ABSTRACT
Rab GTPases, which form the largest branch of the Ras GTPase superfamily, regulate almost every step of vesicle-mediated trafficking. Among them, Rab8 is an essential participant in primary cilium formation. In a report recently published in the Journal of Cell Science, Finetti and colleagues identify Rab8 as a novel player in vesicular traffic in the non-ciliated T lymphocytes, which contributes to the assembly of the specialized signaling platform known as the immune synapse. By interacting with the v-SNARE VAMP-3, Rab8 is indeed responsible for the final docking/fusion step in T cell receptor (TCR) recycling to the immune synapse. A second important take-home message which comes to light from this work is that VAMP-3 also interacts with Rab8 at the base of the cilium in NIH-3T3 cells, where it regulates ciliary growth and targeting of Smoothened at the plasma membrane. Hence the data presented in this report, in addition to identifying Rab8 as a novel player in vesicular traffic to the immune synapse, reveal how both ciliated and non-ciliated cells take advantage of a conserved pathway to build highly specific cellular structures.
KEYWORDS: GTPase, immune synapse, primary cilium, Rab8, receptor recycling, TCR
The primary cilium and the immune synapse as paradigms of polarized cellular structures: The IFT20 connection
What does “polarized cell” mean? As extensively discussed in the literature, it defines cells whose shape changes. This process is mainly primed by the microenvironment: cells, cytokines or the extracellular matrix.1 At the molecular level, the cellular shape is dictated by i) the polymerization/depolymerization of the actin and tubulin cytoskeleton, which is regulated by the activation of the Rho family of small GTPases,2 and ii) the dynamic formation of discrete membrane/sub-membrane domains with specialized functions. This latter mechanism is largely regulated by the ADP-ribosylation factor (Arf) and Rab families of small GTPases, which are responsible for the directional transport of molecules toward specific areas of the plasma membrane to carry out specific functions.3,4
One of the main structures identifying polarized cells is the primary cilium, which is built by almost every cell type usually at the apical cell surface and acts as sensory organelle that transmits signals from the extracellular environment. The assembly of the primary cilium requires the recycling/transport machinery to be polarized toward its base where the centrosome is positioned.1,5
On the other hand, a specialized cell structure exists, knows as the immune synapse, which, as opposed to the widespread cilium, is only built by the non-ciliated T lymphocytes when they encounter an antigen-presenting cell displaying specific antigen associated to the major histocompatibility complex. This membrane area concentrates for a prolonged timeframe all the key molecules responsible for signal transduction and amplification, while keeping out molecules responsible for turning off signals.6
Although these 2 cellular areas are very different in terms of structure and molecular composition, they remarkably share the basic building and maintenance mechanisms, which are guided by the polarization of the centrosome, which represents the key event for the directional transport of molecules and signaling mediators to the nascent structures. Complexes of proteins of the intraflagellar transport (IFT) family are involved in their assembly. In the cilium, they are known to pick up specific cargoes at the base and release them at the tip, transporting then new cargoes back to the cell body.7,8 Of note, a specific member of the IFT complex, IFT20, that is responsible for the assembly and maintenance of cilia and flagella,7,9 in concert with other IFT proteins, is also required for immune synapse assembly.10,11 IFT20 assists indeed polarized recycling of endosomal TCRs from a intracellular pool to the immune synapse,10,11 allowing the replacement of exhausted TCRs as these are cleared from the immune synapse by receptor-mediated endocytosis.8,12
In our recent work published in the Journal of Cell Science, we identify a new component of this shared building machinery, namely the small GTPase Rab8.
