Abstract
Small GTPases function as universal molecular switches due to the nucleotide dependent conformational changes of their switch regions that allow interacting proteins to discriminate between the active GTP-bound and the inactive GDP-bound states. Guanine nucleotide exchange factors (GEFs) recognize the inactive GDP-bound conformation whereas GTPase activating proteins (GAPs), and the GTPase effectors recognize the active GTP-bound state. Small GTPases are linked to each other through regulatory and effector proteins into functional networks that regulate intracellular membrane traffic through diverse mechanisms that include GEF and GAP cascades, GEF-effector interactions, common effectors and positive feedback loops linking interacting proteins. As more structural and functional information is becoming available, new types of interactions between regulatory proteins, and new mechanisms by which GTPases are networked to control membrane traffic are being revealed. This review will focus on the structure and function of the novel Rab11-FIP3-Rabin8 dual effector complex and its implications for the targeting of sensory receptors to primary cilia, dysfunction of which causes cilia defects underlying human diseases and disorders know as ciliopathies.
Keywords: Rab GTPases, GTPase effectors, Golgi, cilium, membrane trafficking
Abbreviations
- TGN
Trans-Golgi Network
Introduction
Small GTPases of the Ras, Rho, Rab and Arf families function as universal molecular switches that regulate effectively all cellular processes including signal transduction, cytoskeleton dynamics and membrane trafficking. Activity of Arf and Rab GTPases is tightly regulated by the guanine nucleotide exchange factors (GEFs), GTPase activating proteins (GAPs), and the GTPase effectors, which cooperate to regulate intracellular membrane traffic through diverse mechanisms that link interacting proteins into functional networks.1-3 Often, several of these regulatory properties are combined within large scaffold proteins, facilitating the ordered recruitment and activation of small GTPases during membrane trafficking.4 Coincidence detection and multiple interactions acting in concert regulate both the localization and the activity of Arf GTPases.5-7 Rab GTPases are specifically localized to different membrane domains where their activity alters the identity of transport membranes, thus connecting the different stages of specific transport pathways.8,9 To carry out their cellular functions, Arfs and Rabs interact with numerous and structurally diverse effector proteins through different but conserved binding modes.10 As more structural and functional information is becoming available, new types of interactions between regulatory proteins, and new mechanisms that link GTPases into regulatory circuits that control membrane traffic are being revealed.
Cilia Targeted Trafficking Complexes
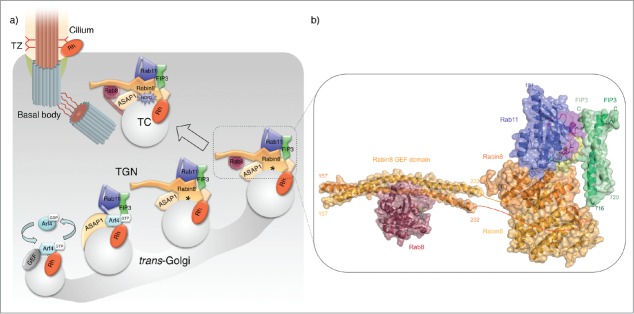
Primary cilia are specialized organelles on the cell surface that have critical roles in sensing the extracellular environment and their dysfunction underlies critical disease pathways in human ciliopathies.11–14 The directional movement of sensory receptors to primary cilia is regulated by the sequential activation of Arf and Rab GTPases at multiple stages of trafficking.4 At least 3 macromolecular complexes regulated by small GTPases are involved in ciliary targeting: the IFT complex, the BBSome, and the ciliary targeting complex that participates in the trafficking of membrane proteins from the TGN to the cilium, which recognizes the conserved ciliary targeting signals of the light receptor rhodopsin and likely other ciliary sensory receptors.15–27 The latter complex serves as an effector of Arf4 and includes 3 other proteins25: i) the Arf GTPase activating protein (GAP) ASAP1,28–30 ii) the small GTPase Rab11a, and iii) the Rab11 and Arf effector FIP3.31,32 Rab11a activates Rab8 through the guanine nucleotide exchange factor (GEF) Rabin8,33 in a cascade of molecular interactions known as the Rab11-Rabin8-Rab8 ciliogenesis cascade.26,34–36 Rab8 is the ultimate Rab GTPase within this cascade and also regulates the final stages of polarized membrane traffic, carrier fusion and lumenogenesis.18,26,37–42 The Arf GAP ASAP1 functions as a GAP and an effector for Arf4, and serves as a platform upon which the ciliary targeting complex is assembled linking the early Arf4-dependent stages of ciliary targeting with the Rab11-Rabin8-Rab8 ciliogenesis cascade25,26 (Fig. 1A). FIP3 regulates ASAP1 interactions with the ciliary cargo, and stabilizes the transient ASAP1-Rab11-FIP3 complex to recruit the Rab8 GEF Rabin8.43 The evolutionary conserved Rab11-Rabin8-Rab8 ciliogenesis cascade parallels Ypt32p-Sec2p-Sec4p cascade in yeast budding.1 Thus, this ciliary targeting pathway combines an ancestral targeting module with the evolutionary more recent functions performed by Arf4, ASAP1, and the Arf and Rab11 effector FIP3, presumably to support the more complex cell organization.
Figure 1.
Golgi to cilia transport via ciliary targeting complexes. (A) Schematic representation of ciliary membrane trafficking in the photoreceptor cell via ciliary targeting complexes. Rh, rhodopsin, a ciliary sensory receptor. TC, transport carriers. At the TGN, the Arf GAP ASAP1 serves as a platform for the stepwise assembly of the Rab11-FIP3-Rabin8 complex. Although the majority of the components that constitute the complex act as dimers, multiple complexes are omitted form the scheme for clarity. Asterisks indicate the potential site where the Rabin8250–290 linker region could approach the membrane to recognize phosphatidylserine (PS). By phosphorylating S272 within this region, NDR2 switches the specificity of Rabin8 binding from PS to Sec15, in preparation for TC fusion with the periciliary plasma membrane. (B) Crystal structure of the human Rab11-FIP3RBD-Rabin8C complex (PDB code: 4UJ3)62 and Rabin8 GEF domain in complex with Rab8 (PDB code: 4LHY)81 are shown in a cartoon representation with a semi-transparent surface. Switch regions are indicated. GMPPNP, GDP and Mg2+ ions are shown as balls-and-sticks.
