Abstract
The pathogenetic mechanism of the mitochondrial tRNA(LeuUUR) gene mutation responsible for the MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes) syndrome was investigated in transformants obtained by transfer of mitochondria from three genetically unrelated MELAS patients into human mitochondrial DNA (mtDNA)-less (rho 0) cells. Marked defects in mitochondrial protein synthesis and respiratory activity were observed in transformants containing virtually pure mutant mtDNA, as compared to the parent of the rho 0 cells (the 143B cell line) or to transformants containing exclusively wild-type mtDNA, derived from one of the patients or a maternally related asymptomatic individual. A striking protective effect against the mutation was exerted in the transformants by levels of residual wild-type mtDNA above 6%. The MELAS mutation occurs within the mtDNA binding site for a protein factor (mTERF) that promotes termination of transcription at the 16S rRNA/tRNA(LeuUUR) gene boundary. A marked decrease in affinity of purified mTERF for the mutant target sequence was observed in in vitro assays. By contrast, RNA transfer hybridization experiments failed to show any significant change in the steady-state amounts of the two rRNA species, encoded upstream of the termination site, and of the mRNAs encoded downstream, in the transformants carrying the MELAS mutation.
Full text
PDF


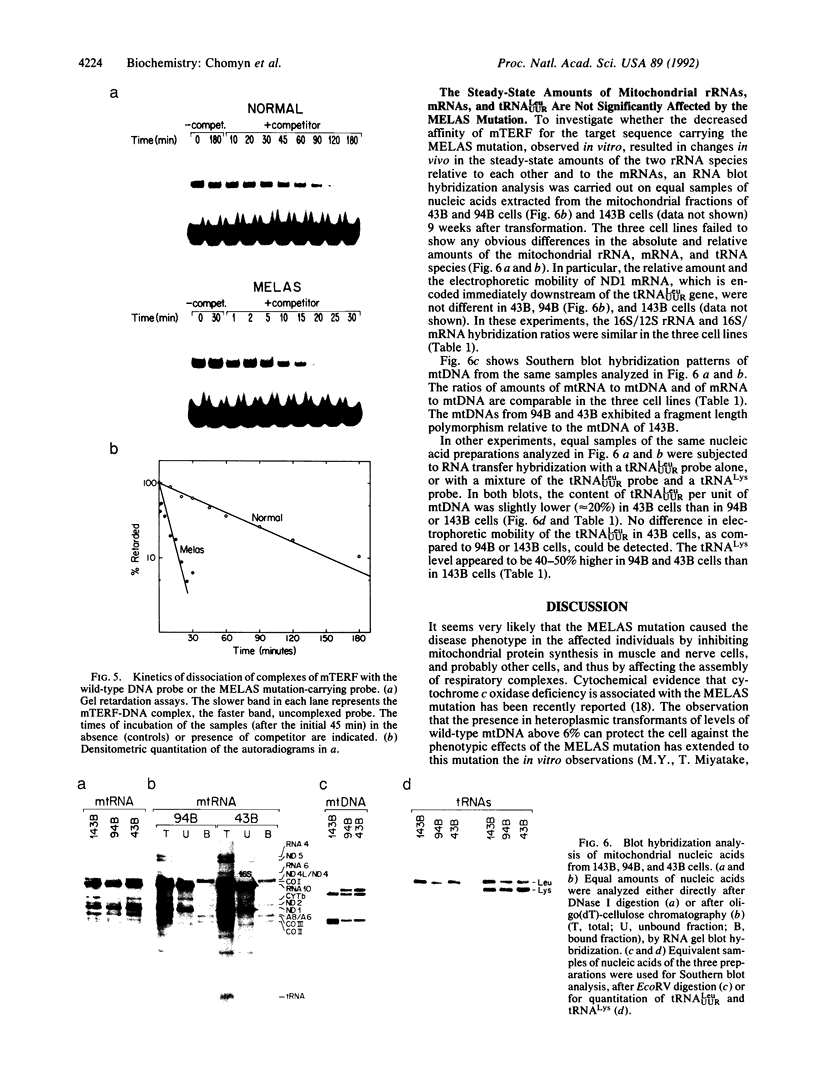

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Askanas V., Engel W. K. A new program for investigating adult human skeletal muscle grown aneurally in tissue culture. Neurology. 1975 Jan;25(1):58–67. doi: 10.1212/wnl.25.1.58. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol. 1991 Apr;11(4):2236–2244. doi: 10.1128/mcb.11.4.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand R., Attardi G. Synthesis and turnover of mitochondrial ribonucleic acid in HeLa cells: the mature ribosomal and messenger ribonucleic acid species are metabolically unstable. Mol Cell Biol. 1981 Jun;1(6):497–511. doi: 10.1128/mcb.1.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Hess J. F., Parisi M. A., Bennett J. L., Clayton D. A. Impairment of mitochondrial transcription termination by a point mutation associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1991 May 16;351(6323):236–239. doi: 10.1038/351236a0. [DOI] [PubMed] [Google Scholar]
- Hurko O., Johns D. R., Rutledge S. L., Stine O. C., Peterson P. L., Miller N. R., Martens M. E., Drachman D. B., Brown R. H., Lee C. P. Heteroplasmy in chronic external ophthalmoplegia: clinical and molecular observations. Pediatr Res. 1990 Nov;28(5):542–548. doi: 10.1203/00006450-199011000-00026. [DOI] [PubMed] [Google Scholar]
