Abstract
Mitochondrial oxidative phosphorylation (OxPhos) induces resistance to MAPK pathway inhibitors in melanoma. However, therapeutic targeting of mitochondria is challenging. In a recent study, we showed that inhibition of mTOR kinase activity resensitized resistant melanomas by indirectly inhibiting OxPhos via a novel mechanism. Here, we discuss the implications of these findings.
Keywords: BRAF, MEK, metabolism, MITF, NRAS, PGC1α
In the 1920s Otto Warburg observed that, unlike normal cells, cancer cells exhibit aerobic glycolysis, i.e., a high rate of glycolysis even in the presence of oxygen. Initially postulated to be the result of mitochondrial dysfunction, it is now recognized that the Warburg effect is a metabolic adaptation in cells with intact mitochondria that is driven by microenvironmental factors or by mutations in classical signaling pathways. For example, mitochondrial respiration in low-oxygen environments produces reactive oxygen species (ROS) and induces oxidative stress. This promotes HIF1α stabilization, which then shifts cellular metabolism toward glycolysis by inactivating pyruvate kinase. However, HIF1α can also be stabilized under normoxic conditions via facilitative gene mutations or by activation of signaling pathways such as PI3K–AKT. Moreover, it has now been demonstrated that tumors employ a variety of metabolic adaptations beyond aerobic glycolysis, including altered glutamine metabolism.1 Recently, Haq et al. demonstrated that activation of the ERK1/2 MAPK pathway by the oncogenic BRAFV600E mutation is associated with metabolic reprogramming of melanoma cells toward elevated glycolysis.2 This switch is mediated by suppression of the lineage-specific transcription factor MITF, a transcriptional activator of PGC1α which is a key regulator of mitochondrial biogenesis and oxidative phosphorylation (OxPhos). Haq and others have further demonstrated that homogenous populations or subsets of melanoma cells may also exhibit elevated OxPhos, an adaptation that confers de novo resistance to both MAPK pathway inhibition (MAPKi) and oxidative stress.2–4 These results support combinatorial inhibition of the MAPK pathway and OxPhos. Although classic inhibitors of mitochondrial respiration are too toxic for clinical implementation some researchers have proposed biguanides such as metformin or phenformin as potential candidates; however, their non-specificity and dosage requirements may limit their use for cancer therapy.5
We recently used 2 unbiased approaches—whole genome siRNA synthetic lethality screening and mRNA expression profiling—to broadly interrogate melanoma resistance to MAPKi and identified elevated OxPhos as a key mediator of resistance.6 Similar to other recent studies, we found that elevated OxPhos significantly correlated with expression of PGC1α and MITF.2,3 In addition to confirming the role of OxPhos in de novo resistance, we demonstrated for the first time that, in approximately half of all examined melanoma cell lines and patient samples with acquired resistance, MAPKi resistance was associated with high OxPhos and high PGC1α.6 We had previously observed that some cell lines with de novo resistance to MAPKi were sensitive to a combination of the MEK inhibitor Selumetinib (MEKi) and the catalytic mTOR inhibitor AZD8055 (mTORC1/2i), which inhibits both Raptor and Rictor complexes of mTOR.7 In the current study, we unexpectedly found that combination treatment with MEKi and mTORC1/2i resulted in synergistic growth inhibition and apoptosis of BRAFV600E-mutant and NRAS-mutant de novo MAPKi-resistant cell lines with high OxPhos/PCG1α. However, this synergy was not observed in resistant lines with low OxPhos/PGC1α. In addition to its efficacy in de novo resistance, this combination was also effective in melanomas with acquired MAPKi resistance that had high OxPhos/PGC1α, but was ineffective in those with low OxPhos/PGC1α. In a BRAFV600E-mutant high OxPhos/PGC1α xenograft mouse model, this combination was significantly more effective in suppressing tumor growth than either inhibitor alone. Although mTORC1 has previously been implicated in mitochondrial activation,8,9 we demonstrated for the first time that inhibition of both mTORC1 and mTORC2 decreases OxPhos more effectively than inhibition of either complex alone. Furthermore, we found that mTORC1/2i markedly reduces PGC1α expression by decreasing nuclear levels of its key transcriptional activator, MITF. Although we did not evaluate its mechanism of action, we hypothesize that mTOR activity enhances nuclear MITF levels by preventing its cytoplasmic extrusion and/or degradation; MITF nuclear levels are further increased by MAPKi-induced nuclear localization and de novo expression, which in turn transcriptionally activates PGC1α (Fig. 1A and B). Conversely, mTOR1/2i treatment promotes cytoplasmic extrusion and/or degradation of MITF, thus decreasing its nuclear levels and the level of PGC1α (Fig. 1C). Elucidation of this mechanism may benefit the development of therapeutic agents.