The small GTPase Rab11 localizes to the trans-Golgi network (TGN) and endosomes, and functions in cytokinesis, membrane receptor recycling, cell polarity and ciliogenesis.4,44-48 The function of Rab11a is essential for the formation and integrity of vertebrate photoreceptors, where it directly interacts with the ciliary receptor rhodopsin, both in the GTP-bound and the GDP-bound state.25,26,43,49,50 The regulatory circuits that link Arf4 to Rab8 involve 2 Rab11a effectors: FIP3 and Rabin8. Rabin8 acts as a Rab11 effector and a scaffold for the Rab11a-Rab8 succession which is essential for ciliogenesis34–36 and fusion of transport carriers.26,37,38,43 During ciliogenesis, Rabin8 interacts directly, through its N-terminal domain, with the TRAPPC3, 9 and 10 subunits of the transport protein particle TRAPPII complex.34 The TRAPPC3, 9 and 10 subunits are homologs of yeast Bet3, Trs120 and Trs130, which are important for the GEF activity toward YPT1 (yeast homolog of Rab1) and YPT31/32 (yeast homolog of Rab11).51,52 Interestingly, the TRAPPII-binding N-terminal domain of Rabin8 is absent from Sec2 (yeast homolog of Rabin8). As TRAPPII mediates nucleotide exchange on the Ypt31 ortholog RabERAB11,53 it could also function as a GEF for vertebrate Rab11. TRAPPC10 has a longin domain (LD) that forms a platform for small GTPases in Rab GEFs,54 and a conserved, centrosome-targeting ASH domain.55 Thus, through its interaction with the TRAPPII complex, Rabin8 may also facilitate the selective activation of Rab11 in ciliogenesis.
The Rab11-FIP3-Rabin8 Dual Effector Complex
Whereas Rabin8 functions as a Rab11 effector at the final stages of ciliary trafficking, FIP3 interacts with Rab11a early in the ciliary pathway.25 FIP3 also functions as a Rab11 effector in cytokinesis56–59 and in the maintenance of the recycling compartment.60,61 The function of FIP3 as a Rab11 effector within the ciliary targeting complex was less clear until now. Given the known sequential interactions within the ciliary targeting complex, the model for its assembly suggested an initial recruitment of FIP3 that is subsequently replaced by Rabin8 to activate Rab8 and facilitate fusion of transport carriers with the plasma membrane.4,24 In this context, by serving as a Rab11 effector upstream of the conserved ciliogenesis cascade, FIP3 could provide a safety valve to block the premature assembly of the Rab11-Rabin8-Rab8 complex. However, recent structural and functional studies reveal that this is not the case because the 2 Rab11a effectors cooperate in carrying out Rab11a-related functions.43,62
The first surprise came from the study showing that FIP3 shapes the Rabin8 binding site within the ciliary targeting complex by significantly increasing Rabin8 interactions with both Rab11a and ASAP1.43 Furthermore, FIP3 and Rab11a bind Rabin8 separately, indicating that the 2 Rab11 effectors directly interact.43
In a concurrent study, the crystal structure of the Rab11-GMPPNP-FIP3-Rabin8 complex revealed simultaneous binding of FIP3 and Rabin8 effectors to activated Rab11.62 Direct interaction between Rabin8 and FIP3 effectors within the dual effector bound complex likely functions to stabilize the transient complexes and create a high avidity hub for cross talk of Arf and Rab GTPases in ciliary trafficking (Fig. 1).
Simultaneous Binding of FIP3 and Rabin8 Effectors to Active Rab11
A hallmark of small GTPases is the nucleotide dependent conformational change of the switch regions that allows effectors to discriminate between the active GTP-bound and the inactive GDP-bound states.63 Small GTPases often have multiple effectors, but because of the limited accessible surface area shaped by the switch regions at the G-site, the binding of different effectors is thought to be sequential and mutually exclusive. Numerous effectors have been described for Rab11 including FIPs, Rabin8, Sec15 and Rab11BP/WDR44.32,35,64–66 Two of these effectors, FIP3 and Rabin8, have been implicated in ciliogenesis and in the trafficking of membrane proteins from the TGN to the cilium.26,35,43 However, recent new data demonstrate that FIP3 and Rabin8 can associate with Rab11 at the same time.43,62 Pull-down experiments, size exclusion chromatography (SEC) and isothermal titration calorimetry (ITC) experiments revealed that the C-terminal Rab11-effector domain of Rabin8 (Rabin8C) binds a preformed Rab11-FIP3 complex to form a ternary Rab11*-GMPPNP-FIP3-Rabin8C complex.43,62 Interestingly, Rabin8C has ∼4-fold higher affinity for Rab11-FIP3 than for Rab11 alone, suggesting that FIP3 and Rabin8 either interact directly, or that the binding of FIP3 to Rab11 induces a conformational change in Rab11 that strengthens the Rabin8 association. Pull-down experiments suggested that the former possibility is most likely correct, as a direct interaction between FIP3 and Rabin8 was observed.43,62 Moreover, the regions of Rab11 that interact with Rabin8 do not undergo conformational changes upon FIP3 effector binding.59,67,68 These results are in agreement with a model where FIP3 and Rabin8 effectors simultaneously associate with Rab11 (Fig. 1).
Rabin8 C-terminal Domain is a Novel Low-Affinity Rab-effector
The fact that FIP3 and Rabin8 are able to bind Rab11 at the same time raises the question of how this is achieved structurally. Previously published crystal structures of Rab11 bound to FIP2 or FIP3 revealed a canonical effector-binding site with extensive contacts with switch I and II regions, which leave little space for simultaneous Rabin8 binding at the G-site.59,68,69 However, although Rabin8 as an effector does have a preference for GTP- vs. GDP-bound Rab11, the Kd of 40μM demonstrates weak affinity that could be the result of a relatively small Rabin8-binding-surface on Rab11*GTP.62 Indeed, the crystal structure of the Rab11-Rabin8 complex revealed a relatively small (∼600Å2) binding interface utilizing mostly non-switch region residues (Fig. 2). The C-terminal domain of Rabin8 adopts a novel fold that interacts with Rab11 via a non-canonical effector-binding site through contacts with 2 residues of switch I (L38 and E39), 4 residues of a non-switch-region loop connecting β5 and α4 of Rab11 (L128, R129, H130 and L131), and no contacts with switch II. Additionally, several main-chain hydrogen bonds are facilitated by β strand β2 of Rabin8 and residues 129–134 of Rab11 (Fig. 2). The Rabin8 binding-site on Rab11 is thus neighboring the canonical effector-binding site suggesting how dual effector binding to Rab11 may be achieved. The Rab11*GMPPNP-FIP3-Rabin8 crystal structure revealed how the 2 FIP3/Rabin8 effectors bind Rab11 at neighboring sites closely approaching and interacting with each other (Fig. 1B).62 FIP3 binds GTP-bound Rab11 with a Kd of ∼0.3μM,59 which is 2 orders of magnitude higher than the affinity of Rabin8 for Rab11.62 It is thus conceivable that Rab11 first binds FIP3 to form a Rab11-FIP3 complex that subsequently recruits Rabin8 to form the Rab11-FIP3-Rabin8 complex. This notion is supported by the fact that Rab11-FIP3 complex has 4-5 fold higher affinity for Rabin8 than Rab11 alone.43,62 In this respect, part of the preference of Rabin8 for GTP-bound Rab11 is indirectly assured via binding of the canonical FIP3 effector. The Rabin8 C-terminal domain thus represents an unusual Rab11 effector that binds at an unconventional effector-binding site with low affinity.
