Abstract
This review addresses the developmental roles of 2 GTPases of the Rho family, RhoV/Chp and RhoU/Wrch. These two GTPases form a distinct subfamily related to Rac and Cdc42 proteins and were detected in a screen for Rho members that are particularly expressed in the neural crest, an embryonic tissue peculiar to vertebrates. The neural crest represents a physiological model of normal epithelial to mesenchymal transition (EMT), in which epithelial cells at the border of neural and non-neural ectoderm differentiate, lose their intercellular connections and migrate throughout the embryo. We showed that RhoV, transiently induced by the canonical Wnt pathway, is required for the full differentiation of neural crest cells, while RhoU, induced later by the non-canonical Wnt pathway, is necessary for the migration process. These two GTPases, which are highly conserved across vertebrates, are thus tightly functionally linked to Wnt signaling, whose implication in embryonic development and cancer progression is well established. In the light of the recent literature, we discuss how RhoV and RhoU may achieve their physiological functions.
Keywords: atypical Rho GTPases, development, neural crest, RhoU, RhoV, Wnt signaling, xenopus
Abbreviations
- NC
neural crest
- BMP
bone morphogenic protein
- FGF
fibroblast growth factor
- EMT
epithelial-mesenchymal transition
- EGFR
epidermal growth factor receptor.
Introduction
Development of the Neural Crest (NC) is probably the most dramatic morphogenetic event of vertebrate embryogenesis. Originating at the boundary between neural and non-neural ectoderm, NC cells differentiate in response to complex inductive cues emanating from the surrounding tissues.1 At this early stage, NC cells express a set of transcription factors, such as Snai1/Snail, Snai2/Slug or Twist, which are known for their pro-invasive activities in stem cells and cancer cells.2 After commitment (specification stage), NC cells migrate throughout the embryo and differentiate to form a broad range of terminal derivatives, including pigment cells, craniofacial skeleton, cartilage, neurons or glia of the peripheral nervous system.3 Among the morphogens required for proper NC development, BMP, FGF, Notch and canonical Wnt pathways have prominent roles in NC induction, while non-canonical Wnt is required for NC migration.4,5 Prior to migration, NC cells undergo a delamination phase, characterized by the loss of epithelial adherens junctions and the acquisition of invasive properties. This developmental process, known as epithelial to mesenchymal transition (EMT), has been proposed to mimic very early events of malignant progression, in which adherent adenoma cells switch to an invasive carcinoma phenotype.6
Because of their impact on adhesion and migration dynamics of many cell types,7 GTPases of the Rho family were suspected to be involved in NC cell dynamics, and several studies pointed to a role of the major Rho family members Rho and Rac1 in NC formation in the Xenopus embryo.8-10 Xenopus represents a model of choice for experimental embryology, mostly because of its rapid embryonic development and the large size of its eggs, which makes them amenable to microinjection and microdissection. Another major advantage of this model is the possibility of manipulating just one side of the embryo, while the other side serves as an internal control of development. Xenopus is also ideal because it contains orthologues for 18 of the 20 Rho family members found in placentals.11 We performed a comprehensive in situ hybridization screen to identify Rho members that are preferentially expressed in NC. Apart from RhoB and Rnd1, we identified RhoV/Chp and RhoU/Wrch, as being expressed sequentially at distinct NC developmental stages.12,13
RhoV and RhoU form an ancient Rho subfamily related to Rac1 and Cdc42 GTPases.11 RhoV and RhoU are atypical in this family as they display a high intrinsic guanine nucleotide exchange activity and are thus thought to be constitutively active whenever they are expressed.14,15 In keeping with their spontaneous activation, they are expressed at very low levels (in particular RhoV) in various tissues and organs.11 Furthermore, they are palmitoylated and not prenylated like most Rho members, suggesting that they act at distinct subcellular locations,16 and they contain additional N-terminal and C-terminal extensions, critical for their activities.15,16 Despite the knowledge of their biochemical properties, little was known about the physiological function of these 2 GTPases, and the work we performed on Xenopus embryos unveiled their roles in NC development.