Rab8: The new player of the game
At the cilium
The existence of a close cross-talk between membrane trafficking and cytoskeletal dynamics is supported by the fact that a subset of molecules regulates both molecular mechanisms. Among them the Rab GTPases, which are involved in almost every step of vesicle-mediated transport.4 Each member of this large subfamily of Ras GTPases has a distinct subcellular localization that correlates with the compartments between which they coordinate transport.13 Rab8 is a paradigm of this complex coordinating activity, as it strongly influences cell morphogenesis through linking membrane recycling to reorganization of the actin and microtubule cytoskeleton.14 Its localization to the primary cilium, centrosome, tubules, macropinosomes and at the leading edge or at the tracking tail of migrating cells confers indeed to this small molecule an important role in mediating cell polarity, cell migration and ciliogenesis.1
Two mammalian Rab8 isoforms have been reported with unknown functional differences, Rab8a and Rab8b which, although encoded by separate genes, harbor high sequence similarity.15,16 Rab8 activity is regulated by specific guanine nucleotide exchange factors (GEFs), that mediate GTP loading, and GTPase activating proteins (GAPs) that convert them into the inactive GDP-bound form.1
At the base of the primary cilium, Rab8 activation relies on Rab11 which contributes, in its GTP-bound form,17 to the recruitment of the Rab8 GEF, Rabin8,18-20 to pericentrosomal vesicles. The transport protein particle (TRAPP) II complex directly associates with Rabin8, thus participating in polarized transport of Rabin8 to the centrosome.21 At the centrosome Rabin8 associates then with Rab8 exploiting its exchange activity.17 Activated Rab8 is now ready to participate in the formation of primary cilium. Furthermore, it also regulates the transport of specific receptors, such as Smoothened22 and fibrocystin23 to the cilium (Figure 1, right).
Figure 1.
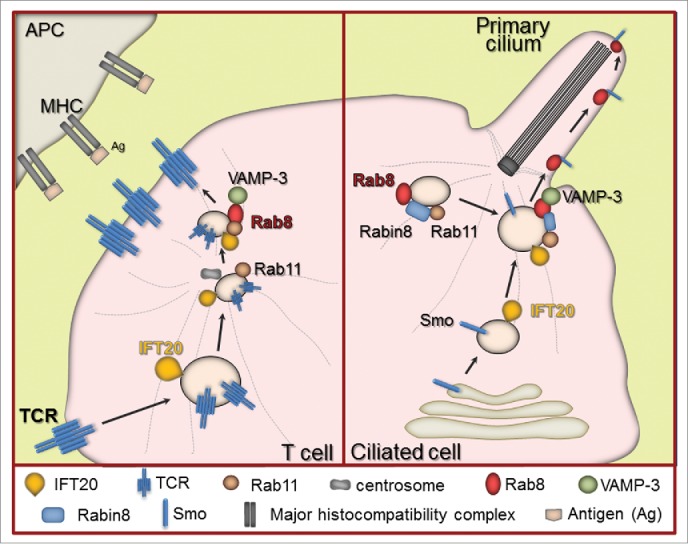
A common vesicular trafficking module in the assembly of the immune synapse and the primary cilium. Left. Following encounter with specific antigen (Ag) associated to the major histocompatibility complex (MHC) on an antigen presenting cell (APC), engaged TCRs initiate a signaling cascade that promotes polarized recycling of the non-engaged TCRs from an intracellular endosome-associated pool to the immune synapse. IFT20 promotes transit of TCR+ vesicles to Rab11+ recycling endosomes that are transported to the centrosome. Rab8 regulates the last step of vesicle transport and fusion with the contact area by interacting with the v-SNARE VAMP3. Right. The delivery of the ciliary receptor Smoothened (Smo) to the ciliary membrane requires IFT20, which is associated with vesicles destined for the primary cilium asnd subsequently directed to a Rab8+ vesicle compartment at the base of the cilium. Rab8 and its GEF Rabin8 are transported to this location in Rab11+ vesicles. Vesicles subsequently dock at and fuse with the periciliary membrane as a result of the interaction of Rab8 with the v-SNARE VAMP3.
At the immune synapse
How does this model fit with the regulation of immune synapse assembly and maintenance?