Figure 2.
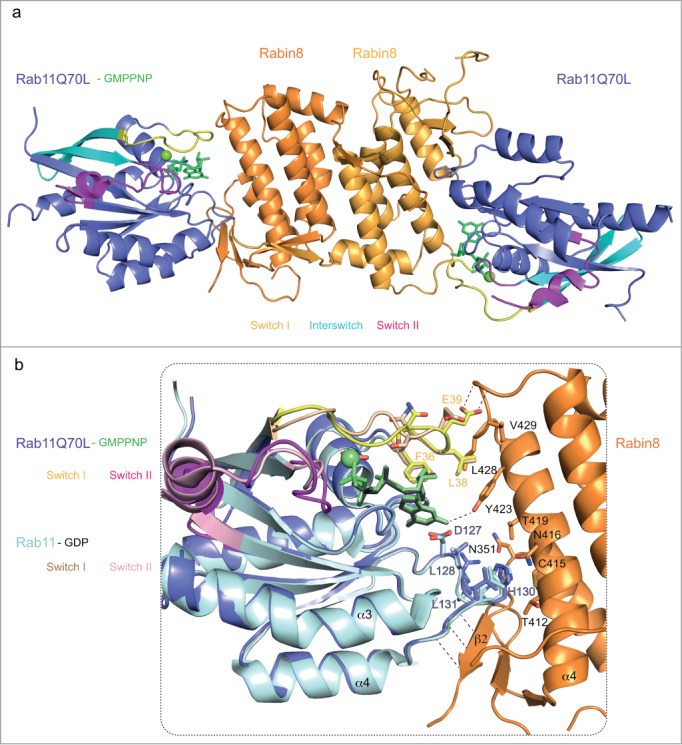
Structural overlay of Rab11-GMPPNP-Rabin8C with Rab11-GDP. (A) Crystal structure of the heterotetrameric Rab11-GMPPNP-Rabin8C complex (PDB code: 4UJ5) shown as a cartoon representation, switch regions are indicated as in Fig. 1. (B) Superimposition of the Rab11-GMPPNP-Rabin8 complex structure onto the Rab11-GDP structure (PDB code: 1OIV). Interacting residues are shown as sticks and labeled according to residue number. Backbone interactions between the γ-carboxyl group of E39 of Rab11 and residues 430–431 of Rabin8, the hydroxyl of Y423 with the guanine base of GMPPNP of Rab11 and hydrogen bonds between β2 of Rabin8C and the loop β5-α4 of Rab11 are indicated with dashed lines.
Rabin8C and PI4KIII Utilize the same Binding Surface on Rab11
Comparison of the Rab11*-*GMPPNP-Rabin8 structure to previously determined structure of Rab11 complexes revealed that Rabin8 binds Rab11 at a similar site to that of the phosphatidylinositol 4-kinase IIIβ (PI4KIIIβ)62,70 (Fig 3). PI4KIIIβ catalyzes the phosphorylation of phosphatidylinositol to generate phosphatidylinositol4-phosphate (PI4P), a reaction important for the formation and function of the Golgi where PI4KIIIβ is localized and where it interacts with Rab11.71 Despite different folds of PI4KIIIβ and Rabin8, both proteins contact a similar set of Rab11 residues including L38, E39, L128, H130 and L131 (Fig. 3). Interestingly, PI4KIIIβ was reported not to be an effector for Rab11 as the binding affinity for Rab11*GTP was only 3-4 fold higher than for Rab11*GDP in agreement with the fact that most contacts are with non-switch-region residues.70 This relatively small but significant preference of PI4KIIIβ for GTP- vs. GDP-bound forms of Rab11 is likely a result of contacts with L38 and E39 of switch I. Given the fact that Rabin8 and PI4KIIIβ bind to similar sites on Rab11 with contacts to L38 and E39 of switch 1 an interesting question is to which degree Rabin8 prefers GTP- vs. GDP-bound Rab11.
Figure 3.
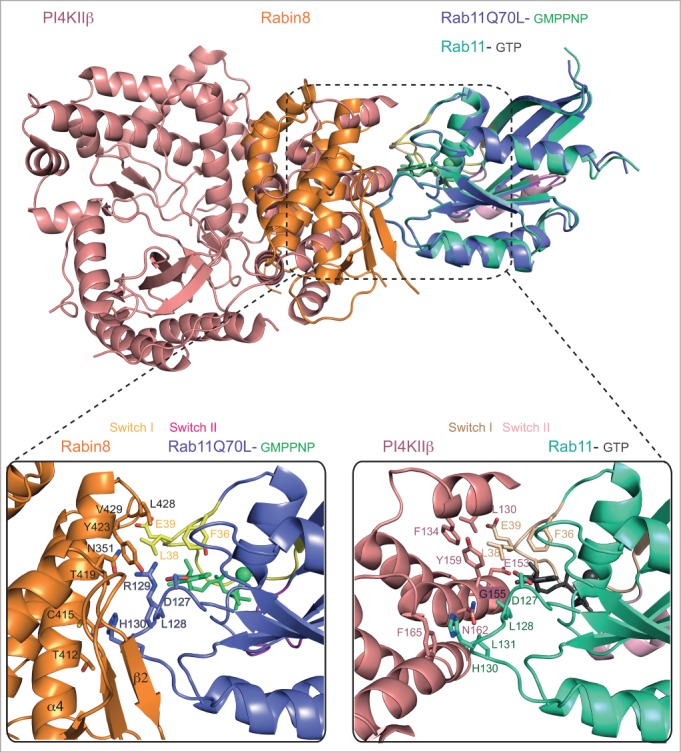
Comparison of the Rab11-Rabin8C and the Rab11-PI4KIIIβ interface. Structural overlay of Rab11-GMPPNP-Rabin8C and Rab11-GTP-PI4KIIIβ (PDB code: 4D0L). Rab11 molecules superimpose well and use the same interface to bind the structurally different Rabin8 and PI4KIIIβ molecules. The boxed-in region represents a zoom-in on the interaction interfaces between Rab11 and Rabin8 (left) and Rab11 and PI4KIIIβ (right). Switch regions are indicated, GMPPNP, GTP and Mg2+ are shown as balls-and-sticks. Interacting residues are shown as sticks and labeled according to residue number.