- Hurko O., McKee L., Zuurveld J. G. Transfection of human skeletal muscle cells with SV40 large T antigen gene coupled to a metallothionein promoter. Ann Neurol. 1986 Nov;20(5):573–582. doi: 10.1002/ana.410200504. [DOI] [PubMed] [Google Scholar]
- Johns D. R., Hurko O. Mitochondrial leucine tRNA mutation in neurological diseases. Lancet. 1991 Apr 13;337(8746):927–928. doi: 10.1016/0140-6736(91)90272-q. [DOI] [PubMed] [Google Scholar]
- King M. P., Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989 Oct 27;246(4929):500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Momoi M. Y., Tominaga K., Momoi T., Nihei K., Yanagisawa M., Kagawa Y., Ohta S. A point mutation in the mitochondrial tRNA(Leu)(UUR) gene in MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes). Biochem Biophys Res Commun. 1990 Dec 31;173(3):816–822. doi: 10.1016/s0006-291x(05)80860-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Momoi M. Y., Tominaga K., Shimoizumi H., Nihei K., Yanagisawa M., Kagawa Y., Ohta S. Respiration-deficient cells are caused by a single point mutation in the mitochondrial tRNA-Leu (UUR) gene in mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS). Am J Hum Genet. 1991 Sep;49(3):590–599. [PMC free article] [PubMed] [Google Scholar]
- Kruse B., Narasimhan N., Attardi G. Termination of transcription in human mitochondria: identification and purification of a DNA binding protein factor that promotes termination. Cell. 1989 Jul 28;58(2):391–397. doi: 10.1016/0092-8674(89)90853-2. [DOI] [PubMed] [Google Scholar]
- Meola G., Scarpini E., Velicogna M., Mottura A., Baron P. L., Beretta S., Scarlato G. Analysis of fibronectin expression during human muscle differentiation. Basic Appl Histochem. 1986;30(2):153–163. [PubMed] [Google Scholar]
- Montoya J., Gaines G. L., Attardi G. The pattern of transcription of the human mitochondrial rRNA genes reveals two overlapping transcription units. Cell. 1983 Aug;34(1):151–159. doi: 10.1016/0092-8674(83)90145-9. [DOI] [PubMed] [Google Scholar]
- Normanly J., Abelson J. tRNA identity. Annu Rev Biochem. 1989;58:1029–1049. doi: 10.1146/annurev.bi.58.070189.005121. [DOI] [PubMed] [Google Scholar]
- Okimoto R., Wolstenholme D. R. A set of tRNAs that lack either the T psi C arm or the dihydrouridine arm: towards a minimal tRNA adaptor. EMBO J. 1990 Oct;9(10):3405–3411. doi: 10.1002/j.1460-2075.1990.tb07542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Ino H., Ohno K., Ohbayashi T., Ikebe S., Sano T., Ichiki T., Kobayashi M., Wada Y., Ozawa T. Mitochondrial DNA mutations in mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS). Biochem Biophys Res Commun. 1991 Jan 31;174(2):861–868. doi: 10.1016/0006-291x(91)91497-z. [DOI] [PubMed] [Google Scholar]