The TCR exploits the recycling machinery, which moves along microtubule tracks, to traffic to the “hot plasma-membrane zone” of the immune synapse following antigen-specific stimulation.8 Although the trafficking machinery underlying this process has as yet not been fully elucidated, it is known that it uses both the slow Rab11-dependent and the fast Rab4-dependent recycling pathways.24,25 Also Rab35, with its specific GAP EPI64C, has been implicated in this process.26
Where does Rab8 come in? A recent report by Soares and colleagues shows that in T lymphocytes Rab8 colocalizes with TCR+ endosomes,27 suggesting a role in TCR trafficking. Finetti and colleagues added a tile to the puzzle by clearly demonstrating that Rab8, which is localized pericentrosomally together with Rab11, is associated with TCR+ endosomes actively recycling to the plasma membrane.28 Hence, similar to IFT20, Rab8 appears as a candidate key molecule in the assembly and maintenance of polarized cell structures. The GTPase activity of Rab8 is required for TCR trafficking at the immune synapse as expression of a dominant negative Rab8 mutant results in a profound impairment in TCR recycling and, conversely, recycling is enhanced in cells transfected with a constitutively active Rab8 mutant.
Interestingly, our data highlight an interplay between Rab8 and IFT20, as the endosomal Rab8+/Rab11+ compartment is also enriched in IFT20, which we have reported to be essential for TCR recycling to the immune synapse.10 Hence Rab8, while not directly interacting, at least coordinates with IFT20 to control TCR recycling.
One of the key steps in the development of our work was the spatio-temporal characterization of the IFT20/Rab8-dependent pathway that regulates TCR recycling. Of the 2 molecules which comes first and which comes later? We capitalized on our previous finding that, while TCR+ endosomes fail to polarize to the immune synapse in the absence of IFT20, the centrosome translocates normally toward the T cell:APC contact under these conditions,10 implicating IFT20 at a very early step in the TCR recycling pathway, which we have later identified as the sorting/traffic from Rab5+ early endosomes.11 By expressing a constitutively active Rab8 mutant in T cells depleted of IFT20 by RNA interference, we observed that it did not rescue the TCR recycling defect, while re-expression of IFT20 in these cells rescued the defect in TCR recycling, demonstrating that IFT20 and Rab8 participate in the same pathway, with IFT20 required for Rab8 to promote this process.28
The regulatory activity of Rab8 can be either IFT20-dependent or IFT20-independent
We recently reported that IFT20 regulates not only TCR recycling, but also recycling of the Transferrin Receptor (TfR),11 which recycles through both the Rab4- and the Rab11-dependent routes.29 Surprisingly and very interestingly, expres of the Rab8 mutants did not affect TfR recycling, notwithstanding the fact that, similar to the TCR, the recycling route of this receptor is regulated by IFT20. In the same report we also showed that, as opposed to the TfR pathway, the Rab11-dependent recycling pathway of the G-protein-coupled receptor chemokine receptor CXCR424 does not require IFT20,11 but is dependent on Rab8 activity.28 Hence the different recycling efficiencies of these 3 membrane receptors are accounted for by their differential recycling pathways, which are defined by the alternative combinatorial usage of various components of the recycling machinery.
Rab8 is required for the final fusion step of recycling vesicles
In T cells
The immunofluorescence analysis of actively recycling TCR receptors in T cells expressing dominant negative Rab8 revealed a distribution that we were initially unable to explain. This Rab8 mutant impaired indeed TCR recycling at the plasma membrane of the immune synapse while, surprisingly, it did not affect the localization of the intracellular TCR+ compartment which appeared to be correctly polarized at the synapse together with the centrosome. This finding led us to map Rab8 in the final steps of the IFT20-dependent pathway that controls polarized TCR recycling, when the TCR cargoes are delivered to the plasma membrane. This step crucially depends on the recruitment to the recycling endosomes of a specific v-SNARE that, by interacting with a cognate membrane-associated t-SNARE, promotes its fusion with the plasma membrane.30 We capitalized on the reports demonstrating that the v-SNAREs VAMP-3 and VAMP-7 are recruited to the immune synapse12,30,31 and decided to focus on VAMP-3, which is known to be required for the accumulation of recycling TCR at the immune synapse.12 Our data reveal that VAMP-3 interacts with Rab8, becoming hence the candidate key mediator of the last fusion step with the plasma membrane of TCR recycling vesicles. This activity requires the GTPase activity of Rab8, since when we used the dominant negative Rab8 mutant we found that the localization of VAMP-3 at the immune synapse was impaired. Collectively, these data depict therefore a scenario where Rab8, while dispensable for the traffic of TCR+ vesicles to the centrosome, controls the last step of fusion with the plasma membrane by transporting the essential v-SNARE VAMP-3.