Specificity of Rabin8 for GTP-bound vs. GDP-bound Rab11
Rabin8 was reported to be an effector of Rab11 as it recognizes the GTP-bound and not the GDP-bound state of the small GTPase in pull-down experiments.35,62 A Kd value for the Rab11*GDP-Rabin8 complex has not been published but is, based on pull-down experiments, clearly much higher than the Kd of 40μM measured for the Rab11*GMPNP-Rabin8 complex.62 Although it is hard to measure the low affinity of Rabin8 for Rab11*GDP accurately, ITC experiments suggest a Kd for the Rab11*GDP-Rabin8 complex in the 200-400μM range (M. Vetter and E. Lorentzen, unpublished data). This indicates that the affinity of Rabin8 for Rab11*GTP is 5-10 fold higher than for Rab11*GDP. The specificity of effectors for the GTP-bound state of small GTPases arises from the unique conformation adopted by the switch regions when bound to GTP, which shape the binding surface to engage different effectors in a specific manner. Although the GDP-bound state often results in disordered switch regions,72 some small GTPases adopt well-ordered but different conformations of the switch regions depending on nucleotide state. One such case is Arl6 where only the GTP-bound conformation can recruit the BBSome to membranes via the BBS1 effector protein because the GDP-bound conformation prevents BBS1-binding due to molecular clashes.19,22 Importantly, the switch regions are also ordered in crystal structures of both GDP- and GTP-bound Rab11.67,73 The conformational differences in switch regions between different nucleotide states of Rab11 are relatively modest with a root-mean-square-deviation (rmsd) between cα-atoms of maximum 5Å (Fig 2). Given the fact that Rabin8 does not contact switch 2 of Rab11, the preference for GTP-bound Rab11 is likely attributed to the contacts with L38/E39 of switch 1 (Fig. 2). By comparing the confirmation of L38/E39 between GDP- and GTP-bound Rab11, it is striking that only the side-chain of E39 but not the side-chain of L38 displays a nucleotide dependent conformational change (Fig 2). In the GTP-bound form of Rab11, the side-chain of E39 points toward residues 430–431 of Rabin8 and makes 2 hydrogen bonds of 3Å in length with backbone NH groups. In the GDP-bound form of Rab11, E39 adopts a different rotameric conformation that points away from Rabin8 and would increase the hydrogen bonding distances to 5–6Å (Fig 2). Notably, the Rabin8-binding competent conformation of E39 is not induced by Rabin8-binding as it adopts the same rotamer in Rab11*GTP not bound to any effectors.67 It appears likely that E39 of Rab11 is important for the nucleotide dependent association with Rabin8.
Rabin8 Effector Binding is Specific for Rab11
Yeast-2-hybrid analysis of Rabin8 binding to a host of different Rab proteins revealed a strong specificity for Rab11.34 To address the molecular basis of this specificity we superimposed the structure of Rab11-Rabin8 with known structures of different Rab family members in the GTP-bound state (Fig 4A). The result reveals that there are no major clashes between Rabin8 and other members of the Rab superfamily such as Rab4, Rab6, Rab8, Rab14 or Rab25 suggesting that complex formation with Rabin8 is in principle possible (Fig 4A). However, the residues utilized by Rab11 to bind Rabin8 are not well conserved in other Rab families (Fig 4B). In particular the Rabin8-interacting switch 1 residues of Rab11 (L398 and E39) are poorly conserved (Fig 4B). E39 is often replaced by an aspartic acid that is not well positioned to make tight hydrogen bonds with residues 430–431 of Rabin8. L38 of Rab11 engages in hydrophobic contacts with residues from Rabin8 but is replaced by a hydrophilic residue in most other Rabs. Additionally, sequence alignment of different Rabs demonstrates that the Rabin8/PI4KIIIβ binding residues are only conserved in Rab11 orthologues and not in Rabs from different families, which suggests that the Rabin8/PI4KIIIβ binding site is unique to Rab11. Interestingly, the Rabin8-binding residues of Rab11 are well conserved in Ypt32, which is the yeast homolog of Rab11 (Fig 4C). This indicates that the molecular mechanism of Sec2 (yeast homolog of Rabin8)-recruitment by Ypt32 to activate Sec4 (yeast homolog of Rab8) is evolutionarily conserved. Surprisingly, the Rabin8 binding residues of Rab11 are also conserved in organisms like Chlamydomonas reinhardtii and Arabidopsis thaliana that do not appear to have a Rabin8 homolog. The genomes of Chlamydomonas reinhardtii and Arabidopsis thaliana do however encode putative PI4KIIIβ homologs with the Rab11-binding helical domain conserved. Given that Rab11 utilized the same residues for Rabin8 and PI4KIIIβ binding it appears likely that the binding site in Chlamydomonas reinhardtii and Arabidopsis thaliana Rab11 is conserved to bind PI4KIIIβ. Collectively, Y2H and bioinformatics analyses suggest that Rabin8 binding is specific to Rab11 and that the recruitment of Rabin8 by Rab11 to activate Rab8 is an evolutionarily ancient pathway in exocytosis.
Figure 4.
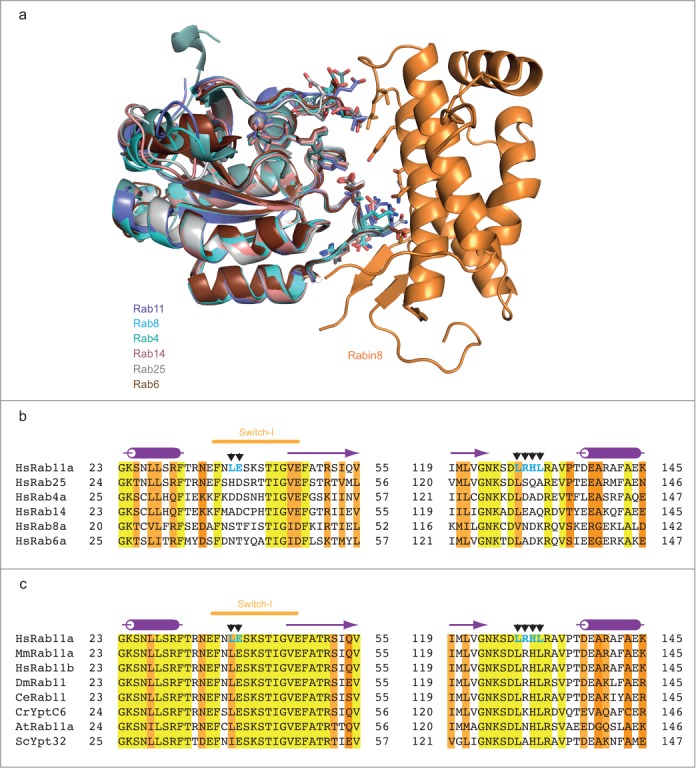
The Rabin8-binding interface is unique to Rab11. (A) Structural overlay of different active Rab GTPases onto the Rab11-GMPPNP-Rabin8 crystal structure shown as a cartoon representation. Rab4 (PDB code: 1YU9), Rab6 (PDB code: 1YZQ), Rab8 (PDB code: 4LHW), Rab14 (PDB code: 4D0G) and Rab25 (PDB code: 3TSO) are cartooned in different colors. Residues utilized by Rab11 to bind Rabin8 and the corresponding residues in different Rab GTPases are shown as sticks. (B) Sequence alignment of Rabin8-interacting regions in human Rab11a with other human Rab GTPases. Rab11a residues that interact with Rabin8 are indicated with black arrows and labeled in bold blue color. Highly conserved residues are highlighted in yellow, similar residues in lighter and darker orange, respectively. Secondary structure derived from human Rab11a is indicated above the sequence. (C) Multiple sequence alignment of Rab11a protein from different species. Rab11a residues that interact with Rabin8 are indicated as shown in Fig 4B. Secondary structure elements from Rab11a structure are indicated above the sequence. Hs: Homo sapiens, Mm: Mus musculus, Dm: Drosophila melanogaster, Ce: Caenorhabditis elegans, Cr: Chlamydomonas reinhardtii, At: Arabidopsis thaliana, Sc: Saccharomyces cerevisiae.
