; 31:256-66; PMID:21078877; http://dx.doi.org/ 10.1128/MCB.00717-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sinsimer KS, Gratacos FM, Knapinska AM, Lu J, Krause CD, Wierzbowski AV, Maher LR, Scrudato S, Rivera YM, Gupta S, et al.. Chaperone Hsp27, a Novel Subunit of AUF1 Protein Complexes, Functions in AU-Rich Element-Mediated mRNA Decay. Mol Cell Biol 2008; 28:5223-37; PMID:18573886; http://dx.doi.org/ 10.1128/MCB.00431-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sandler H, Stoecklin G. Control of mRNA decay by phosphorylation of tristetraprolin. Biochem Soc Trans 2008; 36:491-6; PMID:18481987; http://dx.doi.org/ 10.1042/BST0360491 [DOI] [PubMed] [Google Scholar]
- 116.Maitra S, Chou CF, Luber CA, Lee KY, Mann M, Chen CY. The AU-rich element mRNA decay-promoting activity of BRF1 is regulated by mitogen-activated protein kinase-activated protein kinase 2. RNA 2008; 14:950-9; PMID:18326031; http://dx.doi.org/ 10.1261/rna.983708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fessler MB, Arndt PG, Just I, Nick JA, Malcolm KC, Scott Worthen G. Dual role for RhoA in suppression and induction of cytokines in the human neutrophil. Blood 2006; 109:1248-56; PMID:17018860; http://dx.doi.org/ 10.1182/blood-2006-03-012898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hitti E, Iakovleva T, Brook M, Deppenmeier S, Gruber AD, Radzioch D, Clark AR, Blackshear PJ, Kotlyarov A, Gaestel M. Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element. Mol Cell Biol 2006; 26:2399-407; PMID:16508014; http://dx.doi.org/ 10.1128/MCB.26.6.2399-2407.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dean JLE, Sarsfield SJ, Tsounakou E, Saklatvala J. p38 Mitogen-activated protein kinase stabilizes mRNAs that contain cyclooxygenase-2 and tumor necrosis factor AU-rich elements by inhibiting deadenylation. J Biol Chem 2003; 278:39470-6; PMID:12882963; http://dx.doi.org/ 10.1074/jbc.M306345200 [DOI] [PubMed] [Google Scholar]
- 120.Neininger A, Kontoyiannis D, Kotlyarov A, Winzen R, Eckert R, Volk H-D, Holtmann H, Kollias G, Gaestel M. MK2 targets AU-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J Biol Chem 2002; 277:3065-8; PMID:11741878; http://dx.doi.org/ 10.1074/jbc.C100685200 [DOI] [PubMed] [Google Scholar]
- 121.Hippenstiel S, Soeth S, Kellas B, Fuhrmann O, Seybold J, Krull M, Eichel-Streiber von C, Goebeler M, Ludwig S, Suttorp N. Rho proteins and the p38-MAPK pathway are important mediators for LPS-induced interleukin-8 expression in human endothelial cells. Blood 2000; 95:3044-51; PMID:10807767 [PubMed] [Google Scholar]
- 122.Winzen R, Kracht M, Ritter B, Wilhelm A, Chen CY, Shyu AB, Müller M, Gaestel M, Resch K, Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J 1999; 18:4969-80; PMID:10487749; http://dx.doi.org/ 10.1093/emboj/18.18.4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shimada H, Rajagopalan LE. Rho Kinase-2 Activation in Human Endothelial Cells Drives Lysophosphatidic Acid-mediated Expression of Cell Adhesion Molecules via NF- B p65. J Biol Chem 2010; 285:12536-42; PMID:20164172; http://dx.doi.org/ 10.1074/jbc.M109.099630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nakayama Y, Komuro R, Yamamoto A, Miyata Y, Tanaka M, Matsuda M, Fukuhara A, Shimomura I. RhoA induces expression of inflammatory cytokine in adipocytes. Biochem Biophys Res Commun 2009; 379:288-92; PMID:19101507; http://dx.doi.org/ 10.1016/j.bbrc.2008.12.040 [DOI] [PubMed] [Google Scholar]