In ciliated cells
Rab8, together with the IFT system, is essential for ciliogenesis1,7,9 and, as we have shown, for immune synapse assembly,10,28 suggesting that the mechanisms regulating the assembly of these 2 cellular structures are conserved. Could therefore the VAMP-3/Rab8 interplay, which we characterized in T cells, also be operational in ciliated cells? Definitely yes, as VAMP-3 localizes to a vesicular compartment at the base of the primary cilium in NIH-3T3 cells, where it is required to the delivery of Smoothened cargoes to the ciliary membrane. Indeed, in a clear parallelism with the immune synapse, reducing the levels of VAMP-3 leads to the growth of a shorter cilium and impaired ciliary targeting of Smoothened.
Conclusion
The results reported by Finetti and colleagues highlight a novel role of Rab8, downstream of IFT20, in the pathway that regulates TCR recycling, through recruiting VAMP-3 and promoting the fusion with the synaptic membrane of endosomes carrying TCR cargoes. A crosstalk exists moreover emerges between Rab8 and IFT20 in TCR trafficking and immune synapse assembly, although these 2 molecules are differentially involved in recycling of TfR and CXCR4. These data suggest that Rab8 might help increasing the fidelity of immune synapse targeting of different receptors, whose localization in discrete subdomains of the immune synapse is fundamental to reach the correct structure.6 The function of VAMP-3 as a Rab8 effector in the control of TCR and Smoothened trafficking to the immune synapse and the primary cilium, respectively, further highlights moreover the remarkable conservation in the pathways that orchestrate immune synapse assembly and ciliogenesis.8,32-34
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The work was supported by Telethon - Italy (Grant GGP1102).
References
- [1].Peranen J. Rab8 GTPase as a regulator of cell shape. Cytoskeleton (Hoboken) 2011; 68:527-39; PMID:21850707; http://dx.doi.org/ 10.1002/cm.20529 [DOI] [PubMed] [Google Scholar]
- [2].Sadok A, Marshall CJ. Rho GTPases: masters of cell migration. Small GTPases 2014; 5:e29710; PMID:24978113; http://dx.doi.org/ 10.4161/sgtp.29710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kahn RA, Volpicelli-Daley L, Bowzard B, Shrivastava-Ranjan P, Li Y, Zhou C, Cunningham L. Arf family GTPases: roles in membrane traffic and microtubule dynamics. Biochem Soc Trans 2005; 33:1269-72; PMID:16246095; http://dx.doi.org/ 10.1042/BST0331269 [DOI] [PubMed] [Google Scholar]
- [4].Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2001; 2:107-17; PMID:11252952; http://dx.doi.org/ 10.1038/35052055 [DOI] [PubMed] [Google Scholar]
- [5].Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science 2006; 313:629-33; PMID:16888132; http://dx.doi.org/ 10.1126/science.1124534 [DOI] [PubMed] [Google Scholar]
- [6].Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, Sacristan C, Victora GD, Zanin-Zhorov A, Dustin ML. Functional anatomy of T cell activation and synapse formation. Annu Rev Immunol 2010; 28:79-105; PMID:19968559; http://dx.doi.org/ 10.1146/annurev-immunol-030409-101308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol 2008; 85:23-61; PMID:19147001; http://dx.doi.org/ 10.1016/S0070-2153(08)00802-8 [DOI] [PubMed] [Google Scholar]
- [8].Finetti F, Onnis A, Baldari CT. Regulation of vesicular traffic at the T cell immune synapse: lessons from the primary cilium. Traffic 2015; 16:241-9; PMID:25393976; http://dx.doi.org/ 10.1111/tra.12241 [DOI] [PubMed] [Google Scholar]