RhoV
RhoV is induced in the prospective NC territory as a canonical Wnt response gene, expressed as early as Snai1.13 RhoV induction in response to Wnt is independent of Snai1, since expression of a dominant negative Snai1 mutant in Wnt1-treated embryos did not impair RhoV expression, whereas it blocked the subsequent induction of the Snai2 or Sox9 genes (unpublished data). RhoV expression is transient and is no longer detected at the migration stage. RhoV knockdown by antisense morpholino injection perturbs NC differentiation: while having no effect on the early Snai1 expression, it impaired induction of the Snai2, Twist or Sox9 genes. Consequently, NC-derived cranial structures are strongly inhibited in morphant embryos. Conversely, RhoV overexpression expands the NC territory and increases the expression of Snai1, Snai2 and Twist, indicating that RhoV feeds positively the canonical Wnt pathway. RhoV was shown to activate PAK1,17 a member of a family of versatile kinases involved in cell migration and invasion.18 PAK1 itself can phosphorylate and activate Snai1.19 Since Snai1 activity is critical for NC induction,20 RhoV might thus participate in the propagation and amplification of the canonical Wnt pathway. RhoV activity is also probably relies on its activity on cell adhesion, as recently shown in the zebrafish embryo, wherein RhoV is required for proper localization of E-cadherin and β-catenin at adherens junctions.21 Along the same line, we observed that the neural plate was expanded upon RhoV inhibition and restricted upon moderate RhoV over-expression. This supports a role of RhoV in cell motility since folding of the neural plate is sensitive to the medial migration of NC cells.22
RhoU
As a non-canonical Wnt response gene,23,24 RhoU was expected to be involved in NC cell migration5 and its expression was indeed detected only from the migration stage in NC cells. RhoU depletion impaired NC migration and the subsequent formation of craniofacial cartilages.12 NC cells from RhoU-depleted explants adopted a rounded phenotype and showed reduced adhesion to the substrate. Intriguingly, these effects are in contradiction with the increased density of integrin-dependent adhesive structures observed in RhoU-silenced mammalian cells.25,26 Moderate RhoU overexpression also inhibited NC cell migration but with a distinct mechanism; RhoU-expressing explants readily adhered to the substrate and migratory NC cells scattered at an even higher rate than control cells. However, instead of being polarized, the scattering was isotropic and the persistence of NC cells migration was reduced, indicative of a defect in sensing polarity cues. Overall, these experiments suggest that RhoU controls NC migration through the regulation of polarized cell adhesion.
RhoV and RhoU Signaling in NC Development
Although the signaling pathways used by the 2 GTPases in NC cells remain to be fully determined, several candidates have emerged from the recent literature (Fig. 1). RhoU was shown to associate with EGFR in a Grb2-dependent manner and mediate changes in cell adhesion and migration.27 Grb228 and EGFR29,30 were themselves described as critical for NC adhesion, migration and late differentiation. Another potent RhoU regulator is Src, which can phosphorylate RhoU at its C-terminus thereby modifying its subcellular location.31 Src and its substrate Tks5 are also required for NC migration in zebrafish development.32 Several effectors have been identified for RhoV and RhoU,33 in particular PAKs. PAK1 and PAK2 are expressed in migrating NC cells and indeed their activation or inhibition mimicked the phenotypes observed upon RhoU expression and depletion, respectively.12 The proline-rich tyrosine kinase Pyk2 may also mediate RhoU activity in NC cell migration; indeed Pyk2 interacts with RhoU and the 2 partners cooperate with Src in cytoskeletal dynamics.34 Furthermore, Pyk2 activation triggers EGFR signaling and epithelial cell motility during wound healing.35 Last, RhoU might control polarized migration through interaction with Par6,33,36 a RhoU and Cdc42 partner required for Cdc42-dependent cell polarity.37
Figure 1.
