Abstract
We recently showed that telomeric repeat-binding factor 2 (TRF2) regulates gene expression to promote angiogenesis. We found that TRF2 is highly expressed in tumor vessels and transcriptionally activates platelet-derived growth factor receptor β to promote endothelial cell angiogenic properties independently of its function in telomere protection. This work identifies TRF2 as a promising dual target for cancer therapy.
Keywords: tumor angiogenesis, telomeres, telomeric repeat-binding factor 2 (TRF2), transcriptional regulation, Wilms’ tumor suppressor WT1
Abbreviations
- HS3ST4
heparan sulfate (glucosamine) 3-O-sulfotransferase 4
- HIF1A
hypoxia-inducible factor 1 α subunit
- LEC
lung endothelial cells
- PDGFRB
platelet-derived growth factor receptor β
- TRF2
telomeric repeat-binding factor 2
- TEC
tumor endothelial cell
- VEGF
vascular endothelial growth factor
- WT1
Wilms’ tumor suppressor 1
Angiogenesis is important for cancer progression. Inhibition of tumor angiogenesis is widely accepted as a valuable therapeutic option for cancer treatment and a large number of cancer patients benefit from treatment with inhibitors of the angiogenic vascular endothelial growth factor (VEGF). However, different tumor types show unique features in their vasculature depending on tissue specificity and the expression pattern of pro- and anti-angiogenic molecules and their cognate receptors. Thus, it is not surprising that, in addition to positive antitumor effects, increased invasion and metastasis have also been noted in divergent experimental models targeting VEGF.1 These observations support the notion that other proangiogenic factors play a role in sustained tumor angiogenesis and progression.
We previously found that the Wilms’ tumor suppressor WT1 is highly expressed in vessels of human tumors but not in healthy adjacent tissues, and is required for angiogenesis.2 Furthermore, WT1 is implicated in vessel formation during development and in pathophysiological conditions such as myocardial ischemia as a transcriptional target of hypoxia-inducible factor 1 α (HIF1A).3,4 WT1 encodes a zinc-finger transcription factor with crucial functions in development and was originally identified as a tumor suppressor based on its mutational inactivation in Wilms’ tumor.5 However, because of its overexpression in a variety of human cancers, it is now considered a potential oncogene.
Like WT1, expression of the telomeric repeat-binding factor 2 (TRF2, also known as TERF2), which plays a central role in telomere capping, is frequently increased in human tumors.6 Consistent with a potent oncogenic role of a high level of TRF2, its downregulation in cancer cells reduces tumorigenicity whereas its overexpression favors oncogenesis.7 We showed that the high level of TRF2 found in cancer cells positively regulates the expression of HS3ST4 encoding heparan sulfate (glucosamine) 3-O-sulphotransferase 4, which is involved in the recruitment of NK-cells, thus revealing that TRF2 upregulation can have oncogenic properties through cell non-autonomous mechanisms.7
In our recent work, we identified TRF2 as a transcriptional regulator in tumor endothelial cells (Fig. 1). We found that TRF2 was highly expressed in the tumor vasculature of different human cancer types, but not in the vessels of healthy tissues. Using ex vivo approaches, we demonstrated that tumor-derived endothelial cells (TECs) expressed higher levels of Trf2 than endothelial cells from healthy lung tissues (LECs) and exhibited a higher angiogenic potential. We further showed that upregulation of Trf2 in the tumor endothelium is the result of its transcriptional activation by WT1.8 In agreement with an important angiogenic role of Trf2 overexpression in TECs, depletion of Trf2 in these cells led to loss of their angiogenic properties, and overexpression of Trf2 in LECs conferred on them the high angiogenic potential of TECs.8 Overall, these findings suggest that TRF2 might play a role in vascular development and in cases of ischemia, as has been shown for WT1.3,4
Figure 1.