Regulation of Rabin8
Rabin8 is recruited to the membrane by Rab11a and interacts with specific phospholipids such as phosphatidylserine (PS, strong interaction) and phosphatidic acid (PA, weak interaction).74 The amino acids 251–460 were suggested to encompass a minimum PS-binding domain of Rabin8 based on binding experiments with truncated protein constructs.74 PS recognition is typically mediated by Ca2+-dependent C2 domains or by basic stretches of residues.75 Given that the structure of the C-terminal Rabin8 domain (residues 290–460) does not display a PS-binding domain, residues 250–290 likely encompass the PS-binding region. Rabin8250-290 is a part of the linker region (predicted to be disordered) that connects the central GEF domain to the C-terminal Rab11-effector domain (Fig. 5). The flexible nature of Rabin8250-290 probably allows this region to approach the membrane to recognize PS. Examination of the Rabin8250-290 sequence reveals a basic stretch of residues rich in lysines (260-KTPFKKGHTRNKS-272, human Rabin8 numbering) that could serve as the PS-recognition motif (Fig. 5).
Figure 5.

Putative phosphatidylserine (PS) binding motif in Rabin8. (top) Schematics of the domain architecture of Rabin8 with numbering from the human sequence. (bottom) multiple sequence alignment of the flexible linker region (reside 250–290) of Rabin8 located between the Rab8 GEF domain and the Rab11-binding domain. Serine 272 that is phosphorylated by NDR-2 is colored magenta and the positively charged residues that form a potential PS-binding motif are colored blue. Consensus motif of NDR substrates (HxRxxS/T) is absent from Sec2, nevertheless S186 and S188 in the linker region are also phosphorylated by another kinase.80 Clustal2w was used for the alignment. Abbreviations used are: Hs: Homo sapiens, Bt: Bos Taurus, Mm: Mus musculus, Mv:Manacus vitellinus, Xt: Xenopus tropicalis, Dr: Danio rerio, Sc: Saccharomyces cerevisiae.
Remarkably, NDR2-mediated phosphorylation of Rabin8 regulates the switch in binding specificity of Rabin8 from PS to the Sec15 component of the exocyst complex that mediates carrier tethering at the periciliary plasma membrane.36,74,76 NDR2 (also known as STK38L) was identified as a canine retinal degeneration gene corresponding to human ciliopathy Leber congenital amaurosis (LCA) characterized by early-onset blindness,77,78 indicating that the switch in binding partners of Rabin8 has a crucial role in ciliary membrane trafficking. The site of NDR2 phosphorylation, S272, lies close to the polybasic stretch of residues within the structurally disordered region of Rabin8 (Fig. 5), suggesting that NDR2 phosphorylation directly regulates PS-binding through the introduction of negative charges. Both S272 and the polybasic residues are well conserved in Rabin8 proteins from different organisms (but not in the Sec2 yeast homolog that instead of PS binds PI4P via its C-terminal residues 258–45079) suggesting an evolutionary conserved mechanism of regulation in higher eukaryotes (Fig. 5). Ypt32 and Sec15 compete for binding to Sec2 and phosphorylation of Sec2 within the linker region directs a switch in binding from Ypt32 to Sec15.79,80 This mode of action is not conserved, as Sec15 is a common effector for Rab11 and Rab8,64,65 which differs from Ypt32 that does not associate with the exocyst complex. Interestingly, phosphorylation of both Rabin8 and Sec2 acts as a switch in binding to Sec15, but, unlike in yeast, in higher eukaryotes Sec15, Rab11a and Rabin8 diversify to cooperate in ciliary membrane trafficking.
Concluding Remarks
Interaction networks of small GTPases are organized by regulatory proteins and effectors that form signaling junctions for communication between Arf and Rab GTPases. Comprehensive structural and functional data explain at the molecular level how regulatory circuits that function in ciliary targeting converge to form a signaling junction through the Rab11a-FIP3-Rabin8 dual effector complex. The signaling output of Rab11a through the Rabin8-FIP3 complex may control ciliary targeting by selectively allowing the cargo presented in the context of Arf4 and its effector FIP3 to engage Rabin8 and activate Rab8, ultimately facilitating fusion of transport carriers with the periciliary plasma membrane. Future research will reveal the level of conservation of dual effector complexes in linking interacting proteins to control the activity and output of Rab GTPases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Supported by the NIH grant EY-12421 to DD, and by the European Research Council (ERC grant 310343) and by the EMBO Young Investigator program grant to EL.