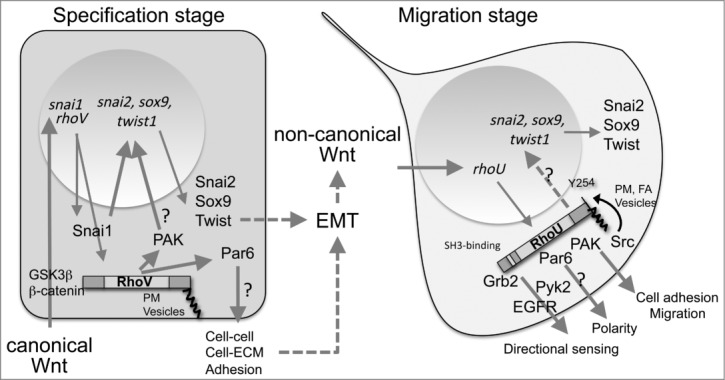
Demonstrated and putative roles of RhoV and RhoU in the developing neural crest. At specification stage, RhoV is induced early by the Wnt canonical pathway (via GSK3β/β−catenin). RhoV exhibits N-terminal and C-terminal extensions (dark gray boxes) required for its activity and are located through palmitoylation at the plasma membrane (PM) and vesicles. RhoV and Snai1 cooperate for the induction of snai2, sox9 and twist, required for EMT. This may be mediated through enhanced PAK activity, shown to phosphorylate and enhance Snai1 transcriptional activity. RhoV might also disrupt NC cell adhesion through Par6 activation. During EMT, RhoV expression is shutdown while the non-canonical Wnt pathway induces RhoU. RhoU also exhibits N-terminal and C-terminal extensions, which contain an SH3-binding domain (white box) and a tyrosine phosphorylated by Src (Y254). RhoU is located at PM, vesicles and focal adhesions (FA). RhoU is involved in NC cell adhesion and migration in a PAK-dependent pathway. RhoU is also required for NC polarity - potentially through its interaction with Par6 - and may participate in directional migration by regulating focal adhesion turnover through interaction with Grb2 or Pyk2 and through phosphorylation by Src. Question marks indicate pathways or interactions not yet validated in NC.
Specific Roles of RhoV and RhoU in NC Development
The specific roles of RhoV and RhoU in NC development remain to be determined. RhoU can rescue RhoV depletion,13 while the reverse is not true.12 Thus RhoU in NC might exert the same functions as RhoV does, plus other functions probably linked to its specific domain; RhoU contains an SH3-binding proline-rich region in its NH2 terminus, that is responsible for its binding to Grb2.15 Another difference between the 2 proteins is the tyrosine that is phosphorylated by Src, which is present at position 254 in RhoU31 but absent in RhoV. Given the functional differences between the 2 GTPases, one can thus propose that RhoV, induced early by the canonical Wnt pathway, initiates the cellular effects necessary for NC formation. These effects are then prolonged by RhoU which is induced later by the non-canonical Wnt pathway and which in addition triggers migration by interacting with partners through its SH3-binding domain. Two clues nevertheless suggest that RhoV may also have specific properties not held by RhoU: i) in the Xenopus embryo, RhoV mRNA is no longer detected in migrating cells,13 indicating the presence of an active shutdown mechanism; ii) the RhoV protein displays an extremely high turnover in mammalian cells (unpublished data), suggesting that its activity is tightly controlled. This strongly suggests that RhoV must not be expressed during migration, which therefore suggests that RhoU cannot substitute for all activities of RhoV.
The sequential expression of the 2 GTPases may therefore be envisioned as follows (Fig. 1): As a canonical Wnt response gene, RhoV cooperates with Snai1 in the induction of NC-specific markers and is probably responsible for disrupting epithelial junctions and modifying cell polarity, potentially through its binding to Par6, as proposed for RhoU in MDCK cells.36 Disruption of cell-cell contacts might then activate the non-canonical Wnt pathway37 and therefore RhoU expression, which in turn could promote polarized cell migration through its SH3-binding domain.
Concluding Remarks
In conclusion, functional analysis of RhoV and RhoU in the Xenopus embryo has revealed their specific roles during development of the neural crest. Although the ‘big 3’ GTPases (RhoA, Rac1 and Cdc42) have already been implicated in Wnt signaling, mostly in non-canonical pathways,38,39 recent literature showed that the conditional invalidation of Rac1 or Cdc42 in mouse NC only induced mitotic and survival defects in post-migratory NC cells. This excludes a role for Rac1 and Cdc42 at early stages of NC development, i.e. in the specification, EMT and migration stages.40,41 This further emphasizes the unique roles of RhoV and RhoU in the high dynamics of this embryonic tissue. Moreover, due to their sensitivity to canonical and non-canonical Wnt pathways, these 2 GTPases might well take a significant contribution in Wnt-related pathologies, in particular tumorigenesis.42
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Julian Venables for critical reading of the manuscript.
