Abstract
The "specialized ribosome" concept proposes that ribosome variants are produced and differentially regulate translation. Examples supporting this notion demonstrated heterogeneity of ribosomal protein composition. However, ribosome translational activity is carried out by rRNA. We, and others, recently showed that rRNA heterogeneity regulates translation to generate distinct translatomes promoting tumorigenesis.
Keywords: fibrillarin, p53, RNA epigenetics, specialized ribosome, translation, tumorigenesis
Abbreviations
- RNA pol I
RNA polymerase I
- snoRNA
small nucleolar RNA
- RP
ribosomal protein
- IRES
internal ribosome entry site
Translation is one of the last steps of gene expression, during which ribosomes synthesize proteins. A growing body of evidence indicates that the translation process per se plays a key role in tumorigenesis.1 Significantly, it was recently revealed that components of the translational machinery play unexpected direct roles in tumorigenesis. For example, haploinsufficiency in ribosomal protein (RP) RPL24 is sufficient to significantly delay tumorigenesis in mice overexpressing the oncogene C-Myc or lacking the tumor suppressor phosphatase and tensin homolog (PTEN).1 In addition, it has been extensively demonstrated that the expression of oncogenes and tumor suppressors is regulated at the protein synthesis level.1,2 Finally, the specific inhibition of RNA polymerase I (RNA pol I) using small molecules selectively kills cancer versus healthy cells in mouse models.3
Human ribosomes are composed of 80 ribosomal proteins and 4 ribosomal RNAs (rRNAs). Although ribosomes were shown to be effectors of translation 40 years ago, it only recently became apparent that they also act as regulators. First, during evolution, ribosomes of higher eukaryotes have selected additional RPs and rRNA segments that are not directly involved in mRNA decoding and peptide formation,4 but are predicted to support regulatory events. Second, ribosomes with variable composition have been identified that support distinct translational activity in response to viral infection or depending on cell type.5 For instance, some ribosomes were shown to more efficiently initiate translation from internal ribosome entry site (IRES) sequences.1 In addition, changes in ribosome composition result in developmental defects in both animals and humans while having no, or only a weak, effect on the survival of cultured cells.1,5 Last, mutations in RP genes or ribosome biogenesis factor genes are associated with ribosomopathies that are characterized by selected developmental defects and by increased cancer susceptibility for particular tumors. These data support the emerging notion of the "specialized ribosome", suggesting that ribosomes exist as different variants that regulate the selection and efficiency of translation of mRNA subsets.
Up to now, most studies have focused on heterogeneity of RP composition. However, key molecular interactions underlying the translational process are carried out by rRNAs, which decode the mRNA and catalyze the peptide bond formation through a ribozyme activity. Similarly to proteins, rRNAs carry chemical modifications, including base methylation, pseudo-uridylation, and ribose methylation at 2′-hydroxyl (2′-O-methylation).6 With 105 sites currently mapped on rRNAs, the most abundant modification is 2′-O-methylation that is catalyzed by fibrillarin (FBL), a conserved methyl-transferase guided by C/D box small nucleolar RNAs (snoRNAs) that ensure site specificity.7 Structural studies suggest that 2′-O-methylation modulates rRNA structure and stability and can thus affect rRNA function. Interestingly, 60% of the 2′-O-methylation sites are within functional regions of rRNAs, and genetic studies in yeast have demonstrated that 2′-O-methylation is indeed important for the translational capacity of ribosomes.7
We reported for the first time that the rRNA 2′-O-methylation pattern modification is associated with changes in translational control during tumorigenesis.8,9 In immortalized human mammary epithelial cells (HMECs), p53 (TP53, best known as p53) knockdown using short hairpin RNA (shRNA) was associated with a modification of the rRNA 2′-O-methylation pattern. This p53-induced alteration in rRNA 2′-O-methylation pattern was linked to increased expression of FBL. We further demonstrated that FBL is a p53 target gene, whose expression is repressed following binding of p53 to the first intron of the FBL gene. Both an increase in FBL expression and modification of rRNA 2′-O-methylation pattern in HMECs-shp53 were associated with changes in the translational activity of ribosomes and in translational control. Reduced p53 expression induced, in an FBL-dependent manner, both a defect in translational fidelity (i.e., amino acid misincorporation and stop codon read-through) and increased translation of a subset of IRES-containing cellular mRNAs (insulin growth factor-1 receptor [IGF-1R], C-Myc, vascular endothelial growth factor-A [VEGF-A], fibroblast growth factor 1 [FGF1] and FGF2). We analyzed in more detail the regulation of IGF-1R mRNA translation using a panel of cell lines, IGF-1R IRES-containing reporter systems, and polysome analysis of endogenous IGF-1R mRNA.