References
- 1.Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem 2012; 81:637-59; PMID:22463690; http://dx.doi.org/ 10.1146/annurev-biochem-052810-093700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stalder D, Antonny B. Arf GTPase regulation through cascade mechanisms and positive feedback loops. FEBS Lett 2013; 587:2028-35; PMID:23684643; http://dx.doi.org/ 10.1016/j.febslet.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 3.Jackson CL. GEF-effector interactions. Cell Logist 2014; 4, e943616; PMID:25610717; http://dx.doi.org/ 10.4161/21592780.2014.943616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deretic D. Crosstalk of Arf and Rab GTPases en route to cilia. Small GTPases 2013; 4:70-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson BC, Fromme JC. Autoregulation of Sec7 Arf-GEF activity and localization by positive feedback. Small GTPases 2012; 3:240-3; PMID:22996016; http://dx.doi.org/ 10.4161/sgtp.21828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonold CM, Fromme JC. Four GTPases differentially regulate the Sec7 Arf-GEF to direct traffic at the trans-golgi network. Dev Cell 2014; 30:759-67; PMID:25220393; http://dx.doi.org/ 10.1016/j.devcel.2014.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright J, Kahn RA, Sztul E. Regulating the large Sec7 ARF guanine nucleotide exchange factors: the when, where and how of activation. Cell Mol Life Sci 2014; 71(18):3419-38; PMID:24728583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2001; 2:107-17; PMID:11252952; http://dx.doi.org/ 10.1038/35052055 [DOI] [PubMed] [Google Scholar]
- 9.Barr FA. Review series: Rab GTPases and membrane identity: causal or inconsequential? J Cell Biol 2013; 202:191-9; PMID:23878272; http://dx.doi.org/ 10.1083/jcb.201306010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan AR, Menetrey J. Structural biology of Arf and Rab GTPases' effector recruitment and specificity. Structure 2013; 21:1284-97; PMID:23931141; http://dx.doi.org/ 10.1016/j.str.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 11.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol 2007; 8:880-93; PMID:17955020; http://dx.doi.org/ 10.1038/nrm2278 [DOI] [PubMed] [Google Scholar]
- 12.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell 2009; 137:32-45; PMID:19345185; http://dx.doi.org/ 10.1016/j.cell.2009.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valente EM, Rosti RO, Gibbs E, Gleeson JG. Primary cilia in neurodevelopmental disorders. Nat Rev Neurol 2014; 10:27-36; PMID:24296655; http://dx.doi.org/ 10.1038/nrneurol.2013.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sang L, Miller JJ, Corbit KC, Giles RH, Brauer MJ, Otto EA, Baye LM, Wen X, Scales SJ, Kwong M, et al.. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell 2011; 145:513-28; PMID:21565611; http://dx.doi.org/ 10.1016/j.cell.2011.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol 2002; 3:813-25; PMID:12415299; http://dx.doi.org/ 10.1038/nrm952 [DOI] [PubMed] [Google Scholar]
- 16.Bhogaraju S, Engel BD, Lorentzen E. Intraflagellar transport complex structure and cargo interactions. Cilia 2013; 2:10; PMID:23945166; http://dx.doi.org/ 10.1186/2046-2530-2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin H, Wang Z, Diener D, Rosenbaum J. Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control. Curr Biol 2007; 17:193-202; PMID:17276912; http://dx.doi.org/ 10.1016/j.cub.2006.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peränen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al.. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 2007; 129:1201-13; PMID:17574030; http://dx.doi.org/ 10.1016/j.cell.2007.03.053 [DOI] [PubMed] [Google Scholar]
- 19.Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell 2010; 141:1208-19; PMID:20603001; http://dx.doi.org/ 10.1016/j.cell.2-010.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liew GM, Ye F, Nager AR, Murphy JP, Lee JS, Aguiar M, Breslow DK, Gygi SP, Nachury MV. The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3. Dev Cell 2014; 31:265-78; PMID:25443296; http://dx.doi.org/ 10.1016/j.devcel.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eguether T, San Agustin JT, Keady BT, Jonassen JA, Liang Y, Francis R, Tobita K, Johnson CA, Abdelhamed ZA, Lo CW, et al.. IFT27 links the BBSome to IFT for maintenance of the ciliary signaling compartment. Dev Cell 2014; 31:279-90; PMID:25446516; http://dx.doi.org/ 10.1016/j.devcel.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mourao A, Nager AR, Nachury MV, Lorentzen E. Structural basis for membrane targeting of the BBSome by ARL6. Nat Struct Mol Biol 2014; 21:1035-41; PMID:25402481; http://dx.doi.org/ 10.1038/nsmb.2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhogaraju S, Taschner M, Morawetz M, Basquin C, Lorentzen E. Crystal structure of the intraflagellar transport complex 25/27. EMBO J 2011; 30:1907-18; PMID:21505417; http://dx.doi.org/ 10.1038/emboj.2011.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Deretic D. Molecular complexes that direct rhodopsin transport to primary cilia. Prog Retin Eye Res 2014; 38:1-19; PMID:24135424; http://dx.doi.org/ 10.1016/j.preteyeres.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J 2009; 28:183-92; PMID:19153612; http://dx.doi.org/ 10.1038/emboj.2008.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Morita Y, Mazelova J, Deretic D. The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. EMBO J 2012; 31:4057-71; PMID:22983554; http://dx.doi.org/ 10.1038/emboj.2012.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward HH, Brown-Glaberman U, Wang J, Morita Y, Alper SL, Bedrick EJ, Gattone VH 2nd, Deretic D, Wandinger-Ness A. A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol Biol Cell 2011; 22:3289-305; PMID:21775626; http://dx.doi.org/ 10.1091/mbc.E11-01-0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown MT, Andrade J, Radhakrishna H, Donaldson JG, Cooper JA, Randazzo PA. ASAP1, a phospholipid-dependent arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol Cell Biol 1998; 18:7038-51; PMID:9819391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Randazzo PA, Hirsch DS. Arf GAPs: multifunctional proteins that regulate membrane traffic and actin remodelling. Cell Signal 2004; 16:401-13; PMID:14709330; http://dx.doi.org/ 10.1016/j.cellsig.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 30.Nie Z, Randazzo PA. Arf GAPs and membrane traffic. J Cell Sci 2006; 119:1203-11; PMID:16554436; http://dx.doi.org/ 10.1242/jcs.02924 [DOI] [PubMed] [Google Scholar]
- 31.Hales CM, Griner R, Hobdy-Henderson KC, Dorn MC, Hardy D, Kumar R, Navarre J, Chan EK, Lapierre LA, Goldenring JR. Identification and characterization of a family of Rab11-interacting proteins. J Biol Chem 2001; 276:39067-75; PMID:11495908; http://dx.doi.org/ 10.1074/jbc.M104831200 [DOI] [PubMed] [Google Scholar]