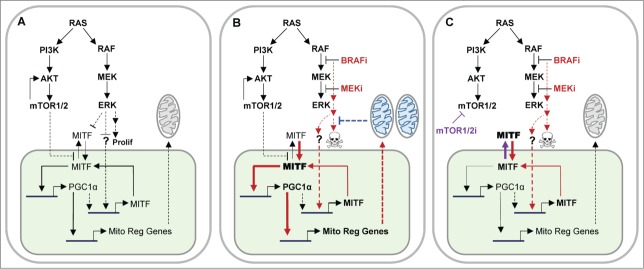
Figure 1.
Mechanism of MAPK inhibitor-induced resistance in melanoma and its reversal with inhibition of mTOR catalytic activity. (A) Activated RAS/RAF/MEK/ERK MAPK pathway in NRAS- and BRAF-mutant melanomas promotes cell proliferation (Prolif) in conjunction with the PI3K/AKT/mTOR pathway. Both pathways may exert homeostatic control over MITF levels by regulating its post-translational modification and nuclear (green) levels. Transcription of MITF may be regulated by PGC1α, independent of the MAPK pathway. (B) In MAPKi-resistant melanomas, inhibition of activated MAPK pathway by a BRAFV600 inhibitor (BRAFi) or a MEK inhibitor (MEKi) increases nuclear levels of MITF, which in turn induces the expression of PGC1α, an activator of mitochondrial regulatory genes (Mito Reg Genes), resulting in elevated oxidative phosphorylation (OxPhos) represented by blue mitochondria. These inhibitors also activate MITF transcription by an unknown (?) mechanism. Elevated OxPhos inhibits mitochondrial apoptotic pathways and prevents BRAFi/MEKi-induced cell death. (C) An mTOR catalytic inhibitor (mTOR1/2i) that inhibits both complexes I and II of mTOR decreases nuclear levels of MITF and inactivates BRAFi/MEKi-induced PGC1α expression. This sensitizes melanoma cells to BRAFi/MEKi-induced cell death. In the above schematics, continuous lines represent direct effects; discontinuous lines, indirect effects; thick lines and bold font, increased activity; red and purple colors, BRAFi/MEKi- and mTOR1/2i-induced effects, respectively.
Although our study has emphasized the significance of high OxPhos in MAPKi resistance, this metabolic phenotype was not universal among resistant cell lines or patient samples. Cell lines that demonstrated low OxPhos/PGC1α were not sensitive to the combination of MEKi plus mTORC1/2i. We did not identify the mechanism of resistance of the low OxPhos MAPKi resistant cell lines, but a recent study by Parmenter et al. described a network of transcription factors including HIF1α and MYC that were downregulated in melanoma cell lines and patient samples in response to MAPKi, resulting in inhibition of glycolysis.10 Although no alterations in OxPhos levels were observed, glycolysis and its associated transcriptional networks were restored in cells and samples from patients that developed resistance.