Funding
This work was supported by CNRS institutional grants and contracts from the Association pour la Recherche contre le Cancer (ARC no. 1048) and from the Ligue Régionale contre le Cancer (Comités ‘Hérault’ and ‘Aude’).
References
- 1.Huang X, Saint-Jeannet JP. Induction of the neural crest and the opportunities of life on the edge. Dev Biol 2004; 275:1-11; PMID:15464568; http://dx.doi.org/ 10.1016/j.ydbio.2004.07.033 [DOI] [PubMed] [Google Scholar]
- 2.Katoh M. Network of WNT and other regulatory signaling cascades in pluripotent stem cells and cancer stem clls. Curr Pharm Biotechnol 2011; 12:160-70; PMID:21044011; http://dx.doi.org/ 10.2174/138920111794295710 [DOI] [PubMed] [Google Scholar]
- 3.Le Douarin NM, Dupin E. Multipotentiality of the neural crest. Curr Opin Genet Dev 2003; 13:529-36; PMID:14550420; http://dx.doi.org/ 10.1016/j.gde.2003.08.002 [DOI] [PubMed] [Google Scholar]
- 4.Betancur P, Bronner-Fraser M, Sauka-Spengler T. Assembling neural crest regulatory circuits into a gene regulatory network. Annu Rev Cell Dev Biol, Vol 26 2010; 26:581-603; PMID:19575671; http://dx.doi.org/ 10.1146/annurev.cellbio.042308.113245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Calisto J, Araya C, Marchant L, Riaz CF, Mayor R. Essential role of non-canonical Wnt signalling in neural crest migration. Development 2005; 132:2587-97; PMID:15857909; http://dx.doi.org/ 10.1242/dev.01857 [DOI] [PubMed] [Google Scholar]
- 6.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139:871-90; PMID:19945376; http://dx.doi.org/ 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 7.Aspenstrom P, Ruusala A, Pacholsky D. Taking Rho GTPases to the next level: the cellular functions of atypical Rho GTPases. Exp Cell Res 2007; 313:3673-9; PMID:17850788; http://dx.doi.org/ 10.1016/j.yexcr.2007.07.022 [DOI] [PubMed] [Google Scholar]
- 8.Broders-Bondon F, Chesneau A, Romero-Oliva F, Mazabraud A, Mayor R, Thiery JP. Regulation of XSnail2 expression by Rho GTPases. Dev Dyn 2007; 236:2555-66; PMID:17676632; http://dx.doi.org/ 10.1002/dvdy.21273 [DOI] [PubMed] [Google Scholar]
- 9.Liu JP, Jessell TM. A role for rhoB in the delamination of neural crest cells from the dorsal neural tube. Development 1998; 125:5055-67; PMID:9811589 [DOI] [PubMed] [Google Scholar]
- 10.Matthews HK, Marchant L, Carmona-Fontaine C, Kuriyama S, Larrain J, Holt MR, Parsons M, Mayor R. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development 2008; 135:1771-80; PMID:18403410; http://dx.doi.org/ 10.1242/dev.017350 [DOI] [PubMed] [Google Scholar]
- 11.Boureux A, Vignal E, Faure S, Fort P. Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol Biol Evol 2007; 24:203-16; PMID:17035353; http://dx.doi.org/ 10.1093/molbev/msl145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fort P, Guemar L, Vignal E, Morin N, Notarnicola C, de Santa Barbara P, Faure S. Activity of the RhoU/Wrch1 GTPase is critical for cranial neural crest cell migration. Dev Biol 2011; 350:451-63; PMID:21156169; http://dx.doi.org/ 10.1016/j.ydbio.2010.12.011 [DOI] [PubMed] [Google Scholar]
- 13.Guemar L, de Santa Barbara P, Vignal E, Maurel B, Fort P, Faure S. The small GTPase RhoV is an essential regulator of neural crest induction in Xenopus. Dev Biol 2007; 310:113-28; PMID:17761159; http://dx.doi.org/ 10.1016/j.ydbio.2007.07.031 [DOI] [PubMed] [Google Scholar]
- 14.Saras J, Wollberg P, Aspenstrom P. Wrch1 is a GTPase-deficient Cdc42-like protein with unusual binding characteristics and cellular effects. Exp Cell Res 2004; 299:356-69; PMID:15350535; http://dx.doi.org/ 10.1016/j.yexcr.2004.05.029 [DOI] [PubMed] [Google Scholar]