Our current model is that modulation of the rRNA 2′-O-methylation pattern induced by changes in FBL expression promotes the translation of a subset of mRNAs encoding proteins with oncogenic properties, which favors tumor initiation and progression (Figure 1). This view is supported by additional observations. We reported that FBL overexpression promotes anchorage-independent cell proliferation and chemoresistance of MCF-7 breast cancer cells.9 Moreover, in breast cancers, a high level of FBL appeared to be an independent marker of poor prognosis, supporting the importance of maintaining a reduced level of FBL to prevent tumorigenesis.8 Following our publication, the Kouzarides group identified FBL as the methyl-transferase that catalyzes methylation of Q105 of histone H2A, a novel histone post-translational modification found to be highly enriched in rDNA chromatin, and enhances RNA pol I activity.10 Together, these data support an important role of FBL in controlling not only ribosome quality, but also ribosome quantity that impacts tumorigenesis.
Figure 1.
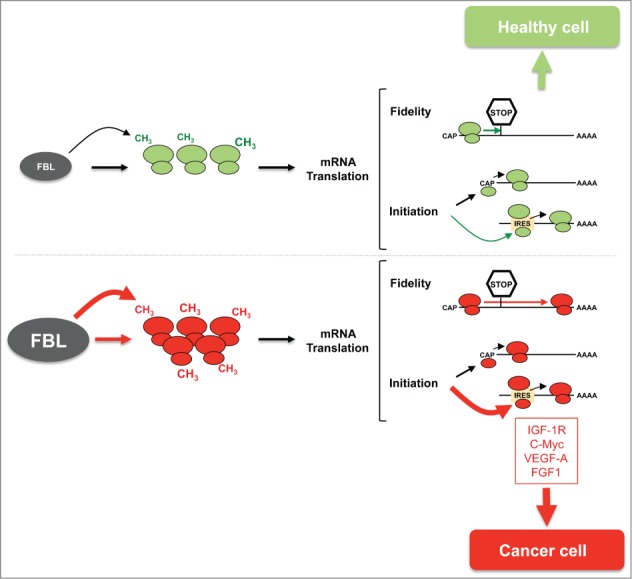
Impact of rRNA 2'-O-methylation on translation and cellular malignant phenotype. Compared to healthy cells (green panel), cancer cells (red panel) express higher levels of fibrillarin (FBL), which promotes an increase in the amount of ribosomes and changes the ribosomal (rRNA) 2'-O-methylation pattern. Alteration of rRNA composition reduces the translational fidelity and enhances internal ribosome entry site (IRES)-dependent translation of a subset of mRNAs encoding proteins that favor tumor development.
The significance of the rRNA perspective in the heterogeneity of ribosomes also has roots in studies of rRNA pseudo-uridylation since mutation of dyskerin (DKC1), the enzyme catalyzing this modification, alters IRES-dependent translation and is associated with cancer susceptibility.1 In the near future the different rRNA chemical modifications will have to be analyzed in concert since the rRNA domains critical for translational activity contain all of the chemical modifications identified to date in rRNA. One must therefore wonder whether the impact of rRNA chemical modifications on translation should be considered RNA epigenetic regulations, as for modification of other classes of RNA and for DNA epigenetics.6 However, several key questions remain to be addressed, including “Are rRNA chemical modifications heritable?” and “Do enzymes exist that are able to demethylate or de-pseudo-uridylate rRNA to dynamically regulate the system?”