- [9].Pazour GJ, Bloodgood RA. Targeting proteins to the ciliary membrane. Curr Top Dev Biol 2008; 85:115-49; PMID:19147004; http://dx.doi.org/ 10.1016/S0070-2153(08)00805-3 [DOI] [PubMed] [Google Scholar]
- [10].Finetti F, Paccani SR, Riparbelli MG, Giacomello E, Perinetti G, Pazour GJ, Rosenbaum JL, Baldari CT. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat Cell Biol 2009; 11:1332-9; PMID:19855387; http://dx.doi.org/ 10.1038/ncb1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Finetti F, Patrussi L, Masi G, Onnis A, Galgano D, Lucherini OM, Pazour GJ, Baldari CT. Specific recycling receptors are targeted to the immune synapse by the intraflagellar transport system. J Cell Sci 2014; 127:1924-37; PMID:24554435; http://dx.doi.org/ 10.1242/jcs.139337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Das V, Nal B, Dujeancourt A, Thoulouze MI, Galli T, Roux P, Dautry-Varsat A, Alcover A. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity 2004; 20:577-88; PMID:15142526; http://dx.doi.org/ 10.1016/S1074-7613(04)00106-2 [DOI] [PubMed] [Google Scholar]
- [13].Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 2011; 91:119-49; PMID:21248164; http://dx.doi.org/ 10.1152/physrev.00059.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Peranen J, Auvinen P, Virta H, Wepf R, Simons K. Rab8 promotes polarized membrane transport through reorganization of actin and microtubules in fibroblasts. J Cell Biol 1996; 135:153-67; PMID:8858170; http://dx.doi.org/ 10.1083/jcb.135.1.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Armstrong J, Thompson N, Squire JH, Smith J, Hayes B, Solari R. Identification of a novel member of the Rab8 family from the rat basophilic leukaemia cell line, RBL.2H3. J Cell Sci 1996; 109:1265-74; PMID:8799816 [DOI] [PubMed] [Google Scholar]
- [16].Chavrier P, Vingron M, Sander C, Simons K, Zerial M. Molecular cloning of YPT1/SEC4-related cDNAs from an epithelial cell line. Mol Cell Biol 1990; 10:6578-85; PMID:2123294; http://dx.doi.org/ 10.1128/MCB.10.12.6578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A 2010; 107:6346-51; PMID:20308558; http://dx.doi.org/ 10.1073/pnas.1002401107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hattula K, Furuhjelm J, Arffman A, Peranen J. A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell 2002; 13:3268-80; PMID:12221131; http://dx.doi.org/ 10.1091/mbc.E02-03-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hattula K, Peranen J. Purification and functional properties of a Rab8-specific GEF (Rabin3) in action remodeling and polarized transport. Methods Enzymol 2005; 403:284-95; PMID:16473595; http://dx.doi.org/ 10.1016/S0076-6879(05)03024-7 [DOI] [PubMed] [Google Scholar]
- [20].Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al.. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 2007; 129:1201-13; PMID:17574030; http://dx.doi.org/ 10.1016/j.cell.2007.03.053 [DOI] [PubMed] [Google Scholar]
- [21].Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, et al.. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A 2011; 108:2759-64; PMID:21273506; http://dx.doi.org/ 10.1073/pnas.1018823108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Boehlke C, Bashkurov M, Buescher A, Krick T, John AK, Nitschke R, Walz G, Kuehn EW. Differential role of Rab proteins in ciliary trafficking: Rab23 regulates smoothened levels. J Cell Sci 2010; 123:1460-7; PMID:20375059; http://dx.doi.org/ 10.1242/jcs.058883 [DOI] [PubMed] [Google Scholar]