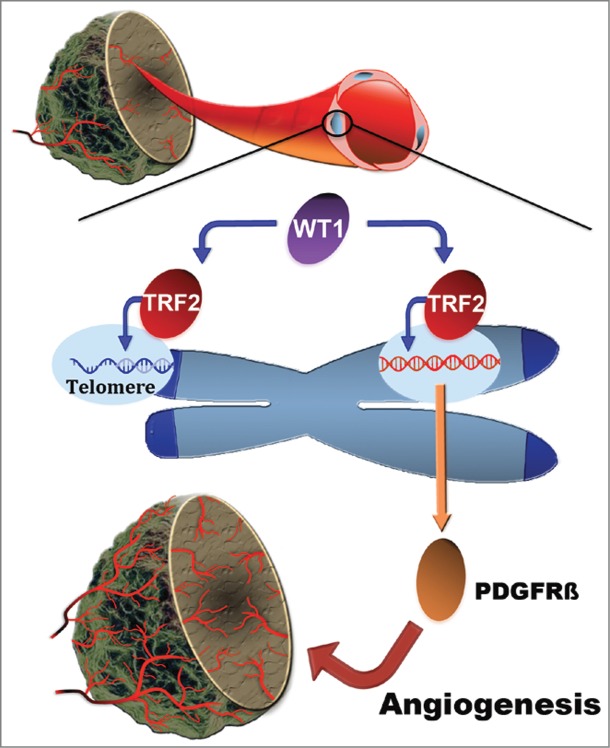
Telomeric repeat-binding factor 2 (TRF2) regulates angiogenesis by acting as a transcription factor independent of its function in telomere protection. TRF2 is overexpressed in many cancer cell types. Furthermore, Trf2 expression is upregulated in tumor-derived endothelial cells, most likely because TRF2 is a transcriptional target of the Wilms’ tumor suppressor 1 (WT1), which is highly expressed in the tumor vasculature. In tumor endothelial cells, Trf2 binds and transactivates the platelet-derived growth factor receptor β (Pdgfrβ) promoter, which stimulates angiogenesis. This represents a non-canonical function of TRF2. Overall, by activating tumor angiogenesis and cancer cell proliferation, TRF2 emerges as a valuable multi-hit target for cancer therapy.
Next, we investigated how a high TRF2 level boosted the angiogenic properties of TECs. First, we did not observe telomere uncapping and initiation of the DNA damage response (DDR) upon modulation of TRF2 levels, indicating that the angiogenic function of TRF2 in endothelial cells is uncoupled from its role in telomere capping. Second, by screening for angiogenic targets of TRF2, we found that TRF2 increases the expression of platelet-derived growth factor receptor β (PDGFRB).8 PDGFRB promotes angiogenesis and is frequently upregulated in cancer endothelia. Autocrine growth stimulation of tumor endothelial cells through PDGFRB that is evoked by interaction with cancer cells has been postulated, underlining the diversity of proangiogenic mechanisms in tumor angiogenesis and the resulting difficulties in establishing adequate antiangiogenic therapies for cancer treatment.9 Third, using different approaches we demonstrated that TRF2 is a direct transcriptional activator of PDGFRB that is capable of binding and activating the PDGFRB promoter. Overexpression of PDGFRB could rescue the inhibition of endothelial cell proliferation caused by depletion of TRF2, but only partially rescued the effects on the migratory potential of these cells.8 This indicates that TRF2 might also activate factors other than PDGFRB that are implicated in endothelial cell migration.
The fact that telomere factors such as TRF2 bind extra-telomeric sites to regulate transcription is not unprecedented (reviewed in10). Our finding that TRF2 binds and activates the PDGFRB promoter should facilitate dissection of the mechanism by which TRF2 acts as a transcriptional regulator and consequently will have strong implications for basic and biomedical research.
Taken together, these findings reveal a novel mechanism by which TRF2 transcriptionally controls tumor angiogenesis independent of its role in telomere protection. They further suggest that pathways controlling replicative potential (telomere) and those controlling energy supply (angiogenesis) co-evolved for a better control of tissue homeostasis and renewal. They also point to TRF2 as a novel multi-hit target in cancer therapy, as it is implicated in both the proliferation of cancer cells through cell autonomous and non-autonomous mechanisms and in the angiogenic properties of TECs. Thus, compared to classic antiangiogenic therapies, the identification of molecules that target TRF2 might be a major advance for cancer treatment through hitting 2 birds with one stone.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The study was supported by the Ligue Nationale contre le Cancer (Equipe labélisée, EG), the Institut National du cancer (INCa) program TELOCHROM, the ANR (National Research Agency) programs INNATELO and the Investments for the Future LABEX SIGNALIFE (reference ANR-11-LABX-0028–01), the Fondation ARC (Association pour la Recherche sur le Cancer), and Fondation de France (KDW). M El Maï is supported by a fellowship from the Ligue Nationale contre le Cancer and The Fondation ARC pour la recherche sur le cancer.