- 15.Shutes A, Berzat AC, Cox AD, Der CJ. Atypical mechanism of regulation of the Wrch-1 Rho family small GTPase. Curr Biol 2004; 14:2052-6; PMID:15556869; http://dx.doi.org/ 10.1016/j.cub.2004.11.011 [DOI] [PubMed] [Google Scholar]
- 16.Chenette EJ, Abo A, Der CJ. Critical and distinct roles of amino- and carboxyl-terminal sequences in regulation of the biological activity of the Chp atypical Rho GTPase. J Biol Chem 2005; 280:13784-92; PMID:15664990; http://dx.doi.org/ 10.1074/jbc.M411300200 [DOI] [PubMed] [Google Scholar]
- 17.Weisz Hubsman M, Volinsky N, Manser E, Yablonski D, Aronheim A. Autophosphorylation-dependent degradation of Pak1, triggered by the Rho-family GTPase, Chp. Biochem J 2007; 404:487-97; PMID:17355222; http://dx.doi.org/ 10.1042/BJ20061696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells CM, Whale A, Hashim FN, Fram S, Jones GE. Signalling to cancer cell invasion through PAK family kinases. Front Biosci Landmark 2011; 16:849-64; PMID:21196207; http://dx.doi.org/ 10.2741/3722 [DOI] [PubMed] [Google Scholar]
- 19.Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail's subcellular localization and functions. Cancer Res 2005; 65:3179-84; PMID:15833848 [DOI] [PubMed] [Google Scholar]
- 20.Aybar MJ, Nieto MA, Mayor R. Snail precedes slug in the genetic cascade required for the specification and migration of the Xenopus neural crest. Development 2003; 130:483-94; PMID:12490555; http://dx.doi.org/ 10.1242/dev.00238 [DOI] [PubMed] [Google Scholar]
- 21.Tay HG, Ng YW, Manser E. A vertebrate-specific Chp-PAK-PIX pathway maintains E-cadherin at adherens junctions during zebrafish epiboly. PLoS One 2011; 5:e10125; PMID:20405038; http://dx.doi.org/ 10.1371/journal.pone.0010125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson LA, Keller RE. Neural tube closure in Xenopus laevis involves medial migration, directed protrusive activity, cell intercalation and convergent extension. Development 1999; 126:4547-56; PMID:10498689 [DOI] [PubMed] [Google Scholar]
- 23.Schiavone D, Dewilde S, Vallania F, Turkson J, Di Cunto F, Poli V. The RhoU/Wrch1 Rho GTPase gene is a common transcriptional target of both the gp130/STAT3 and Wnt-1 pathways. Biochem J 2009; 421:283-92; PMID:19397496; http://dx.doi.org/ 10.1042/BJ20090061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao W, Pennica D, Xu L, Kalejta RF, Levine AJ.. Wrch-1, a novel member of the Rho gene family that is regulated by Wnt-1. Genes Dev 2001; 15:1796-807; PMID:11459829; http://dx.doi.org/ 10.1101/gad.894301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chuang YY, Valster A, Coniglio SJ, Backer JM, Symons M. The atypical Rho family GTPase Wrch-1 regulates focal adhesion formation and cell migration. J Cell Sci 2007; 120:1927-34; PMID:17504809; http://dx.doi.org/ 10.1242/jcs.03456 [DOI] [PubMed] [Google Scholar]
- 26.Ory S, Brazier H, Blangy A. Identification of a bipartite focal adhesion localization signal in RhoU/Wrch-1, a Rho family GTPase that regulates cell adhesion and migration. Biol Cell 2007; 99:701-16; PMID:17620058; http://dx.doi.org/ 10.1042/BC20070058 [DOI] [PubMed] [Google Scholar]
- 27.Zhang JS, Koenig A, Young C, Billadeau DD. GRB2 couples RhoU to epidermal growth factor receptor signaling and cell migration. Mol Biol Cell 2011; 22:2119-30; PMID:21508312; http://dx.doi.org/ 10.1091/mbc.E10-12-0969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxton TM, Cheng AM, Ong SH, Lu Y, Sakai R, Cross JC, Pawson T. Gene dosage-dependent functions for phosphotyrosine-Grb2 signaling during mammalian tissue morphogenesis. Current Biology 2001; 11:662-70; PMID:11369229; http://dx.doi.org/ 10.1016/S0960-9822(01)00198-1 [DOI] [PubMed] [Google Scholar]