- 32.Junutula JR, Schonteich E, Wilson GM, Peden AA, Scheller RH, Prekeris R. Molecular characterization of Rab11 interactions with members of the family of Rab11-interacting proteins. J Biol Chem 2004; 279:33430-7; PMID:15173169; http://dx.doi.org/ 10.1074/jbc.M404633200 [DOI] [PubMed] [Google Scholar]
- 33.Hattula K, Furuhjelm J, Arffman A, Peranen J. A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell 2002; 13:3268-80; PMID:12221131; http://dx.doi.org/ 10.1091/mbc.E02-03-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, et al.. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A 2011; 108:2759-64; PMID:21273506; http://dx.doi.org/ 10.1073/pnas.1018823108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knodler A, Feng S, Zhang J, Zhang X, Das A, Peränen J, Guo W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A 2010; 107(14):6346-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng S, Knödler A, Ren J, Zhang J, Zhang X, Hong Y, Huang S, Peränen J, Guo W. A Rab8 guanine nucleotide exchange factor-effector interaction network regulates primary ciliogenesis. J Biol Chem 2012; 287:15602-9; PMID:22433857; http://dx.doi.org/ 10.1074/jbc.M111.333245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deretic D, Huber LA, Ransom N, Mancini M, Simons K, Papermaster DS. rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci 1995; 108:215-24; PMID:7738098 [DOI] [PubMed] [Google Scholar]
- 38.Moritz OL, Tam BM, Hurd LL, Peränen J, Deretic D, Papermaster DS. Mutant rab8 impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell 2001; 12:2341-51; PMID:11514620; http://dx.doi.org/ 10.1091/mbc.12.8.2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol 2007; 178:363-9; PMID:17646400; http://dx.doi.org/ 10.1083/jcb.200703047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murga-Zamalloa CA, Atkins SJ, Peranen J, Swaroop A, Khanna H. Interaction of retinitis pigmentosa GTPase regulator (RPGR) with RAB8A GTPase: implications for cilia dysfunction and photoreceptor degeneration. Hum Mol Genet 2010; 19:3591-8; PMID:20631154; http://dx.doi.org/ 10.1093/hmg/ddq275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bryant DM, Datta A, Rodríguez-Fraticelli AE, Peränen J, Martín-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 2010; 12:1035-45; PMID:20890297; http://dx.doi.org/ 10.1038/ncb2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Overeem AW, Bryant DM, van ISC. Mechanisms of apical-basal axis orientation and epithelial lumen positioning. Trends Cell Biol 2015; 25(8):476-85; PMID:25941134 [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Deretic D. The Arf/Rab11 effector FIP3 acts synergistically with the Arf GAP ASAP1 to direct Rabin8 in ciliary receptor targeting. J Cell Sci 2015; 128(7):1375-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol 1996; 135:913-24; PMID:8922376; http://dx.doi.org/ 10.1083/jcb.135.4.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen W, Feng Y, Chen D, Wandinger-Ness A. Rab11 is required for trans-golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol Biol Cell 1998; 9:3241-57; PMID:9802909; http://dx.doi.org/ 10.1091/mbc.9.11.3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren M, Xu G, Zeng J, De Lemos-Chiarandini C, Adesnik M, Sabatini DD. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc Natl Acad Sci U S A 1998; 95:6187-92; PMID:9600939; http://dx.doi.org/ 10.1073/pnas.95.11.6187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pellinen T, Ivaska J. Integrin traffic. J Cell Sci 2006; 119:3723-31; PMID:16959902; http://dx.doi.org/ 10.1242/jcs.03216 [DOI] [PubMed] [Google Scholar]
- 48.Knowles BC, Weis VG, Yu S, Roland JT, Williams JA, Alvarado GS, Lapierre LA, Shub MD, Gao N, Goldenring JR. Rab11a regulates syntaxin 3 localization and microvillus assembly in enterocytes. J Cell Sci 2015; 128:1617-26; PMID:25673875; http://dx.doi.org/ 10.1242/jcs.163303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reish NJ, Boitet ER, Bales KL, Gross AK. Nucleotide bound to rab11a controls localization in rod cells but not interaction with rhodopsin. J Neurosci 2014; 34:14854-63; PMID:25378153; http://dx.doi.org/ 10.1523/JNEUROSCI.1943-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grossman GH, Watson RF, Pauer GJ, Bollinger K, Hagstrom SA. Immunocytochemical evidence of Tulp1-dependent outer segment protein transport pathways in photoreceptor cells. Exp Eye Res 2011; 93:658-68; PMID:21867699; http://dx.doi.org/ 10.1016/j.exer.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barrowman J, Bhandari D, Reinisch K, Ferro-Novick S. TRAPP complexes in membrane traffic: convergence through a common Rab. Nat Rev Mol Cell Biol 2010; 11:759-63; PMID:20966969; http://dx.doi.org/ 10.1038/nrm2999 [DOI] [PubMed] [Google Scholar]
- 52.Morozova N, Liang Y, Tokarev AA, Chen SH, Cox R, Andrejic J, Lipatova Z, Sciorra VA, Emr SD, Segev N. TRAPPII subunits are required for the specificity switch of a Ypt-Rab GEF. Nat Cell Biol 2006; 8:1263-9; PMID:17041589; http://dx.doi.org/ 10.1038/ncb1489 [DOI] [PubMed] [Google Scholar]
- 53.Pinar M, Arst HN Jr, Pantazopoulou A, Tagua VG, de los Ríos V, Rodríguez-Salarichs J, Díaz JF, Peñalva MA. TRAPPII regulates exocytic Golgi exit by mediating nucleotide exchange on the Ypt31 ortholog RabERAB11. Proc Natl Acad Sci U S A 2015; 112:4346-51; PMID:25831508; http://dx.doi.org/ 10.1073/pnas.1419168112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levine TP, Daniels RD, Wong LH, Gatta AT, Gerondopoulos A, Barr FA. Discovery of new Longin and Roadblock domains that form platforms for small GTPases in Ragulator and TRAPP-II. Small GTPases 2013; 4:62-9; PMID:23511850; http://dx.doi.org/ 10.4161/sgtp.24262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schou KB, Morthorst SK, Christensen ST, Pedersen LB. Identification of conserved, centrosome-targeting ASH domains in TRAPPII complex subunits and TRAPPC8. Cilia 2014; 3:6; PMID:25018876; http://dx.doi.org/ 10.1186/2046-2530-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson GM, Fielding AB, Simon GC, Yu X, Andrews PD, Hames RS, Frey AM, Peden AA, Gould GW, Prekeris R. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell 2005; 16:849-60; PMID:15601896; http://dx.doi.org/ 10.1091/mbc.E04-10-0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schiel JA, Simon GC, Zaharris C, Weisz J, Castle D, Wu CC, Prekeris R. FIP3-endosome-dependent formation of the secondary ingression mediates ESCRT-III recruitment during cytokinesis. Nat Cell Biol 2012; 14:1068-78; PMID:23000966; http://dx.doi.org/ 10.1038/ncb2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R, Gould GW. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. Embo J 2005; 24:3389-99; PMID:16148947; http://dx.doi.org/ 10.1038/sj.emboj.7600803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eathiraj S, Mishra A, Prekeris R, Lambright DG. Structural basis for Rab11-mediated recruitment of FIP3 to recycling endosomes. J Mol Biol 2006; 364:121-35; PMID:17007872; http://dx.doi.org/ 10.1016/j.jmb.2006.08.064 [DOI] [PubMed] [Google Scholar]