References
- 1.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 2009; 15: 232-9; PMID:19249681; http://dx.doi.org/ 10.1016/j.ccr.2009.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner N, Michiels JF, Schedl A, Wagner KD. The Wilms' tumour suppressor WT1 is involved in endothelial cell proliferation and migration: expression in tumour vessels in vivo. Oncogene 2008; 27:3662-72; PMID:18212735; http://dx.doi.org/ 10.1038/sj.onc.1211044 [DOI] [PubMed] [Google Scholar]
- 3.Wagner N, Wagner KD, Theres H, Englert C, Schedl A, Scholz H. Coronary vessel development requires activation of the TrkB neurotrophin receptor by the Wilms' tumor transcription factor Wt1. Genes Dev 2005; 19:2631-42; PMID:16264195; http://dx.doi.org/ 10.1101/gad.346405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner KD, Wagner N, Wellmann S, Schley G, Bondke A, Scholz H. Oxygen-regulated expression of the Wilms' tumor suppressor Wt1 involves hypoxia-inducible factor-1 (HIF-1). FASEB J 2003; 17:1364-6; PMID:12738801 [DOI] [PubMed] [Google Scholar]
- 5.Haber DA, Buckler AJ, Glaser T, Call KM, Pelletier J, Sohn RL, Douglass EC, Housman DE. An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms' tumor. Cell 1990; 61:1257-69; PMID:2163761; http://dx.doi.org/ 10.1016/0092-8674(90)90690-G [DOI] [PubMed] [Google Scholar]
- 6.Biroccio A, Rizzo A, Elli R, Koering CE, Belleville A, Benassi B, Leonetti C, Stevens MF, D'Incalci M, Zupi G, Gilson E. TRF2 inhibition triggers apoptosis and reduces tumourigenicity of human melanoma cells. Eur J Cancer 2006; 42:1881-8; PMID:16750909; http://dx.doi.org/ 10.1016/j.ejca.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 7.Biroccio A, Cherfils-Vicini J, Augereau A, Pinte S, Bauwens S, Ye J, Simonet T, Horard B, Jamet K, Cervera L, et al. TRF2 inhibits a cell-extrinsic pathway through which natural killer cells eliminate cancer cells. Nat Cell Biol 2013; 15:818-28; PMID:23792691; http://dx.doi.org/ 10.1038/ncb2774 [DOI] [PubMed] [Google Scholar]
- 8.El Mai M, Wagner KD, Michiels JF, Ambrosetti D, Borderie A, Destree S, Renault V, Djerbi N, Giraud-Panis MJ, Gilson E, et al. The Telomeric Protein TRF2 Regulates Angiogenesis by Binding and Activating the PDGFRb Promoter. Cell Rep 2014; 9:1-14; PMID:25437559 [DOI] [PubMed] [Google Scholar]
- 9.Hermansson M, Nistér M, Betsholtz C, Heldin CH, Westermark B, Funa K. Endothelial cell hyperplasia in human glioblastoma: coexpression of mRNA for platelet-derived growth factor (PDGF) B chain and PDGF receptor suggests autocrine growth stimulation. Proc Natl Acad Sci U S A 1988; 85:7748-52; PMID:2845420; http://dx.doi.org/ 10.1073/pnas.85.20.7748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye J, Renault V, Jamet K, Gilson E. Transcriptional outcome of telomere signalling. Nat Rev Genet 2014; 15:491-503; PMID:24913665; http://dx.doi.org/ 10.1038/nrg3743 [DOI] [PubMed] [Google Scholar]