- 29.Budi EH, Patterson LB, Parichy DM. Embryonic requirements for ErbB signaling in neural crest development and adult pigment pattern formation. Development 2008; 135:2603-14; PMID:18508863; http://dx.doi.org/ 10.1242/dev.019299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 1995; 378:394-8; PMID:7477377; http://dx.doi.org/ 10.1038/378394a0 [DOI] [PubMed] [Google Scholar]
- 31.Alan JK, Berzat AC, Dewar BJ, Graves LM, Cox AD. Regulation of the Rho family small GTPase Wrch-1/RhoU by C-terminal tyrosine phosphorylation requires Src. Mol Cell Biol 2010; 30:4324-38; PMID:20547754; http://dx.doi.org/ 10.1128/MCB.01646-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy DA, Diaz B, Bromann PA, Tsai JH, Kawakami Y, Maurer J, Stewart RA, Izpisúa-Belmonte JC, Courtneidge SA. A Src-Tks5 Pathway Is Required for Neural Crest Cell Migration during Embryonic Development. PLoS One 2011; 6:e22499; PMID:21799874; http://dx.doi.org/ 10.1371/journal.pone.0022499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aspenstrom P, Fransson A, Saras J. Rho GTPases have diverse effects on the organization of the actin filament system. Biochem J 2004; 377:327-37; PMID:14521508; http://dx.doi.org/ 10.1042/BJ20031041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruusala A, Aspenstrom P. The atypical Rho GTPase Wrch1 collaborates with the nonreceptor tyrosine kinases Pyk2 and Src in regulating cytoskeletal dynamics. Mol Cell Biol 2008; 28:1802-14; PMID:18086875; http://dx.doi.org/ 10.1128/MCB.00201-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Block ER, Tolino MA, Klarlund JK. Pyk2 activation triggers epidermal growth factor receptor signaling and cell motility after wounding sheets of epithelial cells. J Biol Chem 2010; 285:13372-9; PMID:20215112; http://dx.doi.org/ 10.1074/jbc.M109.083089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brady DC, Alan JK, Madigan JP, Fanning AS, Cox AD. The transforming Rho family GTPase Wrch-1 disrupts epithelial cell tight junctions and epithelial morphogenesis. Mol Cell Biol 2009; 29:1035-49; PMID:19064640; http://dx.doi.org/ 10.1128/MCB.00336-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlessinger K, McManus EJ, Hall A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J Cell Biol 2007; 178:355-61; PMID:17646398; http://dx.doi.org/ 10.1083/jcb.200701083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev 2009; 23:265-77; PMID:19204114; http://dx.doi.org/ 10.1101/gad.1760809 [DOI] [PubMed] [Google Scholar]
- 39.Theveneau E, Mayor R. Integrating chemotaxis and contact-inhibition during collective cell migration: Small GTPases at work. Small Gtpases 2010; 1:113-7; PMID:21686264; http://dx.doi.org/ 10.4161/sgtp.1.2.13673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas PS, Kim J, Nunez S, Glogauer M, Kaartinen V. Neural crest cell-specific deletion of Rac1 results in defective cell-matrix interactions and severe craniofacial and cardiovascular malformations. Dev Biol 2011; 340:613-25; PMID:20184871; http://dx.doi.org/ 10.1016/j.ydbio.2010.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuchs S, Herzog D, Sumara G, Buchmann-Moller S, Civenni G, Wu X, Chrostek-Grashoff A, Suter U, Ricci R, Relvas JB, et al.. Stage-specific control of neural crest stem cell proliferation by the small rho GTPases Cdc42 and Rac1. Cell Stem Cell 2009; 4:236-47; PMID:19265663; http://dx.doi.org/ 10.1016/j.stem.2009.01.017 [DOI] [PubMed] [Google Scholar]
- 42.Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol 2011; 8:97-106; PMID:21151206; http://dx.doi.org/ 10.1038/nrclinonc.2010.196 [DOI] [PubMed] [Google Scholar]