- 60.Horgan CP, Oleksy A, Zhdanov AV, Lall PY, White IJ, Khan AR, Futter CE, McCaffrey JG, McCaffrey MW. Rab11-FIP3 is critical for the structural integrity of the endosomal recycling compartment. Traffic 2007; 8:414-30; PMID:17394487; http://dx.doi.org/ 10.1111/j.1600-0854.2007.00543.x [DOI] [PubMed] [Google Scholar]
- 61.Inoue H, Ha VL, Prekeris R, Randazzo PA. Arf GTPase-activating protein ASAP1 interacts with Rab11 effector FIP3 and regulates pericentrosomal localization of transferrin receptor-positive recycling endosome. Mol Biol Cell 2008; 19:4224-37; PMID:18685082; http://dx.doi.org/ 10.1091/mbc.E08-03-0290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vetter M, Stehle R, Basquin C, Lorentzen E. Structure of Rab11-FIP3-Rabin8 reveals simultaneous binding of FIP3 and Rabin8 effectors to Rab11. Nat Struct Mol Biol 2015; 22(9):695-702; PMID:26258637 [DOI] [PubMed] [Google Scholar]
- 63.Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science 2001; 294:1299-304; PMID:11701921; http://dx.doi.org/ 10.1126/science.1062023 [DOI] [PubMed] [Google Scholar]
- 64.Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol 2005; 12:879-85; PMID:16155582; http://dx.doi.org/ 10.1038/nsmb987 [DOI] [PubMed] [Google Scholar]
- 65.Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem 2004; 279:43027-34; PMID:15292201; http://dx.doi.org/ 10.1074/jbc.M402264200 [DOI] [PubMed] [Google Scholar]
- 66.Zeng J, Ren M, Gravotta D, De Lemos-Chiarandini C, Lui M, Erdjument-Bromage H, Tempst P, Xu G, Shen TH, Morimoto T, et al.. Identification of a putative effector protein for rab11 that participates in transferrin recycling. Proc Natl Acad Sci U S A 1999; 96:2840-5; PMID:10077598; http://dx.doi.org/ 10.1073/pnas.96.6.2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eathiraj S, Pan X, Ritacco C, Lambright DG. Structural basis of family-wide Rab GTPase recognition by rabenosyn-5. Nature 2005; 436:415-9; PMID:16034420; http://dx.doi.org/ 10.1038/nature03798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shiba T, Koga H, Shin HW, Kawasaki M, Kato R, Nakayama K, Wakatsuki S. Structural basis for Rab11-dependent membrane recruitment of a family of Rab11-interacting protein 3 (FIP3)/Arfophilin-1. Proc Natl Acad Sci U S A 2006; 103:15416-21; PMID:17030804; http://dx.doi.org/ 10.1073/pnas.0605357103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jagoe WN, Lindsay AJ, Read RJ, McCoy AJ, McCaffrey MW, Khan AR. Crystal structure of rab11 in complex with rab11 family interacting protein 2. Structure 2006; 14:1273-83; PMID:16905101; http://dx.doi.org/ 10.1016/j.str.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 70.Burke JE, Inglis AJ, Perisic O, Masson GR, McLaughlin SH, Rutaganira F, Shokat KM, Williams RL. Structures of PI4KIIIbeta complexes show simultaneous recruitment of Rab11 and its effectors. Science 2014; 344:1035-8; PMID:24876499; http://dx.doi.org/ 10.1126/science.1253397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Graaf P, Zwart WT, van Dijken RA, Deneka M, Schulz TK, Geijsen N, Coffer PJ, Gadella BM, Verkleij AJ, van der Sluijs P, et al.. Phosphatidylinositol 4-kinasebeta is critical for functional association of rab11 with the Golgi complex. Mol Biol Cell 2004; 15:2038-47; PMID:14767056; http://dx.doi.org/ 10.1091/mbc.E03-12-0862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee MT, Mishra A, Lambright DG. Structural mechanisms for regulation of membrane traffic by rab GTPases. Traffic 2009; 10:1377-89; PMID:19522756; http://dx.doi.org/ 10.1111/j.1600-0854.2009.00942.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pasqualato S, Senic-Matuglia F, Renault L, Goud B, Salamero J, Cherfils J. The structural GDP/GTP cycle of Rab11 reveals a novel interface involved in the dynamics of recycling endosomes. J Biol Chem 2004; 279:11480-8; PMID:14699104; http://dx.doi.org/ 10.1074/jbc.M310558200 [DOI] [PubMed] [Google Scholar]
- 74.Chiba S, Amagai Y, Homma Y, Fukuda M, Mizuno K. NDR2-mediated Rabin8 phosphorylation is crucial for ciliogenesis by switching binding specificity from phosphatidylserine to Sec15. EMBO J 2013; 32(6):874-85; PMID:23435566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caberoy NB, Zhou Y, Alvarado G, Fan X, Li W. Efficient identification of phosphatidylserine-binding proteins by ORF phage display. Biochem Biophys Res Commun 2009; 386:197-201; PMID:19520055; http://dx.doi.org/ 10.1016/j.bbrc.2009.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mazelova J, Ransom N, Astuto-Gribble L, Wilson MC, Deretic D. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J Cell Sci 2009; 122:2003-13; PMID:19454479; http://dx.doi.org/ 10.1242/jcs.039982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berta AI, Boesze-Battaglia K, Genini S, Goldstein O, O'Brien PJ, Szél Á, Acland GM, Beltran WA, Aguirre GD. Photoreceptor cell death, proliferation and formation of hybrid rod/S-cone photoreceptors in the degenerating STK38L mutant retina. PLoS One 2011; 6, e24074; PMID:21980341; http://dx.doi.org/ 10.1371/journal.pone.0024074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goldstein O, Kukekova AV, Aguirre GD, Acland GM. Exonic SINE insertion in STK38L causes canine early retinal degeneration (erd). Genomics 2010; 96:362-8; PMID:20887780; http://dx.doi.org/ 10.1016/j.ygeno.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mizuno-Yamasaki E, Medkova M, Coleman J, Novick P. Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev Cell 2010; 18:828-40; PMID:20493815; http://dx.doi.org/ 10.1016/j.devcel.2010.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stalder D, Mizuno-Yamasaki E, Ghassemian M, Novick PJ. Phosphorylation of the Rab exchange factor Sec2p directs a switch in regulatory binding partners. Proc Natl Acad Sci U S A 2013; 110:19995-20002; PMID:24248333; http://dx.doi.org/ 10.1073/pnas.1320029110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo Z, Hou X, Goody RS, Itzen A. Intermediates in the guanine nucleotide exchange reaction of Rab8 protein catalyzed by guanine nucleotide exchange factors Rabin8 and GRAB. J Biol Chem 2013; 288:32466-74; PMID:24072714; http://dx.doi.org/ 10.1074/jbc.M113.498329 [DOI] [PMC free article] [PubMed] [Google Scholar]