- [23].Follit JA, Li L, Vucica Y, Pazour GJ. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J Cell Biol 2010; 188:21-8; PMID:20048263; http://dx.doi.org/ 10.1083/jcb.200910096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kumar A, Kremer KN, Dominguez D, Tadi M, Hedin KE. Galpha13 and Rho mediate endosomal trafficking of CXCR4 into Rab11+ vesicles upon stromal cell-derived factor-1 stimulation. J Immunol 2011; 186:951-8; PMID:21148034; http://dx.doi.org/ 10.4049/jimmunol.1002019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu H, Rhodes M, Wiest DL, Vignali DA. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity 2000; 13:665-75; PMID:11114379; http://dx.doi.org/ 10.1016/S1074-7613(00)00066-2 [DOI] [PubMed] [Google Scholar]
- [26].Patino-Lopez G, Dong X, Ben-Aissa K, Bernot KM, Itoh T, Fukuda M, Kruhlak MJ, Samelson LE, Shaw S. Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J Biol Chem 2008; 283:18323-30; PMID:18450757; http://dx.doi.org/ 10.1074/jbc.M800056200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Soares H, Henriques R, Sachse M, Ventimiglia L, Alonso MA, Zimmer C, Thoulouze MI, Alcover A. Regulated vesicle fusion generates signaling nanoterritories that control T cell activation at the immunological synapse. J Exp Med 2013; 210:2415-33; PMID:24101378; http://dx.doi.org/ 10.1084/jem.20130150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Finetti F, Patrussi L, Galgano D, Cassioli C, Perinetti G, Pazour GJ, Baldari CT. The small GTPase Rab8 interacts with VAMP-3 to regulate the delivery of recycling T-cell receptors to the immune synapse. J Cell Sci 2015; 128:2541-52; PMID:26034069; http://dx.doi.org/ 10.1242/jcs.171652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mayle KM, Le AM, Kamei DT. The intracellular trafficking pathway of transferrin. Biochim Biophys Acta 2012; 1820:264-81; PMID:21968002; http://dx.doi.org/ 10.1016/j.bbagen.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hay JC. SNARE complex structure and function. Exp Cell Res 2001; 271:10-21; PMID:11697877; http://dx.doi.org/ 10.1006/excr.2001.5368 [DOI] [PubMed] [Google Scholar]
- [31].Larghi P, Williamson DJ, Carpier JM, Dogniaux S, Chemin K, Bohineust A, Danglot L, Gaus K, Galli T, Hivroz C. VAMP7 controls T cell activation by regulating the recruitment and phosphorylation of vesicular Lat at TCR-activation sites. Nat Immunol 2013; 14:723-31; PMID:23666293; http://dx.doi.org/ 10.1038/ni.2609 [DOI] [PubMed] [Google Scholar]
- [32].de la Roche M, Ritter AT, Angus KL, Dinsmore C, Earnshaw CH, Reiter JF, Griffiths GM. Hedgehog signaling controls T cell killing at the immunological synapse. Science 2013; 342:1247-50; PMID:24311692; http://dx.doi.org/ 10.1126/science.1244689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Baldari CT, Rosenbaum J. Intraflagellar transport: it's not just for cilia anymore. Curr Opin Cell Biol 2010; 22:75-80; PMID:19962875; http://dx.doi.org/ 10.1016/j.ceb.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Finetti F, Baldari CT. Compartmentalization of signaling by vesicular trafficking: a shared building design for the immune synapse and the primary cilium. Immunol Rev 2013; 251:97-112; PMID:23278743; http://dx.doi.org/ 10.1111/imr.12018 [DOI] [PubMed] [Google Scholar]