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by CNRS, INSERM, Université Claude Bernard Lyon-1, Center Léon Bérard, Fondation ARC pour la Recherche sur le Cancer (n° SFI20121205802), Ligue Nationale Contre le Cancer Comité Rhône-Alpes-Auvergne et Saône et Loire (n°13-763C) and ANR (13-BSV8-0012-01 Ribometh). VM and JJD are members of Inserm. FC is a member of CNRS.
References
- 1.Ruggero D. Translational control in cancer etiology. Cold Spring Harb Perspect Biol 2013; 5(2): a012336; PMID:22767671; http://dx.doi.org/ 10.1101/cshpe-rspect.a012336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silvera D, Formenti SC, Schneider RJ. Translational control in cancer. Nat Rev Cancer 2010; 10:254-66; PMID:20332778; http://dx.doi.org/ 10.1038/nrc2824 [DOI] [PubMed] [Google Scholar]
- 3.Peltonen K, Colis L, Liu H, Trivedi R, Moubarek MS, Moore HM,Bai B, Rudek MA, Bieberich CJ, Laiho M. A targeting modality for destruction of RNA polymerase I that possesses anticancer activity. Cancer Cell 2014; 25:77-90; PMID:24434211; http://dx.doi.org/ 10.1016/j.ccr.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anger AM, Armache JP, Berninghausen O, Habeck M, Subklewe M, Wilson DN, Beckmann R. Structures of the human and Drosophila 80S ribosome. Nature 2013; 497:80-5; PMID:23636399; http://dx.doi.org/ 10.1038/nature12104 [DOI] [PubMed] [Google Scholar]
- 5.Xue S, Barna M. Specialized ribosomes: a new frontier in gene regulation and organismal biology. Nat Rev Mol Cell Biol 2012; 13:355-69; PMID:22617470; http://dx.doi.org/ 10.1038/nrm3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Therizols G, Laforets F, Marcel V, Catez F, Bouvet P, Diaz JJ. Ribosomal methylation and cancer. Translational Epigenetics Series, In press. [Google Scholar]
- 7.Decatur WA, Fournier MJ. rRNA modifications and ribosome function. Trends Biochem Sci 2002; 27:344-51; PMID:12114023; http://dx.doi.org/ 10.1016/S0968-0004(02)02109-6 [DOI] [PubMed] [Google Scholar]
- 8.Marcel V, Ghayad SE, Belin S, Therizols G, Morel AP, Solano-Gonzalez E, Vendrell JA, Hacot S, Mertani HC, Albaret MA, et al. . p53 acts as a safeguard of translational control by regulating fibrillarin and rRNA methylation in cancer. Cancer Cell 2013; 24:318-30; PMID:24029231; http://dx.doi.org/ 10.1016/j.ccr.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belin S, Beghin A, Solano-Gonzalez E, Bezin L, Brunet-Manquat S, Textoris J, Prats AC, Mertani HC, Dumontet C, Diaz JJ. Dysregulation of ribosome biogenesis and translational capacity is associated with tumor progression of human breast cancer cells. PloS One 2009; 4:e7147; PMID:19779612; http://dx.doi.org/ 10.1371/journal.pone.0007147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tessarz P, Santos-Rosa H, Robson SC, Sylvestersen KB, Nelson CJ, Nielsen ML, Kouzarides T. Glutamine methylation in histone H2A is an RNA-polymerase-I-dedicated modification. Nature 2014; 505:564-8; PMID:24352239; http://dx.doi.org/ 10.1038/nature12819 [DOI] [PMC free article] [PubMed] [Google Scholar]