In summary, recent work has shown that oncogenic signaling pathways can drive metabolic reprogramming in melanoma, and that metabolic adaptation is a mechanism of resistance to targeted therapy. However, there is also heterogeneity of metabolic phenotypes, which appears to drive the response to combinatorial therapy. Specifically, we and others have demonstrated that OxPhos is a major mechanism of both de novo and acquired resistance to MAPKi in melanoma and that this is driven by increased expression of MITF and PCG1α. We have also demonstrated that MAPKi resistance can be overcome by mTORC1/2i acting via nuclear exclusion of MITF, and that this combination is synergistic only in the high OxPhos phenotype. These findings support the rationale for the identification of clinical biomarkers to identify targetable metabolic adaptations such as OxPhos in melanoma and other cancers and thus counteract resistance to targeted therapies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Acknowledgments
We thank Michael A Davies, MD, PhD, for critical reading of this article.
References
- 1.Dang CV, Links between metabolism and cancer. Genes Dev 2012;26:877-90; PMID:22549953; http://dx.doi.org/ 10.1101/gad.189365.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haq R, Shoag J, Andreu-Perez P, Yokoyama S, Edelman H, Rowe GC, Frederick DT, Hurley AD, Nellore A, Kung AL, et al. . Oncogenic BRAF regulates oxidative metabolism via PGC1alpha and MITF. Cancer Cell 2013;23:302-15; PMID:23477830; http://dx.doi.org/ 10.1016/j.ccr.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vazquez F, Lim JH, Chim H, Bhalla K, Girnun G, Pierce K, Clish CB, Granter SR, Widlund HR, Spiegelman BM, et al. . PGC1alpha expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell 2013;23:287-301; PMID:23416000; http://dx.doi.org/ 10.1016/j.ccr.2012.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roesch A, Vultur A, Bogeski I, Wang H, Zimmermann KM, Speicher D, Körbel C, Laschke MW, Gimotty PA, Philipp SE, et al. . Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell 2013;23:811-25; PMID:23764003; http://dx.doi.org/ 10.1016/j.ccr.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollak M, Targeting oxidative phosphorylation: why, when, and how. Cancer Cell, 2013;23:263-4; PMID:23518341; http://dx.doi.org/ 10.1016/j.ccr.2013.02.015 [DOI] [PubMed] [Google Scholar]
- 6. Gopal YN, Rizos H, Chen G, Deng W, Frederick DT, Cooper ZA, Scolyer RA, Pupo GM, Komurov K, Sehgal V, et al. . Inhibition of mTORC1/2 overcomes resistance to MAPK pathway inhibitors mediated by PGC1α and Oxidative Phosphorylation in melanoma. Cancer Res 2014; 74:7037-47; PMID:2529763420959481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gopal YN, Deng W, Woodman SE, Komurov K, Ram P, Smith PD, Davies MA. Basal and treatment-induced activation of AKT mediates resistance to cell death by AZD6244(ARRY-142886) in Braf-mutant human cutaneous melanoma cells. Cancer Res 2010;70:8736-47; PMID:20959481; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-0902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morita M, Gravel SP, Chénard V, Sikström K, Zheng L, Alain T, Gandin V, Avizonis D, Arguello M, Zakaria C, et al. . mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab 2013;18:698-711; PMID:24206664; http://dx.doi.org/ 10.1016/j.cmet.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 9.Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. et al. . mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 2007;450:736-40; PMID:18046414 [DOI] [PubMed] [Google Scholar]
- 10.Parmenter TJ, Kleinschmidt M, Kinross KM, Bond ST, Li J, Kaadige MR, Rao A, Sheppard KE, Hugo W, Pupo GM, et al. . Response of BRAF-mutant melanoma to BRAF inhibition is mediated by a network of transcriptional regulators of glycolysis. Cancer Discov, 20144:23-33; http://dx.doi.org/ 10.1158/2159-8290.CD-13-0440 [DOI] [PMC free article] [PubMed] [Google Scholar]